Abstract
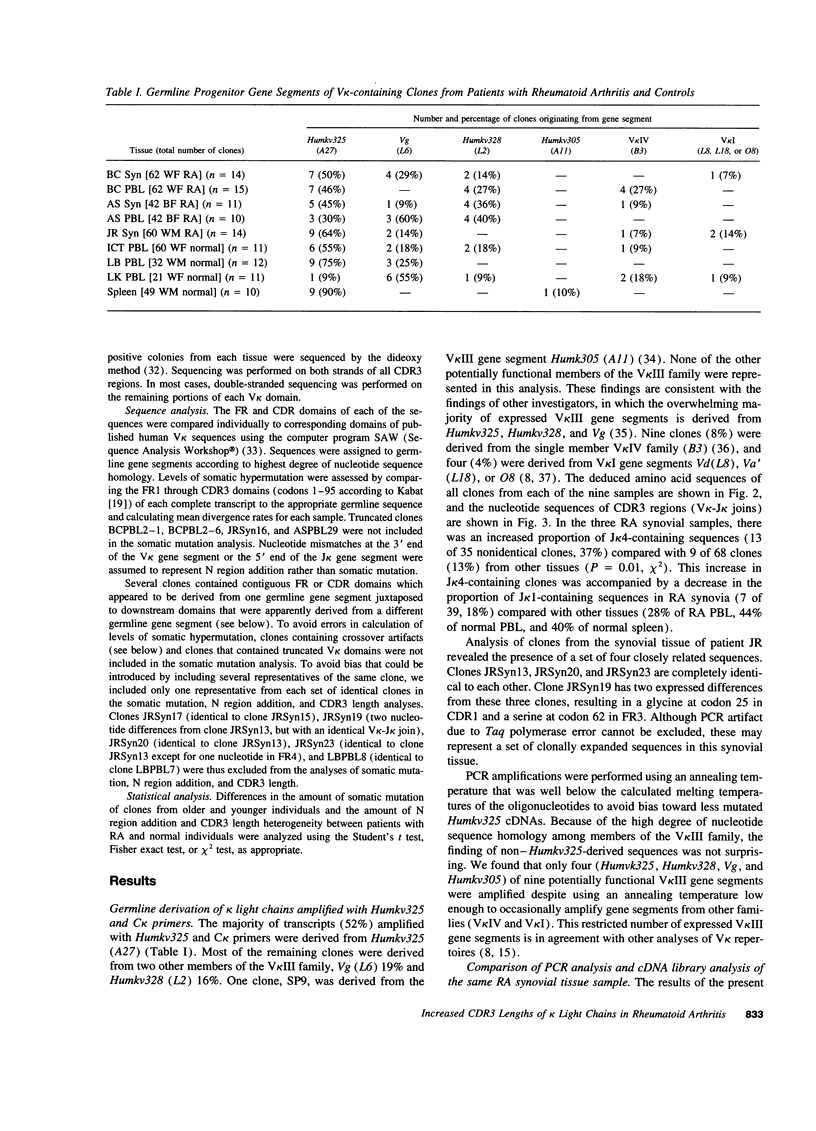
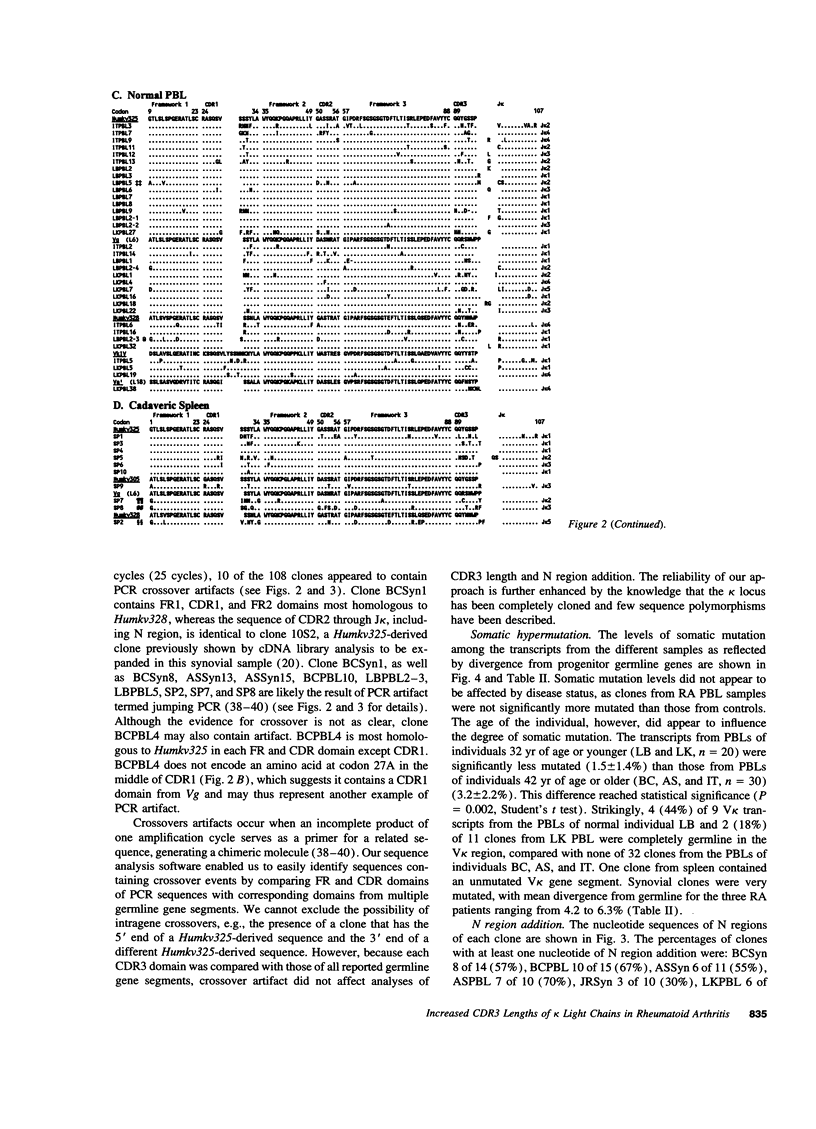
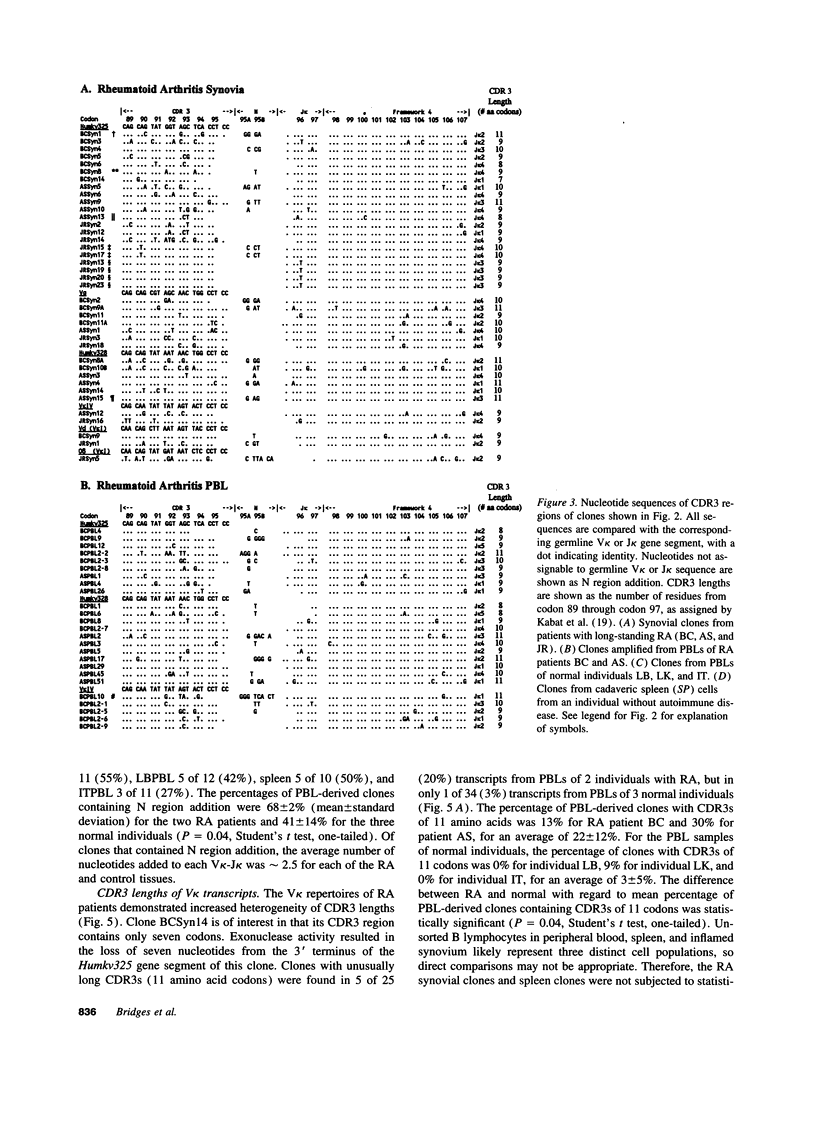
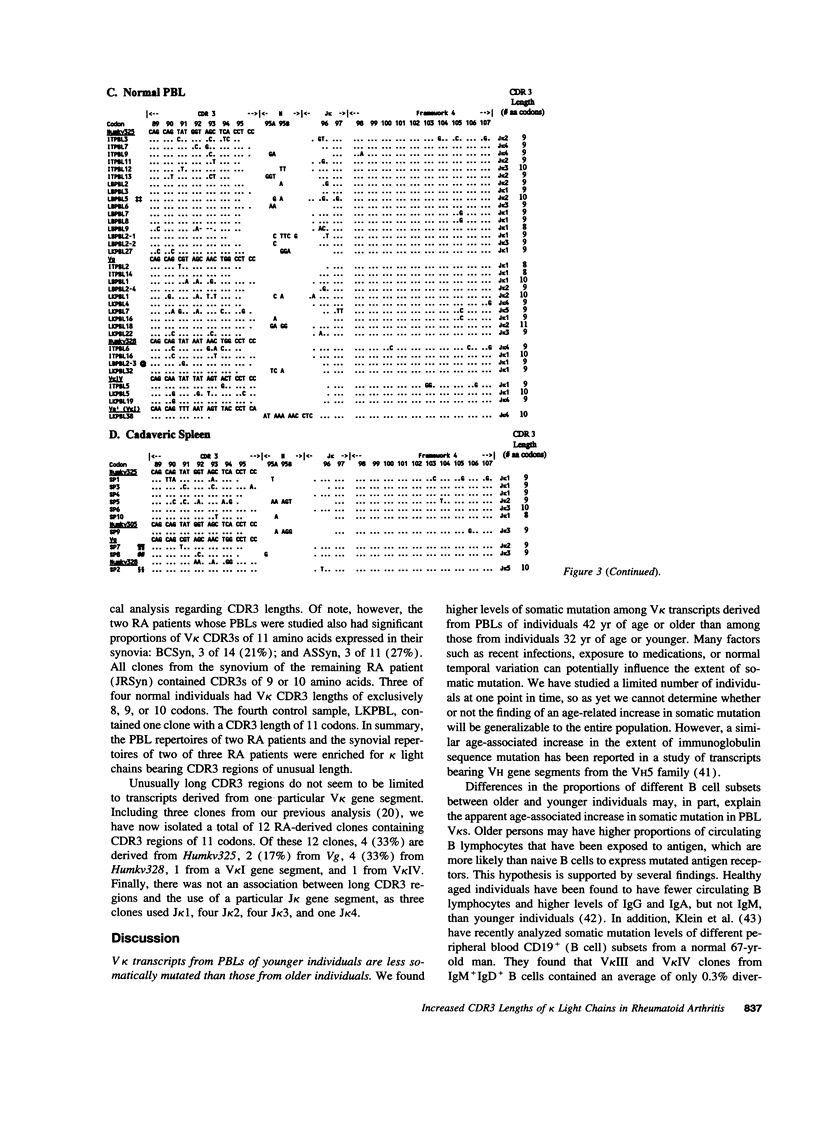
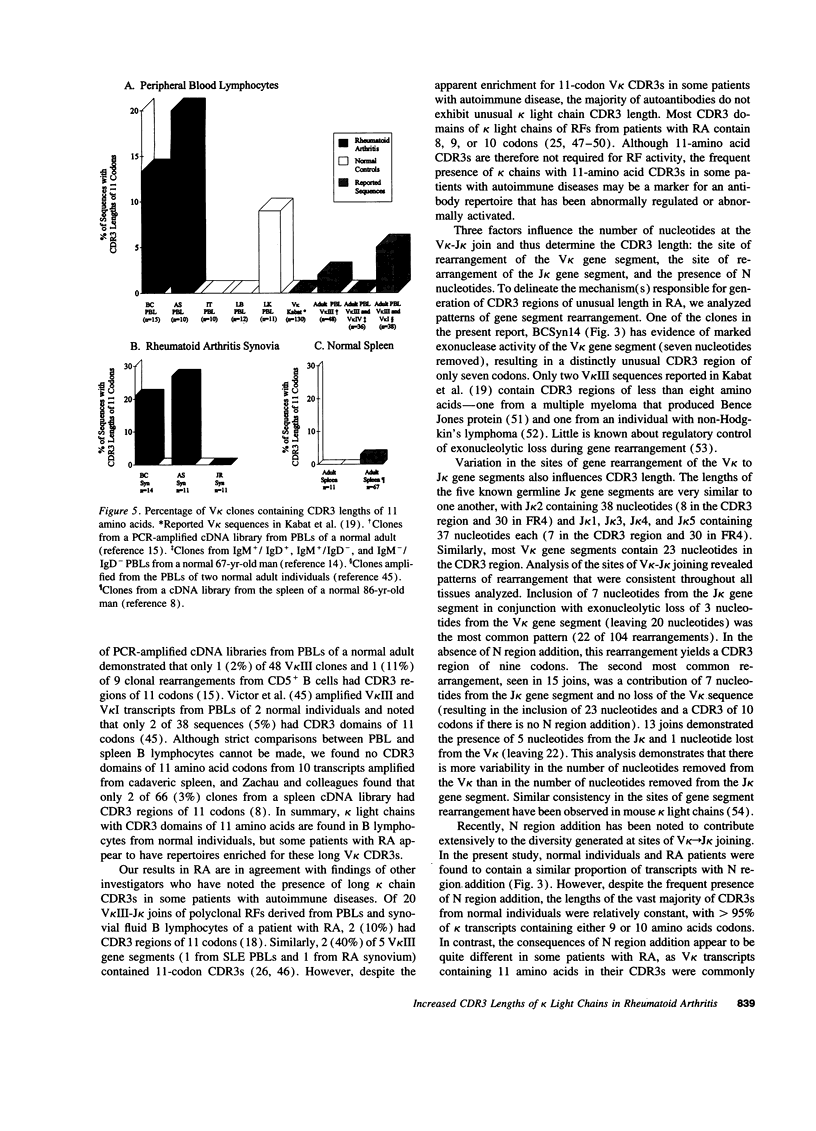
Immunoglobulin secretion by plasma cells infiltrating synovial membranes is a prominent feature of RA. Previous analyses of a cDNA library generated from synovium of RA patient BC revealed immunoglobulin kappa light chain transcripts with extensive somatic mutation, frequent N region addition, and unexpected variation in the lengths of CDR3 regions which form the center of the antigen binding site. To determine if these characteristics are present in other individuals, we performed reverse transcription-polymerase chain reaction amplification and sequenced > or = 10 V kappa-containing amplicons from nine tissue samples: synovia of three individuals with long-standing RA (including patient BC), PBLs of two of these individuals, and PBLs or splenocytes of four normal individuals. Increased levels of somatic mutation in PBLs appeared to correlate with increased age, which may reflect accumulation of circulating memory cells and/or decreased bone marrow production of naive B lymphocytes. Two of three RA synovial samples and both RA PBL samples exhibited increased proportions of clones with unusual CDR3 lengths. Enrichment for these antibody binding sites could be due to abnormal regulation of the emerging repertoire or to selection for B lymphocytes bearing antibodies of unusual specificity, and may play a role in the pathogenesis of RA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón G. S., Schrohenloher R. E., Bartolucci A. A., Ward J. R., Williams H. J., Koopman W. J. Suppression of rheumatoid factor production by methotrexate in patients with rheumatoid arthritis. Evidence for differential influences of therapy and clinical status on IgM and IgA rheumatoid factor expression. Arthritis Rheum. 1990 Aug;33(8):1156–1161. doi: 10.1002/art.1780330816. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Husby G., Williams R. C., Jr Predominance of T cells in the lymphocytic infiltrates of synovial tissues in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):555–562. doi: 10.1002/art.1780190307. [DOI] [PubMed] [Google Scholar]
- Cai J., Humphries C., Lutz C., Tucker P. W. Analysis of VH251 gene mutation in chronic lymphocytic leukemia (CLL) and normal B-cell subsets. Ann N Y Acad Sci. 1992 May 4;651:384–392. doi: 10.1111/j.1749-6632.1992.tb24639.x. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., Zelenetz A. D., Levy S., Levy R. Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Mol Immunol. 1992 Feb;29(2):193–203. doi: 10.1016/0161-5890(92)90100-c. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox J. P., Tomlinson I. M., Winter G. A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur J Immunol. 1994 Apr;24(4):827–836. doi: 10.1002/eji.1830240409. [DOI] [PubMed] [Google Scholar]
- Crowley J. J., Goldfien R. D., Schrohenloher R. E., Spiegelberg H. L., Silverman G. J., Mageed R. A., Jefferis R., Koopman W. J., Carson D. A., Fong S. Incidence of three cross-reactive idiotypes on human rheumatoid factor paraproteins. J Immunol. 1988 May 15;140(10):3411–3418. [PubMed] [Google Scholar]
- Deftos M., Olee T., Carson D. A., Chen P. P. Defining the genetic origins of three rheumatoid synovium-derived IgG rheumatoid factors. J Clin Invest. 1994 Jun;93(6):2545–2553. doi: 10.1172/JCI117265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Riley L. K., Coleman M. S., Cibull M. L., Fuller S. A., Todd E. Expression of terminal deoxynucleotidyl transferase in human thymus during ontogeny and development. J Immunol. 1983 Jul;131(1):195–200. [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Elgavish R. A., Schroeder H. W., Jr SAW: a graphical user interface for the analysis of immunoglobulin variable domain sequences. Biotechniques. 1993 Dec;15(6):1066–1071. [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Ermel R. W., Kenny T. P., Chen P. P., Robbins D. L. Molecular analysis of rheumatoid factors derived from rheumatoid synovium suggests an antigen-driven response in inflamed joints. Arthritis Rheum. 1993 Mar;36(3):380–388. doi: 10.1002/art.1780360314. [DOI] [PubMed] [Google Scholar]
- Ezaki I., Kanda H., Sakai K., Fukui N., Shingu M., Nobunaga M., Watanabe T. Restricted diversity of the variable region nucleotide sequences of the heavy and light chains of a human rheumatoid factor. Arthritis Rheum. 1991 Mar;34(3):343–350. doi: 10.1002/art.1780340312. [DOI] [PubMed] [Google Scholar]
- Gause A., Küppers R., Mierau R. A somatically mutated V kappa IV gene encoding a human rheumatoid factor light chain. Clin Exp Immunol. 1992 Jun;88(3):430–434. doi: 10.1111/j.1365-2249.1992.tb06467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. F., Jr, Schroeder H. W., Jr Developmental regulation of D beta reading frame and junctional diversity in T cell receptor-beta transcripts from human thymus. J Immunol. 1992 Feb 15;148(4):1230–1239. [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenter A. L., Tredup J. High expression of a 3'----5' exonuclease activity is specific to B lymphocytes. Mol Cell Biol. 1991 Sep;11(9):4398–4404. doi: 10.1128/mcb.11.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Carson D. A. Uniform high frequency expression of autoantibody-associated crossreactive idiotypes in the primary B cell follicles of human fetal spleen. J Exp Med. 1990 Jan 1;171(1):189–196. doi: 10.1084/jem.171.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham P. M., Schroeder H. W., Jr Antibody structure and the evolution of immunoglobulin V gene segments. Semin Immunol. 1994 Dec;6(6):347–360. doi: 10.1006/smim.1994.1045. [DOI] [PubMed] [Google Scholar]
- Klein R., Jaenichen R., Zachau H. G. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993 Dec;23(12):3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993 Dec;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Variable region gene analysis of B cell subsets derived from a 4-year-old child: somatically mutated memory B cells accumulate in the peripheral blood already at young age. J Exp Med. 1994 Oct 1;180(4):1383–1393. doi: 10.1084/jem.180.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W. J., Schrohenloher R. E., Crago S. S., Spalding D. M., Mestecky J. IgA rheumatoid factor synthesis by dissociated synovial cells. Characterization and relationship to IgM rheumatoid factor synthesis. Arthritis Rheum. 1985 Nov;28(11):1219–1227. doi: 10.1002/art.1780281105. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Cooper M. D., Carroll A. J., Burrows P. D. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Kirkham P. M., Koopman W. J., Schroeder H. W., Jr Evidence of antigen receptor-influenced oligoclonal B lymphocyte expansion in the synovium of a patient with longstanding rheumatoid arthritis. J Clin Invest. 1994 Jan;93(1):361–370. doi: 10.1172/JCI116968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Koopman W. J., Schroeder H. W., Jr The immunoglobulin kappa light chain repertoire expressed in the synovium of a patient with rheumatoid arthritis. Arthritis Rheum. 1992 Aug;35(8):905–913. doi: 10.1002/art.1780350809. [DOI] [PubMed] [Google Scholar]
- Martin T., Blaison G., Levallois H., Pasquali J. L. Molecular analysis of the V kappa III-J kappa junctional diversity of polyclonal rheumatoid factors during rheumatoid arthritis frequently reveals N addition. Eur J Immunol. 1992 Jul;22(7):1773–1779. doi: 10.1002/eji.1830220716. [DOI] [PubMed] [Google Scholar]
- Meindl A., Klobeck H. G., Ohnheiser R., Zachau H. G. The V kappa gene repertoire in the human germ line. Eur J Immunol. 1990 Aug;20(8):1855–1863. doi: 10.1002/eji.1830200834. [DOI] [PubMed] [Google Scholar]
- Paganelli R., Quinti I., Fagiolo U., Cossarizza A., Ortolani C., Guerra E., Sansoni P., Pucillo L. P., Scala E., Cozzi E. Changes in circulating B cells and immunoglobulin classes and subclasses in a healthy aged population. Clin Exp Immunol. 1992 Nov;90(2):351–354. doi: 10.1111/j.1365-2249.1992.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Victor K., Randen I., Thompson K., Steinitz M., Førre O., Fu S. M., Natvig J. B., Capra J. D. Nucleotide sequence analysis of rheumatoid factors and polyreactive antibodies derived from patients with rheumatoid arthritis reveals diverse use of VH and VL gene segments and extensive variability in CDR-3. Scand J Immunol. 1992 Aug;36(2):349–362. doi: 10.1111/j.1365-3083.1992.tb03108.x. [DOI] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Pratt L. F., Rassenti L., Larrick J., Robbins B., Banks P. M., Kipps T. J. Ig V region gene expression in small lymphocytic lymphoma with little or no somatic hypermutation. J Immunol. 1989 Jul 15;143(2):699–705. [PubMed] [Google Scholar]
- Prelli F., Tummolo D., Solomon A., Frangione B. Novel human light chain V kappa segment: serologic and structural analyses of the kappa III-like Bence Jones protein and IgG kappa light chain REE. J Immunol. 1986 Jun 1;136(11):4169–4173. [PubMed] [Google Scholar]
- Päbo S., Higuchi R. G., Wilson A. C. Ancient DNA and the polymerase chain reaction. The emerging field of molecular archaeology. J Biol Chem. 1989 Jun 15;264(17):9709–9712. [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Radoux V., Chen P. P., Sorge J. A., Carson D. A. A conserved human germline V kappa gene directly encodes rheumatoid factor light chains. J Exp Med. 1986 Dec 1;164(6):2119–2124. doi: 10.1084/jem.164.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden D. A., Paige C. J., Wu G. E. Kappa light chain rearrangement in mouse fetal liver. J Immunol. 1994 Aug 1;153(3):1150–1160. [PubMed] [Google Scholar]
- Ruff-Jamison S., Glenney J. R., Jr Requirement for both H and L chain V regions, VH and VK joining amino acids, and the unique H chain D region for the high affinity binding of an anti-phosphotyrosine antibody. J Immunol. 1993 Apr 15;150(8 Pt 1):3389–3396. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Dighiero G. The pathogenesis of chronic lymphocytic leukemia: analysis of the antibody repertoire. Immunol Today. 1994 Jun;15(6):288–294. doi: 10.1016/0167-5699(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Schäble K. F., Zachau H. G. The variable genes of the human immunoglobulin kappa locus. Biol Chem Hoppe Seyler. 1993 Nov;374(11):1001–1022. [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger B., Huber E., Lorenz W., Osterholzer E., Pargent W., Pech M., Pohlenz H. D., Zimmer F. J., Zachau H. G. The human VK locus. Characterization of a duplicated region encoding 28 different immunoglobulin genes. J Mol Biol. 1988 Jan 5;199(1):23–34. doi: 10.1016/0022-2836(88)90376-2. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Victor K. D., Capra J. D. An apparently common mechanism of generating antibody diversity: length variation of the VL-JL junction. Mol Immunol. 1994 Jan;31(1):39–46. doi: 10.1016/0161-5890(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Victor K. D., Randen I., Thompson K., Forre O., Natvig J. B., Fu S. M., Capra J. D. Rheumatoid factors isolated from patients with autoimmune disorders are derived from germline genes distinct from those encoding the Wa, Po, and Bla cross-reactive idiotypes. J Clin Invest. 1991 May;87(5):1603–1613. doi: 10.1172/JCI115174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. C., Blaison G., Martin T., Knapp A. M., Pasquali J. L. Evidence that the V kappa III gene usage is nonstochastic in both adult and newborn peripheral B cells and that peripheral CD5+ adult B cells are oligoclonal. J Clin Invest. 1994 May;93(5):2093–2105. doi: 10.1172/JCI117204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbart R. H., Wong A. L., Noritake D., Kacena A., Chan G., Ruland C., Chin E., Chen I. S., Rosenblatt J. D. The rheumatoid factor reactivity of a human IgG monoclonal autoantibody is encoded by a variant V kappa II L chain gene. J Immunol. 1991 Oct 15;147(8):2795–2801. [PubMed] [Google Scholar]
- Youngblood K., Fruchter L., Ding G., Lopez J., Bonagura V., Davidson A. Rheumatoid factors from the peripheral blood of two patients with rheumatoid arthritis are genetically heterogeneous and somatically mutated. J Clin Invest. 1994 Feb;93(2):852–861. doi: 10.1172/JCI117040. [DOI] [PMC free article] [PubMed] [Google Scholar]