Abstract
The efficacy of low doses of radiotherapy for the treatment of pituitary corticotroph macrotumors in dogs is evaluated retrospectively. Twelve dogs with pituitary-dependent hyperadrenocorticism and a large pituitary tumor treated with 36 Gy of radiation were included. Radiation was delivered in 12 fractions of 3 Gy over a 4- to 6-week period. Effects of radiation therapy on tumor size were assessed by computed tomography scans; a decrease was observed in 11 dogs (decrease > 50% in 6 dogs). Three dogs were reirradiated due to major tumor regrowth or a lack of tumor decrease (mean total dose: 22 Gy given in 3-Gy fractions over 3 or 4 weeks). The mean and median survival times following the initiation of radiotherapy were 22.6 months (688 days) and 17.7 months (539 days), respectively. These data are consistent with previous findings, based on high-dose radiation, showing that radiotherapy is a useful option for treating pituitary corticotroph macrotumors in dogs. Furthermore, computed tomography follow-up of the treated dogs demonstrates objectively the efficacy of radiotherapy against corticotroph tumors in dogs.
Résumé
Effets de la radiothérapie sur les tumeurs hypophysaires corticotropes de grande taille chez le chien : étude rétrospective sur 12 cas. Une étude rétrospective a été réalisée afin d’évaluer l’efficacité d’un protocole de radiothérapie à faible dose dans le traitement des macroadénomes hypophysaires corticotropes du chien. Douze chiens présentant une maladie de Cushing liée à un macroadénome hypophysaire et traités par radiothérapie à une dose de 36 Gy ont été inclus dans l’étude. La dose totale a été délivrée en 12 séances de 3 Gy réparties sur 4 à 6 semaines. L’efficacité de la radiothérapie a été évaluée par examen tomodensitométrique et une diminution de la taille des tumeurs a été objectivée chez 11 chiens (diminution > 50 % chez 6 chiens). Une réirradiation a été effectuée chez 3 chiens présentant une absence de réponse ou une nouvelle croissance de la tumeur (dose totale moyenne : 22 Gy administrés en séances de 3-Gy pendant 3 à 4 semaines). Les moyenne et médiane de survie calculées à partir du début de l’irradiation pour les 12 chiens ont été respectivement de 22,6 mois (688 jours) et 17,7 mois (539 jours). Ces durées de survie rejoignent les conclusions d’études préalables, utilisant des doses d’irradiation supérieures, en confirmant que la radiothérapie est une option intéressante dans le traitement des macroadénomes hypophysaires corticotropes du chien. Le suivi des chiens traités par examen tomodensitométrique offre par ailleurs une démonstration objective de l’efficacité de la radiothérapie sur les adénomes corticotropes canins.
(Traduit par les auteurs)
Introduction
Canine hyperadrenocorticism usually results from the hyper-secretion of adrenocorticotropic hormone (ACTH) by a pituitary tumor. When large tumors (diameter ≥ 1 cm), macroadenomas or macrocarcinomas, are responsible for pituitary- dependent hyperadrenocorticism (PDH), local control of the tumor is indicated, as these situations are often associated with a more severe clinical pattern [high frequency of neurological signs related to a space-occupying mass effect (1)] and a shorter survival time (2). Pituitary surgery is the treatment of choice for PDH in humans and recently has been shown to be successful with small tumors in dogs (3,4). For pituitary macrotumors, characterized by a major dorsal (suprasellar) extension, in dogs, radiation therapy is considered the treatment of choice. The value of radiotherapy in dogs has been assessed in a few studies (5–7) and these have provided useful data concerning the survival times associated with such procedures. However, in most cases, the efficacy of radiotherapy was evaluated indirectly, based on the progression of neurological signs (6,7). In a minority of dogs, tumor progression was assessed directly at particular time points, by computed tomography (CT) or magnetic resonance imaging (MRI) examinations or necropsy (6,7).
This retrospective study was designed to assess thoroughly the efficacy of pituitary irradiation on 12 dogs with pituitary corticotroph macrotumors by measuring tumor size and survival time after irradiation.
Materials and methods
Records of dogs with adrenocorticotropic hormone (ACTH)-secreting pituitary macrotumors, treated with megavoltage irradiation in a single center, were evaluated retrospectively. Included were all dogs with the following: 1) a pituitary macrotumor (largest diameter ≥ 1 cm) identified by contrast-enhanced CT; 2) an individual history consistent with hyperadrenocorticism, clinical signs, or results of serum biochemical analyses consistent with hyperadrenocorticism; 3) at least 1 endocrine test result (ACTH stimulation test or low-dose dexamethasone suppression test) that corroborated the diagnosis of hyperadrenocorticism; and 4) complete radiotherapy.
Dogs
Twelve dogs (7 female, 5 male) met these inclusion criteria. They were between 4 and 12 y old (mean, 8.6 y) at the beginning of radiotherapy. Ten dogs were purebred, with 9 breeds represented (Table 1). Five dogs displayed clinical neurological signs before irradiation; these included abnormal behavior in 4 dogs (vocalization, sleep disorders, disorientation, head pressing), an altered state of consciousness (depression) in 2 dogs, abnormal gait in 1 dog (mild ataxia), and tremor in 2 dogs. One dog was given mannitol and prednisone before irradiation to lessen the neurological signs.
Table 1.
Description [age at the beginning of radiotherapy, breed, sex, radiation procedure (total dose delivered, type of radiation therapy) and adjuvant control of hypercortisolism] of the 12 dogs included in the study
| Patient | Sex | Age | Breed | Radiation procedure | Adjuvant control of hypercortisolism |
|---|---|---|---|---|---|
| A | F | 9 y | Bearded collie | 36 Gy (cobalt) | mitotane |
| B | M | 4 y | Poodle | 63 Gy (36 + 27) (cobalt) | — |
| C | M | 7.5 y | Boxer | 36 Gy (cobalt) | mitotane |
| D | F | 12 y | Dachshund | 54 Gy (36 + 18) (cobalt) | ketoconazole |
| E | CM | 8 y | Mix breed | 36 Gy (cobalt) | — |
| F | M | 8 y | Bernese mountain dog | 36 Gy (orion) | mitotane |
| G | F | 11.5 y | Mix breed | 36 Gy (orion) | mitotane |
| H | SF | 7 y | Jack Russell terrier | 57 Gy (36 + 21) (orion) | mitotane |
| I | SF | 9 y | Poodle | 36 Gy (orion) | mitotane |
| J | SF | 9.5 y | Labrador retriever | 36 Gy (orion) | mitotane |
| K | M | 8 y | West Highland white terrier | 36 Gy (orion) | mitotane |
| L | M | 10.5 y | Fox terrier | 36 Gy (orion) | mitotane |
F — female, M — male, SF — spayed female, CM — castrated male, y — year
Ten dogs received medical treatment for hyperadrenocorticism after radiotherapy (some dogs had already been treated before). Treatment consisted, according to a described protocol (8), of administration of mitotane (Mitotane; Cooper, Melun, France), induction dose (50 mg/kg body weight (BW)/d PO) followed by a weekly maintenance dose (50–100 mg/kg BW/wk, PO) in 9 dogs, and of ketoconazole (Ketofungol; Janssen-Cilag, Issy-les-Moulineaux, France) (10 mg/kg BW/d, PO) in 1 dog.
Endocrine tests
One of the following 2 screening tests was performed in each dog: a) ACTH stimulation test [determination of plasma cortisol concentration before and 1.5 h after the administration of tetracosactide (Synactène immediat; Novartis, Rueil-Malmaison, France), 0.25 mg, IM], or b) low-dose dexamethasone suppression test [determination of plasma cortisol concentration before and 8 h after the administration of dexamethasone phosphate (Soludecadron; Merck Sharp et Dohme-Chibret, Paris, France), 0.01 mg/kg BW, IV] (8). Hyperadrenocorticism was confirmed by detection of abnormally high post-ACTH serum cortisol concentrations (> 600 nmol/L; reference range, 100 to 600 nmol/L) or inadequate suppression of 8-hour post-dexamethasone plasma cortisol concentrations (≥ 40 nmol/L; reference range, < 30 nmol/L).
Computed tomography
Before CT scans, all dogs were anesthetized with a similar sedation protocol. They were induced with propofol (Rapinovet; Mallinckrodt Veterinary, Levallois-Perret, France), 2 mg/kg BW, IV, and maintained with a mixture of isoflurane (Forene; Zeneca Pharma, Cergy, France), 1.5–2.5%, and oxygen (flow rate: 1 L/min) after intubation with a non-rebreathing anesthetic circuit (dog’s BW < 7 kg) or a semi-closed rebreathing circuit (dog’s BW ≥ 7 kg). With the animal in sternal recumbency, images were acquired with a body scanner (axial scanner — CGR 12000 or General Electric CT Pace- or helicoid scanner — HISPEED Cte/plus; General Electric Medical Systems, General Electric Company, Paris, France) before and after injection of iodinated contrast medium (Télébrix 35; Guerbet, Roissy-Charles de Gaulle, France), 2 mL/kg BW, IV. Contiguous, 3-mm thick, transverse images were obtained, extending from the external occipital protuberance to the cribriform plate, as previously described (9). The height of each tumor was measured from the transverse CT image that contained the greatest pituitary neoplastic area and compared with the height of each cranial vault. Tumor areas were estimated in 7 dogs by using the following equation: height × width × π/4, where: π = 3.14, and height and width were the largest diameters obtained from CT images.
Irradiation procedure
Each dog was anesthetized with propofol (Rapinovet; Mallinckrodt Veterinary), 2 mg/kg BW, IV. Five dogs were treated with cobalt-60 teletherapy (Teletherapy Unit, Ottawa, Ontario) and 7 dogs were irradiated by using a 5 MV linear accelerator (Orion megavoltage; Varian Medical Systems, Buc, France). For the linear accelerator, dose distribution was determined by a software program (Eclipse 3D treatment-planning software; Varian Medical Systems), based on initial CT findings. For cobalt-60 teletherapy, dose profiles were manually calculated by a nuclear physicist. Radiation therapy was delivered to the tumor site by bilaterally opposed portals directed over the temporal region. The planning target volume included the tumor and a 10-mm margin. Eight animals received a total of 36 Gy (unit of absorbed dose of ionizing radiation) given in 12, 3-Gy fractions during 4 wk on an alternate-day schedule (Monday, Wednesday, Friday). The other 4 received the same total dose over 6 wk (Monday, Thursday).
Follow-up and data analysis
The response of the tumor to radiotherapy was evaluated by several CT examinations (1 to 4, depending on survival time and clinical progression, except for 1 dog that died before it could be reexamined). In 9 dogs, the first CT reexaminations took place 1 to 5 mo after the first irradiation session (mean, 3 mo). For 2 dogs (dogs G and I), the first CT reexaminations took place after 12 and 7 mo, respectively.
Tumor progression (tumor size on CT images) was evaluated by comparing tumor height on the repeat CT examination with tumor height on the initial CT examination. When several repeat CT examinations had been performed for the same animal, the one showing the smallest tumor height was used for evaluation of tumor size progression.
Survival time was calculated from the initiation of radiation therapy until death or the end of the study (end of December 2003).
Radiation toxicoses were evaluated in all dogs by means of a neurological examination before each repeat CT examination.
Statistical analysis
Differences between heights of tumors before and after radiotherapy were compared by using the Wilcoxon test.
Survival data and curves (all causes of death) were generated by the product-limit method (Kaplan-Meier). All tests were carried out with describe type of software (Statview 5.0; SAS Institute, Carey, North Carolina, USA).
Results
Tumor progression
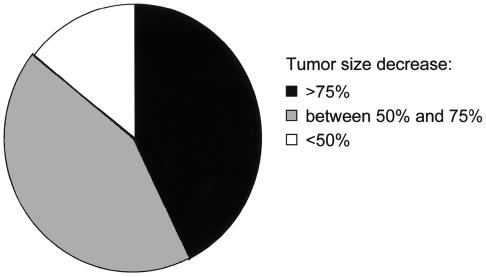
Computed tomography scans were performed in 11 dogs. One dog (dog K) died before any follow-up CT evaluation. Mean tumor heights before and after radiotherapy were significantly different (mean, standard deviation, respectively: 14.1, s = 3.6 mm and 8.8, s = 4.1 mm, P < 0.02). A decrease of more than 30% in pituitary tumor height was observed in 10 of the 11 dogs reexamined. Six macroadenomas decreased in surface area by more than 50% (Figure 1). In 1 dog (dog I), the pituitary tumor was no longer visible on the last CT scan (19 mo).
Figure 1.
Tumor size progression expressed as percentage of area reduction corresponding to the ratio of the tumor area on control CT examination to the tumor area on initial CT examination, estimated in 7 dogs.
In most cases, a decrease was observed during first evaluation after irradiation therapy. In 1 dog (dog A), a response to treatment was visible on CT images only after 16 mo (no progression was visible at 6 mo). The tumor continued to decrease in size during several repeat CT examinations for 3 dogs (last repeat CT after initiation of radiotherapy: 6, 11, and 28 mo for dogs B, L, and J, respectively).
Neurological improvement was observed in all dogs after irradiation, with 1 dog showing improvement during treatment and the others responding progressively over 2 to 4 mo.
Survival time
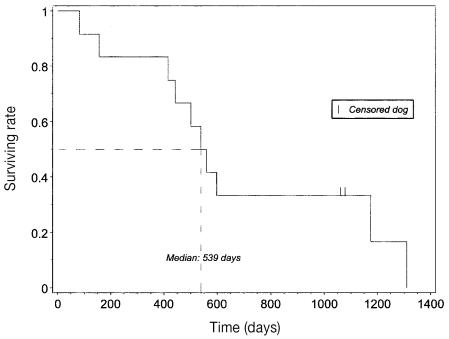
Mean and median survival times were 688, s = 128 d (22.6, s = 4.2 mo) and 539, s = 51 d (17.7, s = 1.7 mo), respectively (Figure 2). Four dogs were euthanized due to the appearance (2 dogs) or recurrence (2 dogs) of neurological signs consistent with tumor regrowth. Four dogs died of tumor-unrelated causes. The cause of death has not been identified for the other 2 dogs.
Figure 2.
Kaplan-Meier survival plot for 12 dogs with pituitary corticotroph macrotumor treated with irradiation. All deaths (tumor-related and tumor-unrelated) were considered events; dogs still alive at the end of the study were censored ( | = censored dogs, – – – = median survival time).
Three dogs (dogs B, D, and H) underwent a 2nd course of irradiation therapy. They were treated with 27, 18, and 21 Gy, respectively on an alternate-day schedule of 3 Gy/fraction and 2 fractions/wk. In dogs B and H, pituitary tumor regrowth had been observed at month 8. The pituitary tumor of dog D remained stable despite irradiation, and tumor-related neurological signs developed at month 14. The response of the tumor to reirradiation was evaluated by CT examination for dogs B and H (2 and 3.5 mo after treatment initiation) and by clinical examination alone for dog D: an improvement of neurological signs was observed. The surface area of the tumor decreased significantly (by more than 50%) in dog B and remained stable in dog H. Survival time after completion of the 2nd course of radiation was 11 mo for dog B, 4.5 mo for dog D, and 5.5 mo for dog H.
The total mean dose per dog was 41.5 Gy.
Toxicoses
The appearance or recurrence of neurological signs was observed in 4 dogs. In all 4 cases, these signs were compared with the results of repeat CT scan showing tumor regrowth, or no tumor decrease despite irradiation for 1 dog. No other new encephalic abnormality was noted on the CT scans of these or the other dogs. None of the other dogs developed severe acute or late adverse effects due to radiation therapy. Dog A had mild anisocoria that resolved without treatment in a few days. Three dogs (E, F, and J) developed more severe bilateral temporal amyotrophia (already present on initial CT).
Discussion
This study evaluated the efficacy of radiotherapy for the treatment of pituitary corticotroph macrotumors in dogs by measuring its effect on tumor size and by determining survival after initiation of treatment. The effect on tumor size was evaluated by means of at least 1 CT reexamination comparing tumor height before and after treatment for 11 of the 12 dogs. This follow-up method was simple and accurate, enabling results to be compared easily. However, these measures reflect a 3-dimensional volume in 1 dimension. For example, if we assume that pituitary tumors are spherical, a 50% decrease in tumor height would represent an 87.5% decrease in tumor volume.
Among the 11 tumors reexamined, the only neoplasm that did not decrease in size had a different CT appearance: a large pituitary mass with heterogeneous contrast enhancement and irregular margins. Thus, the radiosensitivity of tumors may be related to their pathologic appearance (adenoma vs adenocarcinoma), as previously suggested (8,10). Although the appearance of this tumor was suggestive of an undifferentiated state, its histological nature (benign or malignant) could not be determined in this study.
This study is the first in which CT was used exhaustively to follow dogs with pituitary macroadenoma treated with irradiation (2 to 4 examinations per dog). In other studies published on the same subject, the efficacy of radiotherapy was evaluated indirectly by looking at the progression of neurological signs or directly at necropsy (5,7). In a small number of dogs, CT or MRI examinations were carried out (7,10). Dow et al (6) used CT to follow 6 dogs with pituitary macroadenoma that was treated by irradiation. The small number of control CT scans performed (1 or 2 per dog) precluded accurate evaluation of the appearance and duration of effects.
Our results indicate that the effects of radiation therapy appeared quickly (from 1 mo after treatment) and were prolonged: the tumor continued to decrease in size over several months and could remain stable for up to 20 mo after irradiation. Surprisingly, considerable variability was observed in the time at which treatment began to take effect (1 to 16 mo). These findings are relatively new in animals.
Neurological improvement was observed in all dogs after irradiation. Clinical signs of hyperadrenocorticism resolved in all dogs receiving medical treatment for hyperadrenocorticism, except in dog K, which died soon after the course of irradiation. Medical treatment alone (mitotane for example) of Cushing’s disease associated with a corticotroph macroadenoma may resolve the clinical signs of hypercortisolemia, but it has no effect on the space-occupying mass effects of a large pituitary neoplasm (5,11). Neurological dysfunctions may not be tolerable for owners and often result in euthanasia during the first few months after the diagnosis of a macrotumor (2). Thus, mega-voltage irradiation is particularly useful, because it decreases the probability of developing neurological signs and improves existing neurological signs attributed to the compressive effect of the expanding tumor on adjacent structures (altered state of consciousness, abnormal behavior, nystagmus) (1,12). The survival times of dogs with pituitary macrotumor in this study treated with radiotherapy plus medical treatment (mitotane or ketoconazole) were therefore considerably longer than those reported for dogs with pituitary corticotroph macrotumors not treated by radiotherapy (2) and were similar to those in dogs with Cushing’s disease without known macroadenoma and treated with mitotane alone (13).
The general approach in radiation oncology is to achieve tumor control with minimal injury to surrounding healthy tissues. The programming of radiotherapy is essential, as it defines the total dose delivered and its fractionation (14). Higher total doses may improve local control but increase the risk of late encephalic damage, whereas with lower fractions, normal nerve tissue is more likely to be spared (14–16). In most of the schedules used in previous studies of pituitary tumor radio-therapy in dogs, a dose protocol close to the limits of central nervous system tolerance (40 to 48 Gy), delivered in high-dose fractions (4 Gy/fraction) to minimize the number of treatments (6,7,17) was selected. In this study, a different protocol, based on lower dose fractions (3-Gy fractions) reaching a lower total dose (minimal total dose: 36 Gy; mean radiation dose: 41.5 Gy) for a similar number of treatments, was used for most dogs. Doses lower than normal tissue tolerance can be considered, if there is a high probability of tumor control with lower doses (benign tumors) (18). The low frequency of macrocarcinomas within corticotroph tumors encouraged us to adopt this strategy. Although the different studies cannot be compared directly, the mean and median survival times for the population in this study were similar to or higher than those reported previously for pituitary corticotroph macroadenomas treated with different radiotherapy procedures (5–7). However, tumor control was not achieved durably in at least 4 dogs (killed due to the appearance or recurrence of neurological signs.) This lack of long-term tumor control is a major limitation of this protocol, calling into question its efficacy compared with other protocols. Thus, an alternate radiation dose protocol, using a similar dose per fraction (3 Gy) but treating to a higher total dose, may provide an increased probability of tumor control. A direct and prospective comparison of this study’s protocol with previous protocols, based on total doses close to normal tissue tolerance and high-dose fractions or with alternative protocols designed to treat to normal tissue tolerance with similar low fractions by increasing the number of treatments, should be envisaged.
Reirradiation is a therapeutic option in cases of tumor recurrence following a 1st irradiation (19,20). For 3 dogs, major tumor regrowth (8 mo after the 1st procedure) or an absence of tumor decrease led to reirradiation of their pituitary tumors. Despite a low total dose for reirradiation (mean of 22 Gy, given in 3-Gy fractions over 3 or 4 wk), a tumor decrease was demonstrated in 1 dog by CT and was suspected, based on neurological improvement, in another; in a 3rd dog, the tumor remained stable. The decision to administer a 2nd course of treatment may partly account for the high survival rate recorded here. Indeed, recalculation of the median and mean survival times without the 2nd irradiation course (considering a theoretical death on the 1st day of the 2nd procedure) would give results more similar to those of past studies (mean: 20.8 mo and median: 14.5 mo).
No major complication associated with radiotherapy treatment was reported in this study. The appearance or recurrence of neurological signs in 4 of the dogs was attributed to the tumor regrowth identified on a new CT scan. However, as CT is less sensitive than other imaging techniques, such as MRI, or histological examination, we could not totally rule out brain toxicity, for which there is a low probability with the radiation dose protocol administered, based on a lack of change on CT scans of the brain during the follow-up of the 11 dogs (21). In 2 dogs, temporal amyotrophia worsened, despite good medical control of hyperadrenocorticism demonstrated by both clinical improvement of hyperadrenocorticism (regression of polyuria and polydipsia in particular) and results of ACTH stimulation tests (post-ACTH serum cortisol concentration < 150 nmol/L in line with previous recommendations and compatible with our standard therapeutic goal). Although these dogs initially presented temporal amyotrophia, this neurological sign is quite frequent in dogs with Cushing’s syndrome (8,22), we suspect that the worsening amyotrophia may have been linked, at least partly, to the irradiation procedure. Indeed, temporal amyotrophia has not been found to worsen during the follow-up of dogs medically treated for hypercorticism, even when techniques allowing the objective evaluation of this sign are used; for example, no mention of a worsening of temporal amyotrophia was reported during a 1-year period of MRI follow-up in 13 dogs with pituitary-dependent hyperadrenocorticism receiving no radiation therapy (12). There are very few examples of clinical signs associated with Cushing’s syndrome (except pseudomyotonia) that progress despite control of the disease (23). Differences in the susceptibility of cranial nerves to radiation toxicity have already been reported in humans, especially in patients with Cushing’s disease (high radiosensitivity of the optic chiasm for example) (24). For ethical reasons, it was not possible to determine whether the temporal neuromuscular unit (temporal muscle or trigeminal nerve) may be particularly sensitive to radiation in dogs (or in dogs with Cushing’s disease.)
In conclusion, our results support previous studies showing that radiotherapy is a valuable treatment option for pituitary corticotroph macrotumors in dogs. It is well tolerated, allows an objective decrease of pituitary tumor size in most dogs, and results in long-term remission. A prospective comparison of the protocol described in this paper with previously described protocols or with new protocols achieving a higher dose with low dose fractions is needed. The worsening of temporal amyotrophia in some dogs after the completion of radiotherapy was the main probable complication noted during this follow-up study. Strategies to reduce this side effect (hyperfractionation of the total dose, multiplication of fields) should be tested. CVJ
References
- 1.Kipperman BS, Feldman EC, Dybdal NO, Nelson RW. Pituitary tumor size, neurologic signs, and relation to endocrine test results in dogs with pituitary-dependent hyperadrenocorticism: 43 cases (1980–1990) J Am Vet Med Assoc. 1992;201:762–767. [PubMed] [Google Scholar]
- 2.Sarfaty D, Carrillo JM, Peterson ME. Neurologic, endocrinologic, and pathologic findings associated with large pituitary tumors in dogs: Eight cases (1976–1984) J Am Vet Med Assoc. 1988;193:854–856. [PubMed] [Google Scholar]
- 3.Meij BP, Voorhout G, van den Ingh TS, et al. Results of transsphenoidal hypophysectomy in 52 dogs with pituitary-dependent hyperadrenocorticism. Vet Surg. 1998;27:246–261. doi: 10.1111/j.1532-950x.1998.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanson JM, van ‘t HM, Voorhout G, et al. Efficacy of transsphenoidal hypophysectomy in treatment of dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med. 2005;19:687–694. doi: 10.1892/0891-6640(2005)19[687:eothit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Mauldin GN, Burk RL. The use of diagnostic computerized tomography and radiation therapy in canine and feline hyperadrenocorticism. Probl Vet Med. 1990;2:557–564. [PubMed] [Google Scholar]
- 6.Dow SW, LeCouteur RA, Rosychuk RAW, et al. Response of dogs with functional pituitary macroadenomas and macrocarcinomas to radiation. J Small Anim Pract. 1990;31:287–294. [Google Scholar]
- 7.Theon AP, Feldman EC. Megavoltage irradiation of pituitary macrotumors in dogs with neurologic signs. J Am Vet Med Assoc. 1998;213:225–231. [PubMed] [Google Scholar]
- 8.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. Philadelphia: WB Saunders, 2004:252–357.
- 9.Love NE, Fisher P, Hudson L. The computed tomographic enhancement pattern of the normal canine pituitary gland. Vet Radiol Ultrasound. 2000;41:507–510. doi: 10.1111/j.1740-8261.2000.tb01878.x. [DOI] [PubMed] [Google Scholar]
- 10.Goossens MM, Feldman EC, Theon AP, Koblik PD. Efficacy of cobalt 60 radiotherapy in dogs with pituitary-dependent hyperadrenocorticism. J Am Vet Med Assoc. 1998;212:374–376. [PubMed] [Google Scholar]
- 11.Nelson RW, Ihle SL, Feldman EC. Pituitary macroadenomas and macroadenocarcinomas in dogs treated with mitotane for pituitary-dependent hyperadrenocorticism: 13 cases (1981–1986) J Am Vet Med Assoc. 1989;194:1612–1617. [PubMed] [Google Scholar]
- 12.Bertoy EH, Feldman EC, Nelson RW, et al. One-year follow-up evaluation of magnetic resonance imaging of the brain in dogs with pituitary-dependent hyperadrenocorticism. J Am Vet Med Assoc. 1996;208:1268–1273. [PubMed] [Google Scholar]
- 13.Kintzer PP, Peterson ME. Mitotane (o,p’-DDD) treatment of 200 dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med. 1991;5:182–190. doi: 10.1111/j.1939-1676.1991.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilette EL, Gilette SM. Principles of radiation therapy. Semin Vet Med Surg (Small Anim) 1995;10:129–134. [PubMed] [Google Scholar]
- 15.Bley CR, Sumova A, Roos M, Kaser-Hotz B. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med. 2005;19:849–854. doi: 10.1892/0891-6640(2005)19[849:iobtid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Movsas B, Movsas TZ, Steinberg SM, Okunieff P. Long-term visual changes following pituitary irradiation. Int J Radiat Oncol Biol Phys. 1995;33:599–605. doi: 10.1016/0360-3016(95)00221-J. [DOI] [PubMed] [Google Scholar]
- 17.Gilette EL, LaRue SM, Gilette SM. Normal Tissue tolerance and management of radiation injury. Semin Vet Med Surg (Small Anim) 1995;10:209–213. [PubMed] [Google Scholar]
- 18.Smitt MC, Donaldson SS. Radiation therapy for benign disease of the orbit. Semin Radiat Oncol. 1999;9:179–189. doi: 10.1016/s1053-4296(99)80008-3. [DOI] [PubMed] [Google Scholar]
- 19.Schoenthaler R, Albright NW, Wara WM, et al. Re-irradiation of pituitary adenoma. Int J Radiat Oncol Biol Phys. 1992;24:307–314. doi: 10.1016/0360-3016(92)90686-c. [DOI] [PubMed] [Google Scholar]
- 20.Veninga T, Langendijk HA, Slotman BJ, et al. Reirradiation of primary brain tumours: Survival, clinical response and prognostic factors. Radiother Oncol. 2001;59:127–137. doi: 10.1016/s0167-8140(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 21.Benczik J, Tenhunen M, Snellman M, et al. Late radiation effects in the dog brain: Correlation of MRI and histological changes. Radiother Oncol. 2002;63:107–120. doi: 10.1016/s0167-8140(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 22.Saunders JH, Poncelet L, Clercx C, et al. Probable trigeminal nerve schwannoma in a dog. Vet Radiol Ultrasound. 1998;39:539–542. doi: 10.1111/j.1740-8261.1998.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 23.Blot S. Disorders of the skeletal muscles. In: Ettinger SJ, ed. Textbook of Veterinary Internal Medicine. Diseases of the Dog and Cat. Philadelphia: WB Saunders, 2000:684–690.
- 24.Aristizabal SA, Boone ML, Laguna JF. Endocrine factors influencing radiation injury to central nervous tissue. Int J Radiat Oncol Biol Phys. 1979;5:349–353. doi: 10.1016/0360-3016(79)91215-x. [DOI] [PubMed] [Google Scholar]