Abstract
Previous investigations of the effects of clenbuterol have used suprapharmacological doses that induce myocyte death, alter muscle phenotype and do not approximate the proposed therapeutic dose for humans. Recently we reported that smaller doses of clenbuterol induce muscle growth without causing myocyte death. Here, we have used histochemical and proteomic techniques to investigate the molecular effects of this dose. Male Wistar rats (n = 6, per group) were infused with saline or 10 μg.kg-1.d-1 clenbuterol via subcutaneously implanted osmotic pumps. After 14 days the animals’ plantaris muscles were isolated for histochemical and proteomic analyses. Clenbuterol-induced significant muscle growth with concomitant protein accretion and preferential hypertrophy of fast oxidative glycolytic fibers. Clenbuterol reduced the optical density of mitochondrial staining in fast fibers by 20 % and the glycogen content of the muscle by 30 %. Differential analysis of two-dimensional gels showed that heat shock protein 72 and β-enolase were increased whereas aldolase A, phosphogylcerate mutase, and adenylate kinase decreased. Only heat shock protein 72 has previously been investigated in clenbuterol-treated muscles. In conclusion, the clenbutero linduced increase in muscle growth wass concomitant with qualitative changes in the muscle’s proteome that need to be considered when proposing therapeutic uses for this agent.
Keywords: 2D electrophoresis, clenbuterol, histochemistry, mass spectrometry, muscle growth, muscle wasting
Introduction
The β2-adrenergic receptor (β2-AR) agonist clenbuterol has a potent anabolic affect on striated muscles 12, 25, 27, 38. The increased muscle growth is mediated through stimulation of the β2-AR 9, 18 and is associated with an increased rate of protein synthesis 17, 30 and decreased rates of calcium-29 and ATP-dependent 8, 43 proteolysis. Consequently, clenbuterol and similar β2-AR agonists have been proposed as a therapeutic intervention to counter muscle wasting concomitant with aging 39 or chronic disease 1. However, the majority of studies in animals have used large doses (e.g., 1 mg.kg-1) that also induce myocyte death 4, 6, 7. Muscle growth induced by suprapharmacological doses is associated with substantial transformation toward a faster contracting, less fatigue-resistant phenotype as evidenced by alterations in myosin heavy 35 and light 3 chains, myosin ATPase activity 45, energy metabolism 38, and contractile characteristics 12.
It is not entirely clear whether these are inevitable intrinsic effects of β2-AR stimulation or purely a consequence of using suprapharmacological doses of clenbuterol. For example, although the anabolic effect of clenbuterol is reported to be greater in fast muscles 5, 12, 38, clenbuterol-induced anabolism is mediated through the β2-AR which are greater in density in slow than fast muscles 22. Slow-twitch muscles are also more susceptible than fast-twitch muscle to clenbuterol’s myotoxicity 5, and so the shift toward a faster muscle phenotype might be through the selective deletion of slow myofibers. Compatible with this, clenbuterol has more pronounced deleterious effects on the calcium handling of the sarcoplasmic reticulum of slow, compared with fast-twitch fibers 2, and this aberrant calcium handling may be the mechanism by which clenbuterol induces cell death 14.
Recently, we reported that the myotoxic effects of clenbuterol can be separated from its anabolic effects by controlling the dose administered. That is, infusion of large doses (100 μg or 1 mg.kg-1.d-1) induced muscle growth and caused myofibre death whereas a lower dose (10 μg.kg-1.d-1) induced growth without myocyte death 5. Here we attempt to identify the subtle effects of this lower dose of clenbuterol using traditional muscle histochemistry and proteomic analysis, involving differential analysis of two-dimensional (2D) gels and mass spectrometry 13. A significant advantage of this proteomic approach is its inductive nature and inherent ability to identify new effects or processes not previously associated with a particular intervention36. Our hypothesis was based on the ideal of a pure anabolic stimulus, i.e., a quantitative increase in muscle mass through accretion of protein without qualitative changes in protein expression. We sought to determine whether clenbuterol-induced muscle growth differed from this ideal.
Animals, Materials and Methods
Animal husbandry
All experimental procedures complied with the British and national guidelines. Male Wistar rats were bred in-house in a conventional colony and the environmental conditions were controlled at 20 ± 2 °C, 45-50 % humidity and a 12-h light (0600-1800) and dark cycle. Water and food (containing 18.5 % protein) were available ad libitum and the daily consumptions of each individual animal were recorded.
Animals (n = 6, in each group) were infused with either 10 μg clenbuterol.kg-1.d-1 or the saline vehicle only for 14 days, via subcutaneous osmotic pumps implanted under isoflurane anaesthesia, as described previously 5. After 14 days infusion with either clenbuterol or saline, the animals were killed by cervical dislocation. A segment of the mid-belly from the plantaris was resected and mounted in transverse section and supported with liver before being snap-frozen in supercooled isopentane and stored at -80°C. The contralateral plantaris was frozen in liquid nitrogen and stored at -80°C for biochemical analyses.
Muscle histology
Serial cryosections (5 μm) were cut from each muscle specimen and stained using myosin ATPase (after pre-incubation in either acid pH 4.35 or alkali pH 10.4 solutions 16), nicotinamide dinucleotide tetrazolium reductase (NADH-TR), or periodic acid-Schiff (PAS). Cryosections were viewed (x100 magnification) by light microscopy and were digitized using a 12-bit charge-coupled device (1213C; DVC, Austin, TX). One-hundred myofibers from each muscle were randomly selected and identified as being either slow-oxidative (SO), fast-oxidative glycolytic (FOG), or fast-glycolytic (FG) from myosin ATPase-stained cryosections. Calibrated image analysis software (Lucia; LIM, Hostivar, Czech Republic) was used to measure myofibre cross-sectional area (CSA), and the average mitochondrial density and glycogen content were estimated by measuring the optical density of SO, FOG, or FG fibers (100 each) on NADH-TR or PAS-stained cryosections, respectively.
Protein biochemistry
Muscles were pulverised in liquid nitrogen and an accurately weighed portion (∼100 mg) homogenized on ice in 10 volumes of (in mmol) 100 NaCl, 50 Tris, 2 EDTA, 0.5 dithiothreitol pH 7.5, plus complete protease inhibitor (Roche Diagnostics, Lewes, UK). The protein concentration of a 5-μl aliquot of this homogenate was measured using a modified microtiter plate version of the Bradford assay (Sigma; Poole, Dorset, UK). The total protein content of each muscle was then calculated by multiplying protein concentration, homogenate volume, and the fraction of the ground portion relative to total muscle wet weight.
Muscle homogenates were prepared for 2D electrophoresis by centrifugation at 12,000 g, 4 °C for 45 min. An aliquot of the supernatant (sarcoplasmic fraction), containing 250 μg protein, was resuspended in standard rehydration buffer containing 8 mol urea, 2% (w/v) 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, 20 mmol dithiothreitol, 0.5% (v/v) ampholytes and loaded on to 13-cm immobilized pH gradient (IPG) strips (pH 3-10; GE Healthcare, Little Chalfont, UK). Isoelectric focusing (total 52000 volt hours) was conducted and the IPG strips equilibrated in 50 mmol Tris-HCl pH 8.8, containing 6 mol urea, 30 % v/v glycerol, 2 % w/v sodium dodecyl sulfate and a trace of bromophenol blue. Dithiothreitol (10 mg.ml-1) was present as a reducing agent in the first equilibration and iodoacetamide (25 mg.ml-1) in the second. Proteins were electrophorezed through a linear 12 % polyacrylamide gel at 20 °C; initially at a constant current of 15 mA per gel for 30 min and then at 30 mA per gel. Digitized images of colloidal Coomassie- stained gels (Bio-Safe; Bio-Rad, Hercules, CA) were analyzed using 2D expression software (Non Linear Dynamics, Newcastle, UK). Spot volumes were expressed relative to the total integrated spot density, and those spots that changed significantly were excised and subjected to in-gel digestion and peptide extraction using a Mass-Prep digestion robot (Micromass, Manchester, UK). Peptides mixed with matrix (α-cyano-4-hydroxcinnamic acid) and spotted on to a target plate were analyzed using matrix-assisted laser desorption ionisation - time of flight (MALDI-ToF) mass spectrometry (Micromass, Manchester, UK) over the range 1000-3500 thomsons. Proteins were identified from their peptide mass fingerprint by manually searching a locally implemented Mascot server. Search parameters were restricted by taxonomic class (Rodentia) and allowed a single trypsin missed cleavage, carbamidomethyl modification of cysteine, oxidation of methionine and an m/z error of ± 250 ppm.
Statistical analyses
Data are presented as means ± SEM. Statistically significant differences between saline and clenbuterol-treated muscles were determined using Student’s two-tailed independent t-test and differences were considered statistically significant if P < 0.05.
Results
At the beginning of the experiment the average body weight of all rats was 285 ± 7 g. This increased 8 % to 311 ± 10 g after infusion with saline and 13 % to 328 ± 6 g with clenbuterol. Clenbuterol significantly (P<0.05) increased the wet weight (271.9 ± 17 mg vs. 317 ± 23 mg) and total protein content (48 ± 3.2 mg vs. 56.1 ± 4.1 mg) of the plantaris. Histochemistry (Fig. 1) was used to investigate any changes in the myofibre profile, mitochondrial density and glycogen content of the muscles. Clenbuterol increased the CSA of all fibre types (SO, FOG and FG), but only the hypertrophy of the FOG fibers was statistically significant (P<0.05). When calculated as total area (i.e. percent number of myofibers multiplied by their average CSA) the proportion of the muscle composed of SO fibers was identical between saline- and clenbuterol-treated muscles, but the calculated total areas of FOG and FG fibers increased by 24 % and 6 %, respectively (Table 1). The density of NADH-TR staining, indicative of mitochondrial content, decreased (range 10%-25%) in FOG and FG fibers whereas the density of PAS staining of muscle glycogen decreased (range 16%-38%) in all 3 myofibre types (Table 1). Differential analysis of 2D gels matched 87 protein spots, 5 of which were significantly (P<0.01) altered in abundance by clenbuterol (Fig. 2). Peptide ion spectra were collected using MALDI-ToF mass spectrometry and used to identify each protein based on its peptide mass fingerprint (Table 2).
Table 1.
Plantaris muscle characteristics after infusion of saline or clenbuterol.
| SO | FOG | FG | |
|---|---|---|---|
| Fiber type proportion (%) | |||
| Saline | 12 ± 0.3 | 28 ± 0.6 | 60 ± 0.6 |
| Clenbuterol | 10 ± 0.9 | 27 ± 1.1 | 63 ± 2 |
| Cross-sectional area (μm2) | |||
| Saline | 2751 ± 93 | 2301 ± 73 | 4053 ± 71 |
| Clenbuterol | 3292 ± 37 | 2962 ± 81* | 4086 ± 64 |
| Calculated total area (arbitrary units) | |||
| Saline | 330 ± 13 | 644 ± 14 | 2432 ± 44 |
| Clenbuterol | 329 ± 33 | 800 ± 19* | 2575 ± 68* |
| Change in calculated area (%) | - 0.3 | + 24 | + 6 |
| NADH-TR staining (optical density) | |||
| Saline | 0.189 ± 0.005 | 0.182 ± 0.005 | 0.134 ± 0.003 |
| Clenbuterol | 0.200 ± 0.002 | 0.136 ± 0.007* | 0.122 ± 0.02* |
| PAS staining (optical density) | |||
| Saline | 0.151 ± 0.003 | 0.259 ± 0.017 | 0.230 ± 0.012 |
| Clenbuterol | 0.127 ± 0.005* | 0.160 ± 0.006* | 0.153 ± 0.004* |
All data are presented as means ± SEM (n = 6, in each group). Calculated total area represents the average cross-sectional area for each fiber type multiplied by fiber type proportion (%). Data were analysed using Student’s two-tailed t-test,
P<0.05 significantly different from the saline control.
Discussion
This work deepens our knowledge of the effects of clenbuterol on skeletal muscle. By using this lower dose, derived from our previous studies 4, 5, we have avoided the confounding influences of myocyte death 5 and gross perturbations in metabolism 12 that are associated with the use of larger doses of clenbuterol. Proteomic analysis revealed five proteins that were significantly altered in response to 10 μg clenbuterol.kg-1.d-1 (Table 2). Only one, heat shock protein 72 (HSP 72), has previously been investigated in relation to β2-agonist administration32.
Large doses of clenbuterol significantly increase the abundance of type IIb myosin heavy chain (MHC) in the rat plantaris and other muscles with mixed fibre types 32, 35, 40, thereby increasing speed of contraction 12 and providing a useful model to investigate changes in muscle phenotype 3. In skeletal muscle, HSP 72 is localized to SO myofibers expressing predominantly type I MHC 31. Interventions, such as functional overload 33 or endurance exercise 11, that increase the proportion of type I fibers also increase the abundance of HSP 72. In contrast, the administration of large doses of clenbuterol decreases the expression of HSP 72 32, and this is related to the reduced proportion of type I MHC in the clenbuterol-treated muscles. In this study, the total area of the plantaris occupied by SO fibers (Table 1) was not changed by clenbuterol, but the abundance of HSP 72 was increased (Table 2). Possibly, this increase in HSP 72 is one of the mechanisms by which the muscle is able to resist the cell death induced by larger doses of this agent 5.
Clenbuterol has been reported to increase the uptake of glucose and the accumulation of glycogen in insulin-resistant skeletal muscles 34. However, in normal healthy animals clenbuterol caused a striking decrease in the glycogen content of the plantaris and this was particularly evident in the FOG and FG fibers (Fig. 1 and Table 1). Previously, glycolytic enzymes such as phosphofructokinase have been reported to either increase 35 or decrease 12 after administration of large doses of clenbuterol. Here, we observed opposing changes in the abundance of three glycolytic enzymes; while both fructose-bisphosphate aldolase A (aldolase A) and phosphogylcerate mutase (PGM) decreased, β-enolase increased, making it difficult to determine the net effect of these changes on carbohydrate metabolism. Changes in some glycolytic enzymes may relate to aspects of muscle physiology other than energy metabolism. For example, β-enolase is localized to the perinuclear region 28, and similar to other glycolytic enzymes, such as lactate dehydrogenase and phosphogylcerate kinase 37, β-enolase might have an auxiliary role in transcription and DNA replication or repair. The expression of β-enolase is decreased in denervated muscle 41 and in a model of muscle damage and regeneration the abundance of β-enolase decreases immediately after the induction of damage, but is restored during regeneration 28. Therefore, the clenbuterol-induced increase in the abundance of β-enolase might be more closely associated with its hypertrophic effects, rather than its effects on energy metabolism.
Clenbuterol’s lipolytic and anabolic (repartitioning) effects may be desirable as a therapy against metabolic disorders 42 and some of the evidence collected here supports the therapeutic potential of clenbuterol. In particular, the observed decreased in adenylate kinase and aldolase A (Table 2) oppose the increased concentrations of these proteins measured in skeletal muscles of obese individuals 19. Similarly, the expression of HSP 72 is lower in the muscle of type II diabetic individuals 24, but was elevated here (Table 2) after administration of clenbuterol. However, several of our observations could also be used to portray a less desirable image of clenbuterol-induced muscle growth. That is, the clenbuterol-induced decrease in aldolase A opposes the increased expression of this enzyme in endurance-trained muscle 44, phosphoglycerate mutase deficiency is associated with exercise intolerance 10, and a decreased abundance of adenylate kinase could deleteriously affect the muscle’s resistance to fatigue 21. Such observations seem to correlate well with the decreased exercise capacity that is commonly reported 15, 20, 23, 25 after administration of larger doses of clenbuterol and suggest that this effect might still be evident after administration of this low non-myotoxic dose.
The dose of clenbuterol used here in rats is thought to be equivalent to the safe therapeutic dose in humans 26 and, as opposed to larger doses, does not induce myocyte death 5. Contrary to our original hypothesis, the muscle growth induced by this dose was associated with alterations in the muscle’s proteome. To some extent the alterations oppose those observed in insulin-resistant and type II diabetic muscle and support the idea that clenbuterol might be beneficial in such circumstances. However, clenbuterol caused preferential hypertrophy of FOG fibers and reduced the oxidative potential and glycogen content of the muscle, suggesting that the deleterious effects of clenbuterol on muscle function may not have been negated by use of this low non-myotoxic dose.
Fig. 1.
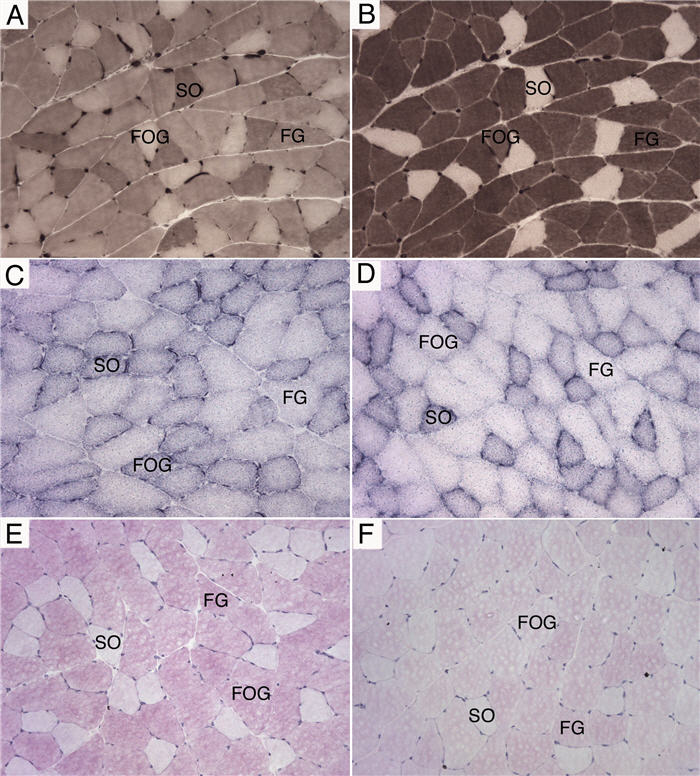
Histochemical analysis of muscle adaptation.
Serial cryosections of plantaris muscle stained for myosin ATPase using an acid (A) or alkali (B) pre-incubation and used to assign myofiber types as slow-oxidative (SO), fast-oxidative glycolytic (FOG), and fast-glycolytic (FG). Nicotinamide dinucleotide tetrazolium reductase (C and D) and periodic acid-Schiff (E and F) staining demonstrating the marked differences in mitochondrial density and glycogen content, respectively, between saline-treated (C and E) and clenbuterol-treated (D and F) plantaris muscles. All images are x 200 magnification.
Fig. 2.
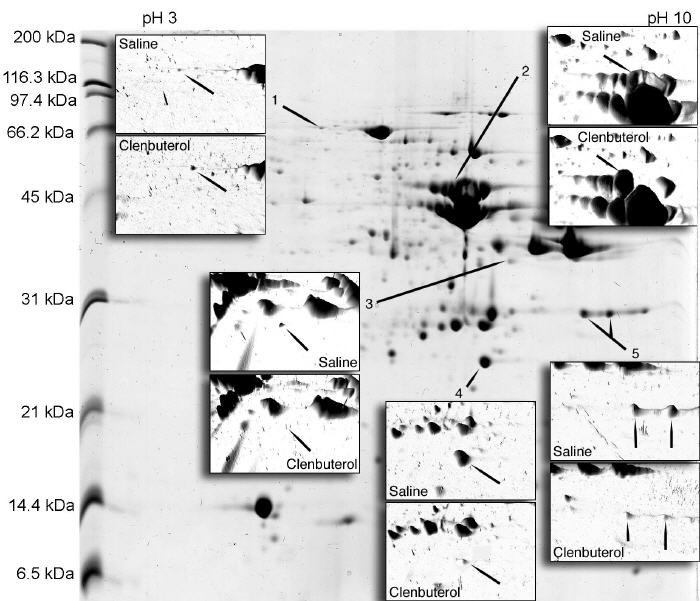
Two-dimensional electrophoretic separation of sarcoplasmic proteins.
Representative gel image of the soluble protein fraction (250 μg) of plantaris muscle focused on a 13-cm IPG strip (pH3-10) and electrophoresed through a 16 cm, 12 % polyacrylamide gel. Inserts show spot densities as three-dimensional images and those protein spots (arrows) significantly different between saline- and clenbuterol-treated muscles. Spot numbers are consistent with Table 2.
Acknowledgments
This research was funded by a British Heart Foundation Junior Research Fellow (FS/04/028) awarded to JGB.
Abbreviations
- AR
adrenergic receptor
- CSA
cross sectional area
- FG
fast glycolytic
- FOG
fast oxidative glycolytic
- HSP 72
heat shock protein 72
- MALDI-ToF
matrix assisted laser desorption ionisation-time of flight
- MHC
myosin heavy chain
- NADH-TR
nicotinamide dinucleotide-tetrazolium reductase
- PAS
periodic acid Schiff
- PGM
phosphoglycerate mutase
- SO
slow oxidative
References
- 1.Argiles J, Almendro V, Busquets S, Lopez-Soriano FJ. The pharmacological treatment of cachexia. Curr Drug Targets. 2004;5:265–277. doi: 10.2174/1389450043490505. [DOI] [PubMed] [Google Scholar]
- 2.Bakker AJ, Head SI, Wareham AC, Stephenson DG. Effect of clenbuterol on sarcoplasmic reticulum function in single skinned mammalian skeletal muscle fibres. Am J Physiol. 1998;274:C1718–C1726. doi: 10.1152/ajpcell.1998.274.6.C1718. [DOI] [PubMed] [Google Scholar]
- 3.Bozzo C, Stevens L, Toniolo L, Mounier Y, Reggiani C. Increased phosphorylation of myosin light chain associated with slow-to-fast transition in rat soleus. Am J Physiol. 2003;285:C575–583. doi: 10.1152/ajpcell.00441.2002. [DOI] [PubMed] [Google Scholar]
- 4.Burniston JG, Chester N, Clark WA, Tan L-B, Goldspink DF. Dose-dependent apoptotic and necrotic myocyte death induced by the β2-adrenergic receptor agonist, clenbuterol. Muscle Nerve. 2005;32:767–774. doi: 10.1002/mus.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burniston JG, Clark WA, Tan L-B, Goldspink DF. Dose-dependent separation of the hypertrophic and myotoxic effects of the β2-adrenergic receptor agonist clenbuterol in rat striated muscles. Muscle Nerve. 2006;33:655–663. doi: 10.1002/mus.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burniston JG, Ng Y, Clark WA, Colyer J, Tan L-B, Goldspink DF. Myotoxic effects of clenbuterol in the rat heart and soleus muscle. J Appl Physiol. 2002;93:1824–1832. doi: 10.1152/japplphysiol.00139.2002. [DOI] [PubMed] [Google Scholar]
- 7.Burniston JG, Tan L-B, Goldspink DF. β2-Adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. J Appl Physiol. 2005;98:1379–1386. doi: 10.1152/japplphysiol.00642.2004. [DOI] [PubMed] [Google Scholar]
- 8.Busquets S, Figueras MT, Fuster G, Almendro V, Moore-Carrasco R, Ametiller E, et al. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 2004;64:6725–6731. doi: 10.1158/0008-5472.CAN-04-0425. [DOI] [PubMed] [Google Scholar]
- 9.Choo JJ, Horan MA, Little RA, Rothwell NJ. Anabolic effects of clenbuterol in skeletal muscle are mediated by beta2-adrenoceptor activation. Am J Physiol. 1992;263:E50–E56. doi: 10.1152/ajpendo.1992.263.1.E50. [DOI] [PubMed] [Google Scholar]
- 10.Cornelio F, Di Donato S. Myopathies due to enzyme deficiencies. J Neurol. 1985;232:329–340. doi: 10.1007/BF00313831. [DOI] [PubMed] [Google Scholar]
- 11.Desplanches D, Ecochard L, Sempore B, Mayet-Sornay MH, Favier R. Skeletal muscle HSP72 response to mechanical unloading: influence of endurance training. Acta Physiol Scand. 2004;180:387–394. doi: 10.1111/j.1365-201X.2003.01255.x. [DOI] [PubMed] [Google Scholar]
- 12.Dodd SL, Powers SK, Vrabas IS, Criswell D, Stetson S, Hussain R. Effects of clenbuterol on the contractile and biochemical properties of skeletal muscle. Med Sci Sport Exerc. 1996;28:669–676. doi: 10.1097/00005768-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Doherty MK, McLean L, Hayter JR, Pratt JM, Robertson DH, El-Shafei A, et al. The proteome of chicken skeletal muscle: changes in soluble protein expression during growth in a layer strain. Proteomics. 2004;4:2082–2093. doi: 10.1002/pmic.200300716. [DOI] [PubMed] [Google Scholar]
- 14.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol (Lond) 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan ND, Williams DA, Lynch GS. Deleterious effects of chronic clenbuterol treatment on endurance and sprint exercise performance in rats. Clin Sci. 2000;98:339–347. [PubMed] [Google Scholar]
- 16.Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970;28:365–367. [PubMed] [Google Scholar]
- 17.Hesketh JE, Campbell GP, Lobley GE, Maltin CA, Acamovic F, Palmer RM. Stimulation of actin and myosin synthesis in rat gastrocnemius muscle by clenbuterol; evidence for translational control. Comp Biochem Physiol. 1992;102C:23–27. doi: 10.1016/0742-8413(92)90037-8. [DOI] [PubMed] [Google Scholar]
- 18.Hinkle RT, Hodge KMB, Cody DB, Sheldon RJ, Kobilka BK, Isfort R. Skeletal muscle hypertrophy and anti-hypertrophic effects of clenbuterol are mediated by the beta2-adrenergic receptor. Muscle Nerve. 2002;25:729–734. doi: 10.1002/mus.10092. [DOI] [PubMed] [Google Scholar]
- 19.Hittel DS, Hathout Y, Hoffman EP, Houmard JA. Proteome analysis of skeletal muscle from obese and morbidly obese women. Diabetes. 2005;54:1283–1288. doi: 10.2337/diabetes.54.5.1283. [DOI] [PubMed] [Google Scholar]
- 20.Ingalls CP, Barnes WS, Smith SB. Interaction between clenbuterol and run training: effects on exercise performance and MLC isoform content. J Appl Physiol. 1996;80:795–801. doi: 10.1152/jappl.1996.80.3.795. [DOI] [PubMed] [Google Scholar]
- 21.Janssen E, Dzeja PP, Oerlemans F, Simonetti AW, Heerschap A, de Haan A, et al. Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. Embo J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen J, Brors O, Dahl HA. Different beta-adrenergic receptor density in different rat skeletal muscle fibre types. Pharmacol Toxicol. 1995;76:380–385. doi: 10.1111/j.1600-0773.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 23.Kearns CF, McKeever KH. Clenbuterol diminishes aerobic performance in horses. Med Sci Sports Exerc. 2002;34:1976–1985. doi: 10.1097/00005768-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- 25.Lynch GS, Hayes A, Campbell SP, Williams DA. Effects of beta2-agonist administration and exercise on contractile activation of skeletal muscle fibres. J Appl Physiol. 1996;81:1610–1618. doi: 10.1152/jappl.1996.81.4.1610. [DOI] [PubMed] [Google Scholar]
- 26.Maltin CA, Delday MI, Hay SM, Baillie AG. Denervation increases clenbuterol sensitivity in muscle from young rats. Muscle Nerve. 1992;15:188–192. doi: 10.1002/mus.880150210. [DOI] [PubMed] [Google Scholar]
- 27.Maltin CA, Hay SM, Delday MI, Lobley GE, Reeds JP. The action of the beta-agonist clenbuterol on protein metabolism in innervated and denervated phasic muscles. Biochem J. 1989;261:965–971. doi: 10.1042/bj2610965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkulova T, Dehaupas M, Nevers MC, Creminon C, Alameddine H, Keller A. Differential modulation of alpha, beta and gamma enolase isoforms in regenerating mouse skeletal muscle. Eur J Biochem. 2000;267:3735–3743. doi: 10.1046/j.1432-1327.2000.01408.x. [DOI] [PubMed] [Google Scholar]
- 29.Navegantes C, Resanino NMZ, Migliorini RH, Kettelhut I. Catechoalimes inhibit Ca+2- dependent proteolysis in rat skeletal muscle through beta2-adrenoceptors and cAMP. Am J Physiol. 2001;281:E449–454. doi: 10.1152/ajpendo.2001.281.3.E449. [DOI] [PubMed] [Google Scholar]
- 30.Navegantes C, Resano NM, Baviera AM, Migliorini RH, Kettelhut IC. Effect of sympathetic denervation on the rate of protein synthesis in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E642–647. doi: 10.1152/ajpendo.00371.2003. [DOI] [PubMed] [Google Scholar]
- 31.Ogata T, Oishi Y, Roy RR, Ohmori H. Endogenous expression and developmental changes of HSP72 in rat skeletal muscles. J Appl Physiol. 2003;95:1279–1286. doi: 10.1152/japplphysiol.00353.2003. [DOI] [PubMed] [Google Scholar]
- 32.Oishi Y, Imoto K, Ogata T, Taniguchi K, Matsumoto H, Fukuoka Y, et al. Calcineurin and heat-shock proteins modulation in clenbuterol-induced hypertrophied rat skeletal muscles. Pflugers Arch. 2004;448:114–122. doi: 10.1007/s00424-003-1225-6. [DOI] [PubMed] [Google Scholar]
- 33.Oishi Y, Ogata T, Ohira Y, Taniguchi K, Roy RR. Calcineurin and heat shock protein 72 in functionally overloaded rat plantaris muscle. Biochem Biophys Res Commun. 2005;330:706–713. doi: 10.1016/j.bbrc.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Pan SJ, Hancock J, Ding Z, Fogt D, Lee M, Ivy JL. Effects of clenbuterol on insulin resistance in conscious obese Zucker rats. Am J Physiol. 2001;280:E554–561. doi: 10.1152/ajpendo.2001.280.4.E554. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrino MA, D'Antona G, Bortolotto S, Boschi F, Pastoris O, Bottinelli R, et al. Clenbuterol antagonizes glucocorticoid-induced atrophy and fibre type transformation in mice. Exp Physiol. 2004;89:89–100. doi: 10.1113/expphysiol.2003.002609. [DOI] [PubMed] [Google Scholar]
- 36.Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19:1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 37.Popanda O, Fox G, Thielmann HW. Modulation of DNA polymerases alpha, delta and epsilon by lactate dehydrogenase and 3-phosphoglycerate kinase. Biochim Biophys Acta. 1998;1397:102–117. doi: 10.1016/s0167-4781(97)00229-7. [DOI] [PubMed] [Google Scholar]
- 38.Rajab P, Fox J, Riaz S, Tomlinson D, Ball D, Greenhaff PL. Skeletal muscle myosin heavy chain isoforms and energy metabolism after clenbuterol treatment in the rat. Am J Physiol. 2000;279:R1076–R1081. doi: 10.1152/ajpregu.2000.279.3.R1076. [DOI] [PubMed] [Google Scholar]
- 39.Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. Beta 2-agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol (Lond) 2004;555:175–188. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens L, Ricart-Firinga C, Gohlsch B, Bastide B, Mounier Y, Pette D. Effects of unweighting and clenbuterol on myosin light and heavy chains in fast and slow muscle of rat. Am J Physiol. 2000;279:C1558–15663. doi: 10.1152/ajpcell.2000.279.5.C1558. [DOI] [PubMed] [Google Scholar]
- 41.Tang H, Cheung WM, Ip FC, Ip NY. Identification and characterization of differentially expressed genes in denervated muscle. Mol Cell Neurosci. 2000;16:127–140. doi: 10.1006/mcne.2000.0864. [DOI] [PubMed] [Google Scholar]
- 42.Torgan CE, Etgen GJ, Kang HY, Ivy JL. Fibre type-specific effects of clenbuterol and exercise training on insulin-resistant muscle. J Appl Physiol. 1995;79:163–167. doi: 10.1152/jappl.1995.79.1.163. [DOI] [PubMed] [Google Scholar]
- 43.Yimlamai T, Dodd SL, Borst SE, Park S. Clenbuterol induces muscle-specific attenuation of atrophy through effects on the ubiquitin-proteasome pathway. J Appl Physiol. 2005;91:71–80. doi: 10.1152/japplphysiol.00448.2004. [DOI] [PubMed] [Google Scholar]
- 44.Yoshioka M, Tanaka H, Shono N, Snyder EE, Shindo M, St-Amand J. Serial analysis of gene expression in the skeletal muscle of endurance athletes compared to sedentary men. FASEB J. 2003;17:1812–1819. doi: 10.1096/fj.02-1200com. [DOI] [PubMed] [Google Scholar]
- 45.Zeman RJ, Ludemann R, Easton TG, Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2-receptor agonist. Am J Physiol. 1988;254:E726–732. doi: 10.1152/ajpendo.1988.254.6.E726. [DOI] [PubMed] [Google Scholar]


