Abstract
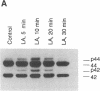
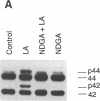
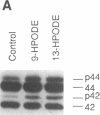
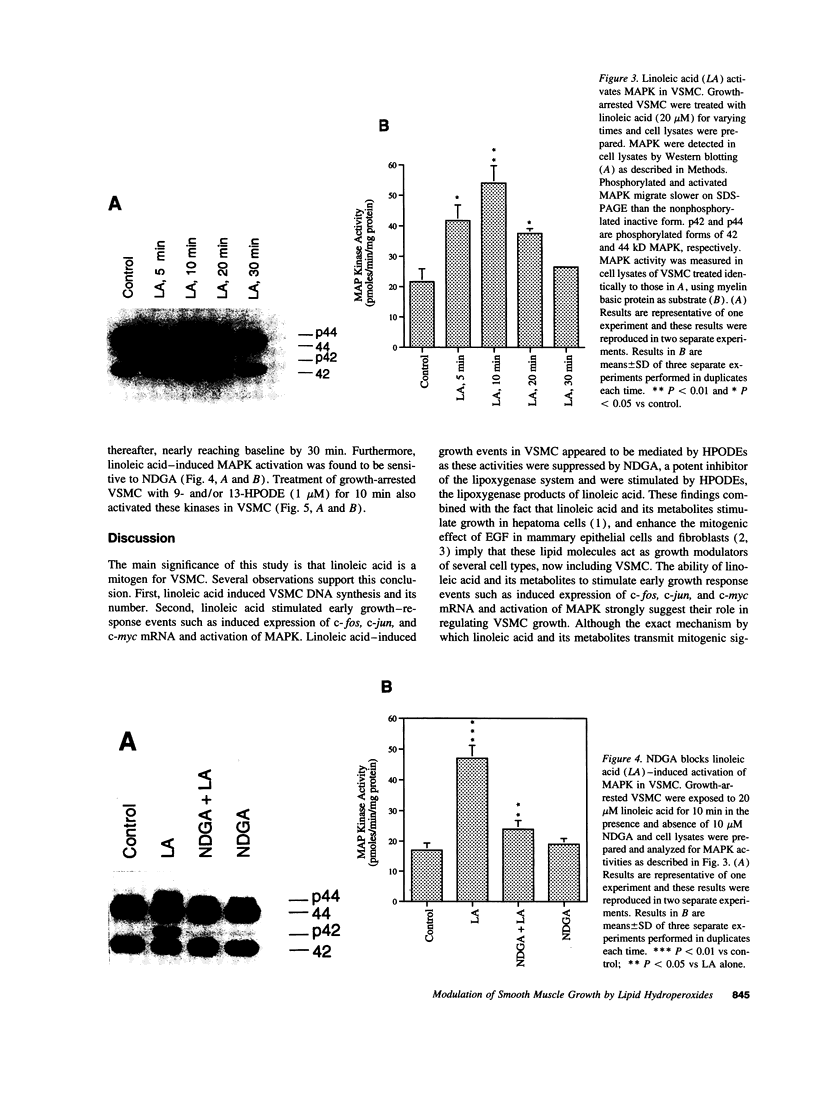
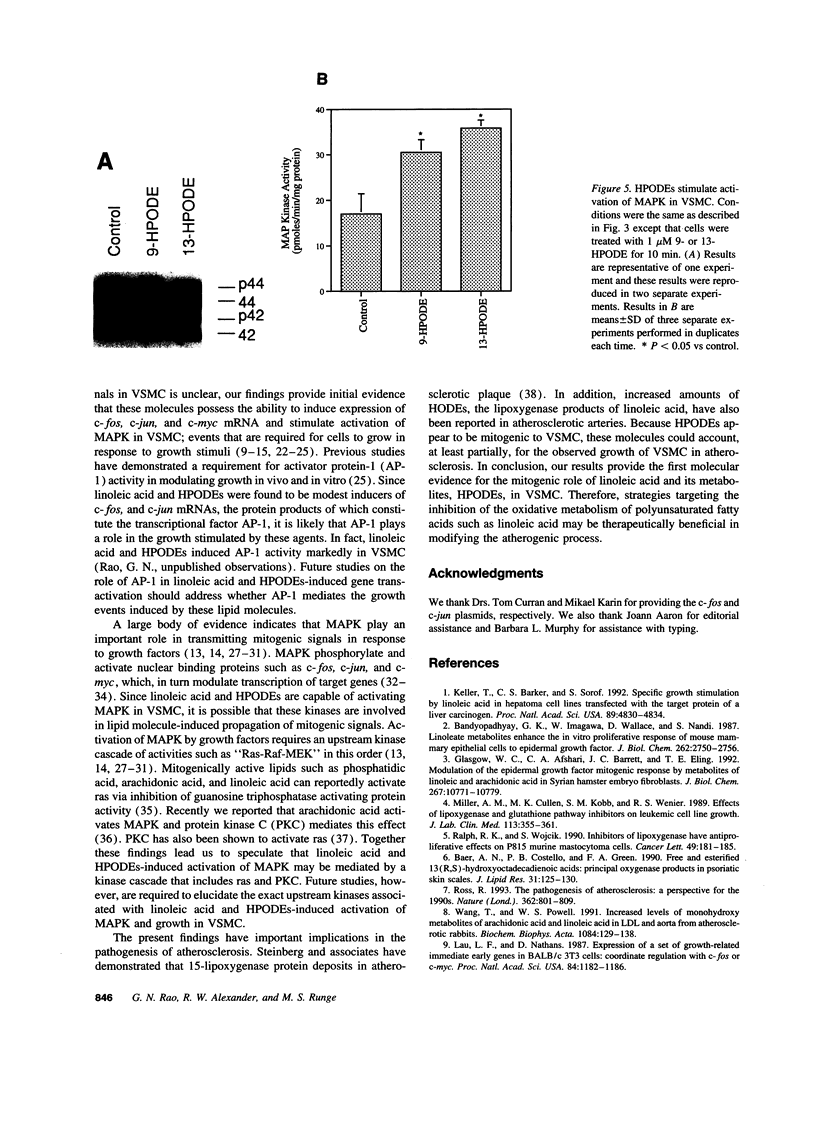
Previous studies from other laboratories suggest that linoleic acid and its metabolites, hydroperoxyoctadecadienoic acids, play an important role in modulating the growth of some cells. A correlation has been demonstrated between hydroperoxyoctadecadienoic acids and conditions characterized by abnormal cell growth such as atherosclerosis and psoriasis. To determine if linoleic acid and its metabolites modulate cell growth in atherosclerosis, we measured DNA synthesis, protooncogene mRNA expression, and mitogen-activated protein kinase (MAPK) activation in vascular smooth muscle cells (VSMC). Linoleic acid induces DNA synthesis, c-fos, c-jun, and c-myc mRNA expression and MAPK activation in VSMC. Furthermore, nordihydroguaiaretic acid, a potent inhibitor of the lipoxygenase system, significantly reduced the growth-response effects of linoleic acid in VSMC, suggesting that conversion of linoleic acid to hydroperoxyoctadecadienoic acids (HPODEs) is required for these effects. HPODEs also caused significant induction of DNA synthesis, protooncogene mRNA expression, and MAPK activation in growth-arrested VSMC, suggesting that linoleic acid and its metabolic products, HPODEs, are potential mitogens in VSMC, and that conditions such as oxidative stress and lipid peroxidation which provoke the production of these substances may alter VSMC growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Baer A. N., Costello P. B., Green F. A. Free and esterified 13(R,S)-hydroxyoctadecadienoic acids: principal oxygenase products in psoriatic skin scales. J Lipid Res. 1990 Jan;31(1):125–130. [PubMed] [Google Scholar]
- Bandyopadhyay G. K., Imagawa W., Wallace D., Nandi S. Linoleate metabolites enhance the in vitro proliferative response of mouse mammary epithelial cells to epidermal growth factor. J Biol Chem. 1987 Feb 25;262(6):2750–2756. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Funk C. D., Powell W. S. Release of prostaglandins and monohydroxy and trihydroxy metabolites of linoleic and arachidonic acids by adult and fetal aortae and ductus arteriosus. J Biol Chem. 1985 Jun 25;260(12):7481–7488. [PubMed] [Google Scholar]
- Gardner A. M., Vaillancourt R. R., Johnson G. L. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase by G protein and tyrosine kinase oncoproteins. J Biol Chem. 1993 Aug 25;268(24):17896–17901. [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Glasgow W. C., Afshari C. A., Barrett J. C., Eling T. E. Modulation of the epidermal growth factor mitogenic response by metabolites of linoleic and arachidonic acid in Syrian hamster embryo fibroblasts. Differential effects in tumor suppressor gene (+) and (-) phenotypes. J Biol Chem. 1992 May 25;267(15):10771–10779. [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Alessandrini A., Crews C. M., Erikson R. L. Raf-1 forms a stable complex with Mek1 and activates Mek1 by serine phosphorylation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10947–10951. doi: 10.1073/pnas.90.23.10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. S., van Lingen B., Papaioannou V. E., Spiegelman B. M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993 Jul;7(7B):1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Keler T., Barker C. S., Sorof S. Specific growth stimulation by linoleic acid in hepatoma cell lines transfected with the target protein of a liver carcinogen. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4830–4834. doi: 10.1073/pnas.89.11.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. Behind the Fos and Jun leucine zipper. Cancer Cells. 1989 Nov;1(3):71–76. [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S. G., Crews C. M., Wu L., Driller J., Clark R., Erikson R. L., McCormick F. Reconstitution of the Raf-1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol. 1993 Nov;13(11):6615–6620. doi: 10.1128/mcb.13.11.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Miller A. M., Cullen M. K., Kobb S. M., Weiner R. S. Effects of lipoxygenase and glutathione pathway inhibitors on leukemic cell line growth. J Lab Clin Med. 1989 Mar;113(3):355–361. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Ralph R. K., Wojcik S. Inhibitors of lipoxygenase have antiproliferative effects on P815 murine mastocytoma cells. Cancer Lett. 1990 Mar;49(3):181–185. doi: 10.1016/0304-3835(90)90156-r. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Baas A. S., Glasgow W. C., Eling T. E., Runge M. S., Alexander R. W. Activation of mitogen-activated protein kinases by arachidonic acid and its metabolites in vascular smooth muscle cells. J Biol Chem. 1994 Dec 23;269(51):32586–32591. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sellmayer A., Uedelhoven W. M., Weber P. C., Bonventre J. V. Endogenous non-cyclooxygenase metabolites of arachidonic acid modulate growth and mRNA levels of immediate-early response genes in rat mesangial cells. J Biol Chem. 1991 Feb 25;266(6):3800–3807. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travo P., Barrett G., Burnstock G. Differences in proliferation of primary cultures of vascular smooth muscle cells taken from male and female rats. Blood Vessels. 1980;17(2):110–116. doi: 10.1159/000158240. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- Wang T., Powell W. S. Increased levels of monohydroxy metabolites of arachidonic acid and linoleic acid in LDL and aorta from atherosclerotic rabbits. Biochim Biophys Acta. 1991 Jul 9;1084(2):129–138. doi: 10.1016/0005-2760(91)90211-y. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]