Abstract
This study evaluated the use of bulk milk as a diagnostic tool for estimation of herd-level Neospora caninum exposure in Atlantic Canada; it was used to estimate the prevalence of dairy farms with a within-herd N. caninum-seroprevalence ≥ 15% in Prince Edward Island (PEI). The variation over time of N. caninum antibodies in bulk milk is also reported. Skimmed bulk milk and individual serum samples were analyzed for N. caninum antibodies by using an enzyme-linked immunosorbent assay (ELISA). Bulk milk samples were collected in May 2004 (n = 235), May 2005 (n = 189), and June 2005 (n = 235). The prevalence of dairy farms with a within-herd seroprevalence ≥ 15% on PEI was 6.4% in May 2004. In May and June 2005, respectively, 10.1% and 10.2% of farms had a ≥ 15% within-herd seroprevalence. In 11 farms that were considered positive based on bulk milk samples, blood samples were collected from all adult cows in September 2005, in conjunction with a 4th bulk milk sample on the same day. The correlation coefficient between serology and bulk milk ELISA was 0.87. The results of this study demonstrate that the prevalence of N. caninum in dairy farms can be estimated by using a bulk milk ELISA.
Résumé
Titrage immunoenzymatique du lait en vrac par antigène adsorbé pour estimer la prévalence de Neospora caninum dans des fermes laitières de l’Île du Prince-Édouard au Canada. Cette étude avait pour but d’évaluer l’utilisation du lait en vrac comme outil diagnostique afin d’estimer le niveau d’exposition des troupeaux du Canada atlantique à Neospora caninum. Ce travail a servi à mesurer la prévalence des fermes laitières de l’Île-du-Prince-Édouard (IPE) présentant une séroprévalence de troupeaux à N. caninum ≥ à 15 %. La variation dans le temps des anticorps à N. caninum dans le lait en vrac est également rapportée. Du lait en vrac écrémé ainsi que des échantillons individuels de sérum ont été analysés pour mesurer les anticorps à N. caninum par titrage immunoenzymatique utilisant un antigène adsorbé (ELISA). Les échantillons de lait en vrac ont été recueillis en mai 2004 (n = 235), mai 2005 (n = 189) et juin 2005 (n = 235). La prévalence des fermes laitières de l’IPE présentant une séroprévalence de troupeaux ≥ à 15 % était de 6,4 % en mai 2004. En mai et juin 2005, cette même séroprévalence était respectivement de 10,1 et 10,2 %. En septembre 2005, dans 11 fermes considérées positives selon des résultats de l’analyse des échantillons de lait en vrac, des échantillons sanguins ont été prélevés chez toutes les vaches adultes, conjointement avec le 4ième échantillon de lait en vrac de la journée. Le coefficient de corrélation entre la sérologie et l’ELISA sur le lait en vrac était de 0,87. Les résultats de cette étude démontrent que la séroprévalence de N. caninum dans les fermes laitières peut être estimée par ELISA sur le lait en vrac.
(Traduit par Docteur André Blouin)
Introduction
N eospora caninum is an important cause of sporadic, epidemic, and endemic abortion in cattle worldwide (1). The infection usually has a chronic course and persists throughout the life of an infected animal (2). Thus far, no vaccine is available that limits endogenous transplacental infection (3), and there is no treatment that prevents or cures N. caninum infection. Consequently, the strategy to reduce the prevalence and the losses caused to the farming industry by N. caninum is to break the life cycle of the parasite and eliminate infected animals (4,5).
Definitive diagnosis of Neospora-associated abortions is based on examination of the aborted fetus, including observation of characteristic lesions, combined with immunoperoxidase staining or polymerase chain reaction (PCR) in fetal tissues. However, in many instances, fetal material is not available. In these situations, a presumptive diagnosis can be made based on the detection of antibodies to N. caninum in serum or milk. Previous research has suggested that farms with a within-herd seroprevalence ≥ 15% have an increased risk for reproductive losses (6,7). Therefore, a 15% within-herd seroprevalence is considered an appropriate cut-off value for identifying a herd with substantial reproductive losses due to neosporosis (8).
Compared with individual serum samples, the collection of bulk milk samples (comprising a pooled sample of milk from all lactating cows in a herd) is a noninvasive, convenient, and economical method of sampling. Diagnostic tests adapted for use with bulk milk have been developed for several viral (9–13), bacterial (14–20), and parasitic (21–23) bovine diseases. Today, bulk milk analysis is routinely used as a tool in the diagnosis of Bovine herpesvirus-1 and Bovine viral diarrhea virus infections in dairy herds in Scandinavia (24–26).
In Europe and Asia, studies have evaluated test characteristics of enzyme-linked immunosorbent assays (ELISAs) for N. caninum on individual and bulk milk samples (8,27–31). In previous work, an indirect ELISA on individual milk samples demonstrated a sensitivity of 90% and a specificity of 90%, relative to serum, and a linear correlation between milk and serum antibody results was characterized by an R2 = 0.70 (29). Bartels et al (8) evaluated the application of the same indirect ELISA on bulk milk samples and found a sensitivity and specificity of 61% and 92%, respectively, and a negative and positive predictive value of 86% and 84%, respectively.
A recent study in Thailand showed that repeated bulk milk testing at regular intervals provided better information about a herd’s N. caninum status than did a single test (32). However, no studies have been performed in North America to evaluate test characteristics of antibodies to N. caninum in bulk milk.
In a serological survey performed in 1998, 63% of the Prince Edward Island (PEI) dairy farms had at least 2 N. caninum-positive cows (33). Due to the potential economic impact of N. caninum, particularly on the breeding and selling of high quality dairy cattle in PEI (34), there is great interest in screening dairy farms for the presence of N. caninum. Thus, a diagnostic test utilizing bulk milk rather than individual serum samples would be a valuable, cost-effective tool for identifying farms that may benefit from further investigation and implementation of control measures.
The objectives of this study were as follows: 1) to evaluate the use of bulk milk as a diagnostic tool for the estimation of herd-level exposure to N. caninum; 2) to estimate the prevalence of dairy farms in PEI with a ≥ 15% within-herd N. caninum seroprevalence, using bulk milk; and 3) to study the titer variation over time of antibodies to N. caninum in bulk milk.
Materials and methods
Sample population and collection of samples and data
Bulk milk samples from PEI dairy farms were collected in May 2004, May 2005, and June 2005. In May 2004 and June 2005, 20-mL bulk milk samples from 235 farms (all but 2 of the dairies in PEI in June 2005) were obtained for laboratory testing. In May 2005, because of technical difficulties, bulk milk samples were obtained from only 189 farms. No information on abortion history was available from these herds.
The bulk milk samples were centrifuged at 1000 × g for 10 min. Skimmed milk from beneath the cream layer was obtained from the milk samples and stored at −20°C until analyzed. At analysis, thawed skimmed milk aliquots of bulk milk samples were assayed for the presence of antibodies to N. caninum, using a commercially available indirect ELISA (Herd Check Anti-Neospora ELISA, IDEXX Laboratories, Westbrook, Maine, USA) and the manufacturer’s recommendations, with 1 exception; milk samples were diluted 1:2, instead of the 1:100 recommended for serum, as described by Bartels et al (8).
The positive and negative control sera provided with the test kit were used as controls at the 1:100 dilution recommended by the manufacturer. Test results were expressed as a sample-to-positive (S/P) ratio. The S/P ratio was defined as the optical density (OD) of the sample (S) minus the OD of the negative control (NC), all divided by the OD of the positive control (PC) minus the OD of the negative control ([S-NC]/[PC-NC]). Milk samples with a S/P ratio ≥ 0.6 were considered positive, thus indicating a farm with an estimated within-herd seroprevalence of 15% or more, suggesting that these farms have an increased risk for reproductive losses, as reported by Bartels et al (8).
To evaluate the use of the ELISA on bulk milk as a diagnostic tool for estimation of herd-level N. caninum exposure in Atlantic Canada, an Atlantic Veterinary College (AVC) validation process of this ELISA under AVC laboratory conditions was conducted. The validation utilized 31 reference bulk milk samples provided by the Animal Health Service (AHS) in Deventer, The Netherlands.
For 11 farms that were considered positive for N. caninum on one or more bulk milk samples, 10-mL blood samples were collected from all adult cows (ranging from 34 to 104 per farm) in September 2005. At this visit, a 4th bulk milk sample was also collected. During the visit, the stage of lactation and whether each cow’s milk was included in the bulk tank was recorded.
The blood samples were centrifuged at 1000 × g for 10 min. Serum was harvested from the blood samples and stored at −20°C. Serum samples were analyzed for antibodies to N. caninum by using a commercially available competitive-inhibition ELISA (VMRD, Pullman, Washington, USA) according to the manufacturer’s recommendations. Sera with an inhibition value > 30% were considered positive. The percentage inhibition was calculated as 100-([OD of test sera/mean OD of negative reference sera] × 100). The reported sensitivity and specificity of this test (using serum) are 97.6% and 98.6%, respectively (35). Samples from the 4th bulk milk sampling were handled and tested in a similar manner to the first 3 bulk milk samples.
Serum and milk samples were stored at −20°C and analyzed on 10 separate days during the study period when batches of at least 45 samples would be available for analysis. Serum and milk samples were analyzed in duplicate and the OD was measured in a microplate spectrophotometer (Spectramax 384plus; Molecular Devices Corporation, Sunnyvale, California, USA) at a wavelength of 650 nm.
Statistical analyses
A concordance correlation coefficient (CCC) (36) was calculated to estimate the level of agreement between reference bulk milk samples performed in the 2 laboratories, and between the bulk milk ELISA results from the different sampling dates. A Pearson’s correlation coefficient was calculated to determine the correlation between within-herd seroprevalence and bulk milk ELISA results of the 11 farms that were test-positive on bulk tank milk and were individually tested for cows positive for N. caninum infection. A one-way repeated measures analysis of variance (ANOVA) was used to determine a difference between the mean S/P ratio on the 3 sampling dates. Scatterplots were created to assist in the visual representation of the data. A statistical software program (Stata version 8; Stata Corp LP, College Station, Texas, USA) was used for the statistical analyses of the data.
Results
Reference bulk milk samples
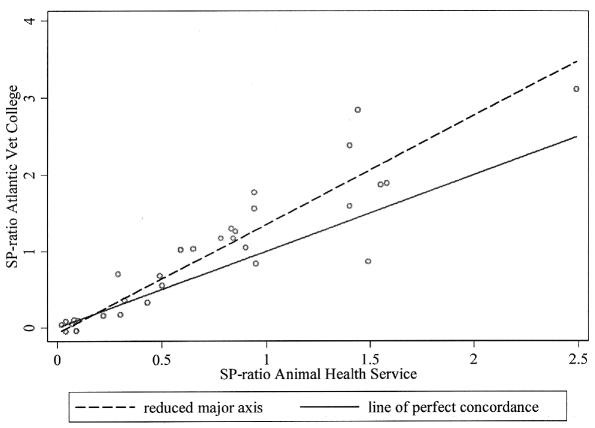
The ELISA results on the 31 reference samples correlated well between the 2 laboratories; the CCC was 0.82 (95% CI: 0.73–0.91) (Figure 1). Only 3 samples were classified as negative at the AHS but positive at the AVC. All 3 samples had ELISA results very close to the cut-off value (0.60) for 1 of the 2 tests (0.70, 0.68, and 0.59), making their interpretation challenging.
Figure 1.
Scatterplot of results from a Neospora caninum ELISA performed on 31 reference bulk milk samples analyzed at the Atlantic Veterinary College and at the Animal Health Service, The Netherlands. The solid line represents perfect agreement, whereas the dashed line indicates the line of best fit for the relationship between the test results.
Bulk milk from dairy farms on PEI
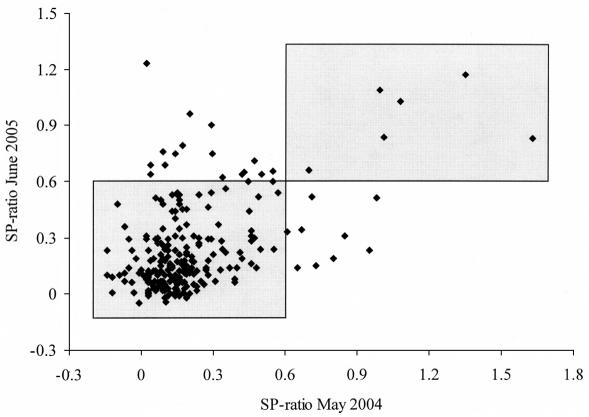
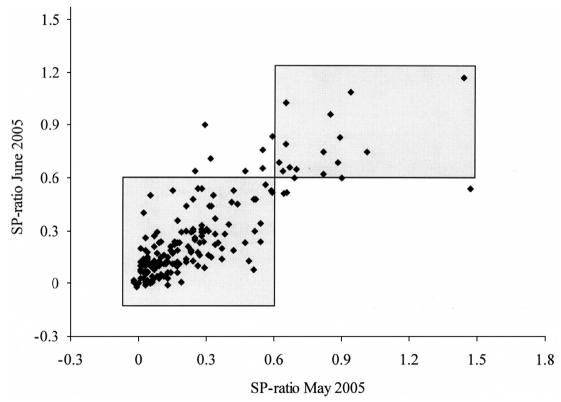
In May 2004, 15 of 235 bulk milk samples (6.4%, 95% CI: 3.2–9.5%) were ELISA-positive, while in May 2005 and June 2005, respectively, 19 of 189 (10.1%, 95% CI: 6.2–15.3%) and 24 of 235 (10.2%, 95% CI: 6.3–14.1%) farms were found to be positive (Figures 2 and 3). A one-way repeated measures ANOVA revealed a slight trend in increase in S/P ratio over time (P = 0.11), when comparing S/P ratios in May 2004 with May 2005 and June 2005. The CCC was 0.25 (95% CI: 0.13–0.37) on samples collected in May 2004 and June 2005. The CCC increased to 0.36 (95% CI: 0.23–0.48) and 0.73 (95% CI: 0.67–0.80), comparing samples collected in May 2004 and May 2005, and in May 2005 and June 2005, respectively. From 15 ELISA-positive farms in May 2004, 5 farms remained positive in both May 2005 and June 2005. Seven farms that were positive in 2004 were negative on both samplings in 2005.
Figure 2.
Scatterplot of results from a Neospora caninum ELISA performed on 2 bulk milk samples collected more than 1 y apart (May 2004 and June 2005) from 235 dairy farms in Prince Edward Island. The shaded areas contain farms that had the same test result (positive or negative) on both occasions, based on a cut-off value of ≥ 0.60.
Figure 3.
Scatterplot of results from a Neospora caninum ELISA performed on 2 bulk milk samples collected 1 mo apart (May and June 2005) from 189 dairy farms in Prince Edward Island. The shaded areas contain farms that had the same test result (positive or negative) on both occasions, based on a cut-off value of ≥ 0.60.
Regarding variation in test results from the bulk milk, 161 farms of the 189 farms that had results for May 2004 and May 2005 and June 2005 showed consistent test results, with 5 (3%) farms being positive for all 3 samplings and 156 (83%) farms being negative on all 3 samplings.
Individual serology and bulk milk from 11 selected farms
On the 11 selected farms, the total number of lactating animals contributing milk to the bulk tank ranged from 26 to 84 cows per farm. Although more than 11 farms were positive on one or more occasions, financial constraints limited whole herd serum testing to 11 farms.
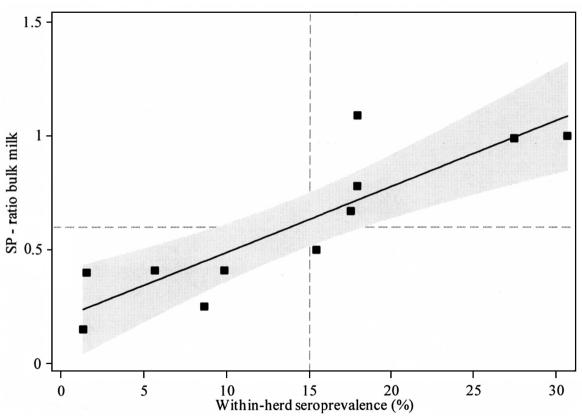
Results for the 11 selected farms that were ELISA-positive on bulk milk for 1 or more occasions in May 2004, May 2005, and June 2005 are shown in Table 1. The Pearson’s correlation coefficient between serology and bulk milk samples collected in September 2005 was 0.87 (95% CI: 0.57–0.97) (Figure 4). When the interpretation by Bartels et al (8) of bulk milk ELISA results with respect to seroprevalence levels was used, 10 of the 11 herds were classified correctly, when the established cut-off value of 0.60 was used (Table 1). Only Farm 6 did not classify correctly; Farm 6 was negative according to the 4th bulk milk sample (S/P ratio = 0.5), but it had a within-herd seroprevalence of slightly more than 15%, at 15.4%.
Table 1.
Comparison of Neospora caninum ELISA results from serum (cow level, 1 sampling date) and bulk milk (herd level, 4 sampling dates) from 11 dairy farms
| Bulk Milk ELISA S/P Ratioa |
Cow Level Serum ELISA Test Resultsb |
||||||
|---|---|---|---|---|---|---|---|
| Herd | May ’04 | May ’05 | June ’05 | Sept ’05 | Prevalence (%) | Number of positive cows | Total number of cows |
| 1 | 0.04 | 0.25 | 0.64 | 0.15 | 1.3 | 1 | 77 |
| 2 | 0.34 | 0.82 | 0.62 | 0.25 | 5.7 | 2 | 35 |
| 3 | 0.85 | 0.28 | 0.31 | 0.40 | 1.5 | 1 | 66 |
| 4 | 0.61 | 0.28 | 0.33 | 0.41 | 5.6 | 4 | 72 |
| 5 | 0.50 | 0.47 | 0.64 | 0.41 | 9.8 | 4 | 41 |
| 6 | 0.65 | 0.21 | 0.14 | 0.50 | 15.4 | 4 | 26 |
| 7 | 0.43 | 0.70 | 0.65 | 0.67 | 17.5 | 7 | 40 |
| 8 | 0.67 | 0.54 | 0.34 | 0.78 | 17.9 | 7 | 39 |
| 9 | 0.42 | 0.64 | 0.64 | 0.99 | 27.4 | 23 | 84 |
| 10 | 0.55 | 0.69 | 0.60 | 1.00 | 30.6 | 22 | 72 |
| 11 | 0.14 | 1.01 | 0.75 | 1.09 | 17.9 | 12 | 67 |
Sample-to-Positive Ratio — Bold printed numbers indicate a positive bulk milk result (S/P ≥ 0.60)
Individual serum samples were collected on the same day as the bulk milk sample in September 2005. All numbers refer only to lactating cows contributing to the bulk tank on the sampling date
Figure 4.
Scatterplot of bulk milk results from a Neospora caninum ELISA versus within-herd seroprevalence results from a Neospora caninum ELISA of the lactating cows that were contributing to the bulk tank, from 11 dairy farms in Prince Edward Island. Bulk milk and blood sample collection were performed on the same day in September 2005. Dashed lines represent a cutoff value of 0.60 for the S/P ratio and 15% for within-herd seroprevalence. The shaded area represents a 95% confidence interval around the best line of fit.
Discussion
The results of this study confirm that the prevalence of N. caninum in dairy farms can be estimated with the bulk milk ELISA that was used. A previous evaluation of this test determined a high specificity (92%), but a limited sensitivity (61%), when applying a cut-off value of 0.6 (8). Because of the moderate sensitivity, a considerable number of dairy herds tested will be incorrectly classified as negative. Depending on the purpose of a screening test, this can be a disadvantage. The test sensitivity can be increased by repeated testing (32). The frequency of repeated testing required to increase the sensitivity will depend on the dynamics of the infection and the dynamics in the lactating herd. The association between seroprevalence level and risk for reproductive losses may be different in different dairy industry situations. The herd-prevalence of N. caninum infection in The Netherlands is 76% in dairy herds (37), comparable with the 79% in dairy farms in Atlantic Canada (33). As the dairy industries in The Netherlands and Atlantic Canada are comparable, this cut-off value was considered appropriate in this study. The CCC of 0.82 between the 2 laboratories suggests that extrapolation of the S/P ratio cut-off value of 0.6, as proposed by Bartels et al (8), is adequate for an Atlantic Canadian context. Differences in S/P ratios may be caused by different laboratory techniques in performing an ELISA, such as automated versus manual washing steps. The use of more reference samples to optimize a cut-off value for each laboratory situation would be ideal, but it may not be feasible in a practical setting.
Investigating 11 farms by using serology in combination with bulk milk further confirmed that the cut-off value of 0.6 for bulk milk corresponded well with a within-herd seroprevalence of 15% (correlation coefficient = 0.87). Our main concern was in correctly classifying bulk milk ELISA-positive farms, and for this reason, bulk milk ELISA-positive farms were selected for individual animal serologic testing. Because of economic reasons, a competitive ELISA was used to test serum, while an indirect ELISA was used for the bulk milk analysis (in-kind contribution of competitive ELISA-kits made it possible to analyze sera at low cost). A recent study in which several N. caninum-assays were compared by using cattle sera demonstrated similar Se and Sp for the indirect and competitive ELISAs that were used in this study (38).
There was individual farm variability in S/P ratios in bulk milk over time. The difference over time in bulk milk ELISA results is likely the consequence of test-positive cows leaving or entering the milking herd. In addition, an active infection in the herd, such as a point source infection, may lead to a sudden increase in S/P ratio caused by an increased antibody titer. The bulk milk samples represent only cows that contributed milk to the bulk tank on the day of sampling, not dry cows, sick cows, or cows in the colostral period. If these cows, or the young stock of the herd, form the majority of seropositive animals, the bulk milk test may not detect the herd as being positive. However, performing multiple tests per year will compensate for this scenario. Another reason for dissimilarity between prevalence of antibody positive cows and total antibodies in bulk milk could be antibody levels, lactation stage, and milk yield per animal. This is likely to be more important in small herds with few cows contributing to the bulk milk (27). Lactation stage was identified as a factor that was associated with an increase in the milk result relative to the serum result in individual animals (29). We know from previous studies that Neospora-antibody titers in serum can fluctuate over time (39); therefore, it is likely that antibody titers in milk will also fluctuate. Despite these factors, there was good correlation between seroprevalence and the bulk milk ELISA S/P ratio performed on samples collected on the same day.
The proportion of bulk milk ELISA-positive farms on PEI tended to increase (P = 0.11) between May 2004 and June 2005. However, other factors besides increased seroprevalence may have played a role. Variation between ELISA-plates and variation between test days has to be considered, as well as other farm-level factors. The CCC increased considerably when sampling rounds that were closer in time (0.73 when 1 mo apart) were compared with those that were further apart (0.36 and 0.25 when 12 and 13 mo apart, respectively), due to dynamics in a dairy herd. A more detailed study (including factors at the farm and laboratory level) in which N. caninum antibody levels in milk and serum were monitored over time could provide more insight into the variability observed in this study.
A monitoring program where farms are tested on a regular basis in connection with already existing milk quality testing will be an economical way in which to identify farms that will benefit from intervention. The investment for the producer to monitor Neospora-status on the farm can therefore be minimal. The Dutch Animal Health Service started a Neospora-monitoring program in 2004 that has been received well by producers (40). In conclusion, the bulk milk ELISA used in this study will identify dairy farms with a ≥ 15% within-herd seroprevalence in Atlantic Canada. Repeated sampling is recommended, and more research is needed to possibly control factors involved in the observed variability. CVJ
Acknowledgments
The authors thank the dairy producers of Prince Edward Island for participating in this study. CVJ
Footnotes
In kind contribution was provided by IDEXX Laboratories Inc., Westbrook, Maine, USA and VMRD Inc., Pullman, Washington, USA.
References
- 1.Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkman C, Johansson O, Stenlund S, Holmdahl OJ, Uggla A. Neospora species infection in a herd of dairy cattle. J Am Vet Med Assoc. 1996;208:1441–1444. [PubMed] [Google Scholar]
- 3.Trees AJ, Williams DJ. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 2005;21:558–561. doi: 10.1016/j.pt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Frossling J, Uggla A, Bjorkman C. Prevalence and transmission of Neospora caninum within infected Swedish dairy herds. Vet Parasitol. 2005;128:209–218. doi: 10.1016/j.vetpar.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Wouda W, Moen AR, Schukken YH. Abortion risk in progeny of cows after a Neospora caninum epidemic. Theriogenology. 1998;49:1311–1316. doi: 10.1016/S0093-691X(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 6.Wouda W, Bartels CJ, Moen AR. Characteristics of Neospora caninum-associated abortion storms in dairy herds in The Netherlands (1995 to 1997) Theriogenology. 1999;52:233–245. doi: 10.1016/s0093-691x(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 7.Dijkstra T, Barkema HW, Eysker M, Wouda W. Evidence of post-natal transmission of Neospora caninum in Dutch dairy herds. Int J Parasitol. 2001;31:209–215. doi: 10.1016/s0020-7519(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 8.Bartels CJ, van Maanen C, van der Meulen AM, Dijkstra T, Wouda W. Evaluation of three enzyme-linked immunosorbent assays for detection of antibodies to Neospora caninum in bulk milk. Vet Parasitol. 2005;131:235–246. doi: 10.1016/j.vetpar.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Pritchard GC, Kirkwood GM, Sayers AR. Detecting antibodies to infectious bovine rhinotracheitis and BVD virus infections using milk samples from individual cows. Vet Rec. 2002;150:182–183. doi: 10.1136/vr.150.6.182. [DOI] [PubMed] [Google Scholar]
- 10.Paton DJ, Christiansen KH, Alenius S, Cranwell MP, Pritchard GC, Drew TW. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Vet Rec. 1998;142:385–391. doi: 10.1136/vr.142.15.385. [DOI] [PubMed] [Google Scholar]
- 11.Forschner E, Bunger I, Krause HP, Kuttler D. [Control measures in officially acknowledged brucellosis-free and leukosis unsuspected dairy herds on the basis of bulk milk samples in combination with ELISA tests] Dtsch Tierarztl Wochenschr. 1989;96:475–486. [PubMed] [Google Scholar]
- 12.Traven M, Bjornerot L, Larsson B. Nationwide survey of antibodies to bovine coronavirus in bulk milk from Swedish dairy herds. Vet Rec. 1999;144:527–529. doi: 10.1136/vr.144.19.527. [DOI] [PubMed] [Google Scholar]
- 13.Elvander M, Edwards S, Naslund K, Linde N. Evaluation and application of an indirect ELISA for the detection of antibodies to bovine respiratory syncytial virus in milk, bulk milk and serum. J Vet Diagn Invest. 1995;7:177–182. doi: 10.1177/104063879500700202. [DOI] [PubMed] [Google Scholar]
- 14.Thoen CO, Haas CA, Angus RD, Townsend AS. Evaluation of a potassium chloride extract of Brucella abortus in an ELISA for detecting Brucella antibodies in bulk tank milk samples from cows. Vet Microbiol. 1995;45:185–189. doi: 10.1016/0378-1135(94)00120-l. [DOI] [PubMed] [Google Scholar]
- 15.Dom PP, Haesebrouck F, Vandermeersch R, Descamps J, Van OK. Prevalence of Leptospira interrogans serovar hardjo antibodies in milk in Belgian dairy herds. Vet Q. 1991;13:118–120. doi: 10.1080/01652176.1991.9694294. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen SS, Thamsborg SM, Houe H, Bitsch V. Bulk-tank milk ELISA antibodies for estimating the prevalence of paratuberculosis in Danish dairy herds. Prev Vet Med. 2000;44:1–7. doi: 10.1016/s0167-5877(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 17.Paiba GA, Green LE, Lloyd G, Patel D, Morgan KL. Prevalence of antibodies to Coxiella burnetii (Q fever) in bulk tank milk in England and Wales. Vet Rec. 1999;144:519–522. doi: 10.1136/vr.144.19.519. [DOI] [PubMed] [Google Scholar]
- 18.Grove TM, Jones GM. Use of an enzyme-linked immunosorbent assay to monitor the control of Staphylococcus aureus mastitis. J Dairy Sci. 1992;75:423–434. doi: 10.3168/jds.S0022-0302(92)77778-9. [DOI] [PubMed] [Google Scholar]
- 19.Veling J, van Zijderveld FG, van Zijderveld-van Bemmel AM, Schukken YH, Barkema HW. Evaluation of two enzyme-linked immunosorbent assays for detecting Salmonella enterica subsp. enterica serovar dublin antibodies in bulk milk. Clin Diagn Lab Immunol. 2001;8:1049–1055. doi: 10.1128/CDLI.8.6.1049-1055.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen LR, Ersboll AK. Factors associated with variation in bulk-tank-milk Salmonella Dublin ELISA ODC% in dairy herds. Prev Vet Med. 2005;68:165–179. doi: 10.1016/j.prevetmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Kloosterman A, Ploeger HW, Pieke EJ, Lam TJ, Verhoeff J. The value of bulk milk ELISA Ostertagia antibody titres as indicators of milk production response to anthelmintic treatment in the dry period. Vet Parasitol. 1996;64:197–205. doi: 10.1016/0304-4017(95)00919-1. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez J, Dohoo I. A bulk tank milk survey of Ostertagia ostertagi antibodies in dairy herds in Prince Edward Island and their relationship with herd management factors and milk yield. Can Vet J. 2002;43:454–459. [PMC free article] [PubMed] [Google Scholar]
- 23.Charlier J, Claerebout E, Duchateau L, Vercruysse J. A survey to determine relationships between bulk tank milk antibodies against Ostertagia ostertagi and milk production parameters. Vet Parasitol. 2005;129:67–75. doi: 10.1016/j.vetpar.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg AL, Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Vet Microbiol. 1999;64:197–222. doi: 10.1016/s0378-1135(98)00270-3. [DOI] [PubMed] [Google Scholar]
- 25.Niskanen R, Alenius S, Larsson B, Jacobsson SO. Determination of level of antibodies to bovine virus diarrhoea virus (BVDV) in bulk tank milk as a tool in the diagnosis and prophylaxis of BVDV infections in dairy herds. Arch Virol Suppl. 1991;3:245–251. doi: 10.1007/978-3-7091-9153-8_30. [DOI] [PubMed] [Google Scholar]
- 26.Nylin B, Stroger U, Ronsholt L. A retrospective evaluation of a Bovine Herpesvirus-1 (BHV-1) antibody ELISA on bulk-tank milk samples for classification of the BHV-1 status of Danish dairy herds. Prev Vet Med. 1999;47:91–105. doi: 10.1016/s0167-5877(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 27.Chanlun A, Naslund K, Aiumlamai S, Bjorkman C. Use of bulk milk for detection of Neospora caninum infection in dairy herds in Thailand. Vet Parasitol. 2002;110:35–44. doi: 10.1016/s0304-4017(02)00315-1. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkman C, Holmdahl OJ, Uggla A. An indirect enzyme-linked immunoassay (ELISA) for demonstration of antibodies to Neospora caninum in serum and milk of cattle. Vet Parasitol. 1997;68:251–260. doi: 10.1016/s0304-4017(96)01076-x. [DOI] [PubMed] [Google Scholar]
- 29.Schares G, Barwald A, Staubach C, et al. Adaptation of a commercial ELISA for the detection of antibodies against Neospora caninum in bovine milk. Vet Parasitol. 2004;120:55–63. doi: 10.1016/j.vetpar.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Schares G, Barwald A, Staubach C, et al. Regional distribution of bovine Neospora caninum infection in the German state of Rhineland-Palatinate modelled by logistic regression. Int J Parasitol. 2003;33:1631–1640. doi: 10.1016/s0020-7519(03)00266-2. [DOI] [PubMed] [Google Scholar]
- 31.Frossling J, Lindberg A, Bjorkman C. Evaluation of an iscom ELISA used for detection of antibodies to Neospora caninum in bulk milk. Prev Vet Med. 2006;74:120–129. doi: 10.1016/j.prevetmed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Chanlun A, Emanuelson U, Chanlun S, Aiumlamai S, Bjorkman C. Application of repeated bulk milk testing for identification of infection dynamics of Neospora caninum in Thai dairy herds. Vet Parasitol. 2006;136:243–250. doi: 10.1016/j.vetpar.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Keefe GP, VanLeeuwen JA. Neospora then and now: Prevalence of Neospora caninum in Maritime Canada in 1979, 1989, and 1998. Can Vet J. 2000;41:864–866. [PMC free article] [PubMed] [Google Scholar]
- 34.Trees AJ, Davison HC, Innes EA, Wastling JM. Towards evaluating the economic impact of bovine neosporosis. Int J Parasitol. 1999;29:1195–1200. doi: 10.1016/s0020-7519(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 35.Baszler TV, Adams S, Vander-Schalie J, Mathison BA, Kostovic M. Validation of a commercially available monoclonal antibody-based competitive-inhibition enzyme-linked immunosorbent assay for detection of serum antibodies to Neospora caninum in cattle. J Clin Microbiol. 2001;39:3851–3857. doi: 10.1128/JCM.39.11.3851-3857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 37.Bartels CJ, Arnaiz-Seco JI, Ruiz-Santa-Quitera A, et al. Supranational comparison of Neospora caninum seroprevalences in cattle in Germany, The Netherlands, Spain and Sweden. Vet Parasitol. 2006;137:17–27. doi: 10.1016/j.vetpar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Wapenaar W, Barkema HW, VanLeeuwen JA, et al. Comparison of serological methods for the diagnosis of Neospora caninum infection in cattle. Vet Parasitol. 2007;143:166–173. doi: 10.1016/j.vetpar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins MC, Wouda W, Dubey JP. Serological response over time to recombinant Neospora caninum antigens in cattle after a neosporosis-induced abortion. Clin Diagn Lab Immunol. 1997;4:270–274. doi: 10.1128/cdli.4.3.270-274.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dijkstra T, Bartels CJM, Wouda W. Control of bovine neosporosis: Experiences from the Netherlands. Proc World Assoc Adv Vet Parasitol. 2005:191. [Google Scholar]