Abstract
Patients with schwannomatosis develop multiple schwannomas but no vestibular schwannomas diagnostic of neurofibromatosis type 2. We report an inactivating germline mutation in exon 1 of the tumor-suppressor gene INI1 in a father and daughter who both had schwannomatosis. Inactivation of the wild-type INI1 allele, by a second mutation in exon 5 or by clear loss, was found in two of four investigated schwannomas from these patients. All four schwannomas displayed complete loss of nuclear INI1 protein expression in part of the cells. Although the exact oncogenetic mechanism in these schwannomas remains to be elucidated, our findings suggest that INI1 is the predisposing gene in familial schwannomatosis.
Schwannomatosis (MIM 162091) is characterized by the development of multiple spinal, peripheral, and cranial-nerve schwannomas in the absence of vestibular schwannomas.1 The presence of vestibular schwannomas is diagnostic of neurofibromatosis type 2 (NF2 [MIM 101000]). Molecular analyses identified somatically acquired mutations in the NF2 gene in schwannomas of patients with schwannomatosis.2–4 However, linkage studies performed in families affected with schwannomatosis excluded NF2 as the germline-transmissible schwannomatosis gene and suggested a location of this gene near marker D22S1174, which is in the region on chromosome 22 centromeric to NF2.4 In this region is the CABIN1 gene, in which missense mutations may contribute to the pathogenesis of both schwannomatosis and NF2.5
We investigated whether INI1—also known as SMARCB1, hSNF5, and BAF47—is involved in familial schwannomatosis. INI1 is an attractive candidate gene, because it is a tumor-suppressor gene that is located within a short distance (i.e., <360 kb) of marker D22S1174 (University of California Santa Cruz [UCSC] Genome Browser). INI1 is a member of the ATP-dependent SWI-SNF chromatin remodeling complex and affects the expression of genes that regulate cell cycle, growth, and differentiation.6 The involvement of INI1 in schwannoma tumorigenesis has been investigated in one earlier study, but no mutations were found in 23 tumors. However, only exons 2–8 of INI1 were examined (i.e., not exons 1 or 9), and it was not specified whether tumors of patients with schwannomatosis were included.7 Thus far, INI1 is known to be involved in the development of malignant rhabdoid tumors that typically occur in the brain and kidney of infants.8–10 Only two families with an INI1 germline mutation, an exon 4 frameshift mutation,11 and an exon 7 donor splice site mutation12 have been described in which multiple generations were affected by malignant (rhabdoid) tumors in infancy. In both of these families, clear cases of nonexpressing obligate carriers of the INI1 mutation were seen. Recently, INI1 alterations have been reported to occur in the proximal type of epithelioid sarcomas.13
To determine the possible involvement of INI1 in familial schwannomatosis, we examined the mutational status of this gene in constitutional and tumor DNA of a proband and her father, both of whom fulfill the diagnostic criteria for schwannomatosis.14 Additionally, we investigated INI1 protein expression in these tumors by immunohistochemistry. Finally, we sequenced the NF2 gene to determine whether these neoplasms carried somatically acquired NF2 mutations as well.
The proband, a 22-year-old female, presented with pain in her back that had been increasing for 3 years. A magnetic resonance imaging (MRI) scan of the lumbar spine revealed intradural tumors at L1 and L2–L3. After laminectomy, three tumors arising from lumbar spinal nerve roots were removed and diagnosed histopathologically as schwannomas. MRI scans of the cervical and thoracic spine showed multiple intradural, extramedullary lesions of variable size. The most cranial lesion was at C6–C7. MRI scans of the brain (1-mm T2 weighted slices and, after contrast, 3-mm T1 weighted slices) were normal; in particular, vestibular schwannomas were absent. Three years after surgery, except for some local numbness in the right thigh, the patient has not had any more symptoms. As part of the counseling procedure, the NF2 gene in blood DNA of the patient was sequenced in the laboratory of Dr. Olschwang (Institut Paoli-Calmettes, Marseilles), but no mutation was found. The father of the proband had a history of diabetes mellitus and had surgery at the age of ∼35 years because of Wolff-Parkinson-White syndrome. When he was 49 years of age, subcutaneous tumors were removed from his right thumb, right index finger, and the first web space of his left hand and were diagnosed histopathologically as schwannomas. In subsequent years, additional schwannomas were removed from his right upper arm and right thumb, and two seborrheic keratosis lesions from his forehead. A CT scan of the lumbar spine that was performed because of a herniated intervertebral disc when he was 50 years old revealed no tumors, nor did an MRI scan of the brain (3-mm T2 weighted slices and, after contrast, 3-mm T1 weighted slices) that was performed because of a mild cerebrovascular accident when he was 51 years old. In both the father and his daughter (who was his only child), the dermatologist and ophthalmologist did not find other signs of neurofibromatosis. The father died at the age of 57 years from cardiac arrest. An autopsy was not performed. In both patients, hearing loss, tinnitus, and balance dysfunction were absent. The parents, one brother, and one sister of the father had no obvious signs of schwannomatosis.
Informed consent of the proband and her mother was obtained for using their body materials and those of the deceased father. Frozen tumor material was available from one schwannoma from the sacral region of the proband. Peripheral blood was taken from the mother. From the father, formalin-fixed and paraffin-embedded tissue was available from three schwannomas (right index finger, right upper arm, and right thumb) and from one seborrheic keratosis lesion. Genomic DNA was extracted from blood and tissues by use of commercially available kits (Qiagen). The INI1 and NF2 genes were sequenced by use of genomic DNA as substrate for amplification by PCR. Primer sequences for mutation analysis of the 9 INI1 exons and the 17 NF2 exons were kindly provided by, respectively, Dr. J. A. Biegel15 and Dr. S. Olschwang (personal communication) and are listed in tables 1 and 2, respectively. Sequence reactions were performed using ABI Big Dye v3.1 chemistry, and the products were sequenced with an ABI 3730 capillary system (Applied Biosystems). Sequences were analyzed with CodonCode Aligner (CodonCode).
Table 1. .
Primers for Amplification of the INI1 Exons[Note]
| Primer Sequence |
|||
| Exon | Product Size (bp) |
Forward | Reverse |
| 1 | 140 | 5′-CCC TCC TGA TCC CTC GCA GC-3′ | 5′-CGG GCT ACC TCG GAG CCG AT-3′ |
| 2 | 213 | 5′-CTG CGA CCC TTA TAA TGA GC-3′ | 5′-GCG AGT GGT TTT GAA ACA GG-3′ |
| 3 | 195 | 5′-ACC AGC AGA GTG ACC CAG TG-3′ | 5′-AGA GAT GCC CTG GCC AGG AA-3′ |
| 4 | 225 | 5′-GGA TCA GGT CCT ATA CTG AC-3′ | 5′-AAC TAA GGC GGA ATC AGC AC-3′ |
| 5 | 201 | 5′-TTG CAT ACC TAG GGC TCC GG-3′ | 5′-GCC CGA CTG CCT TGT ACC AT-3′ |
| 6 | 277 | 5′-TGG TGC AAT CTC TTG GCA TC-3′ | 5′-TCA GTG CTC CAT GAT GAC AC-3′ |
| 7 | 312 | 5′-TGG GCT GCA AAA GCT CTA AC-3′ | 5′-CGC TCA CAC AGA GAA GTC TT-3′ |
| 8 | 313 | 5′-ATC CAC TGG GTG CCA GCA GT-3′ | 5′-TCT GCC TGG AAA GCC AGG TG-3′ |
| 9 | 200 | 5′-CCC TGT AGA GCC TTG GGA AG-3′ | 5′-GCC TCT GTC CTT GCC AGA AG-3′ |
Note.— Annealing temperature was 60°C.
Table 2. .
Primers for Amplification of the NF2 Exons[Note]
| Primer Sequence |
|||
| Exon | Product Size (bp) |
Forward | Reverse |
| 1 | 225 | 5′-GGC TAA AGG GCT CAG AGT GC-3′ | 5′-CTT CCA CCT CGA CTG TCA CC-3′ |
| 2 | 222 | 5′-ATT TTT GCT CAC AGT GTC CTT CC-3′ | 5′-ACT GGA AAG CTC ACG TCA GCC-3′ |
| 3 | 179 | 5′-GTC TTT TGC TCT GCA ATT CTG C-3′ | 5′-AGA ACT GGG GGG TAG CCT TGA-3′ |
| 4 | 170 | 5′-AGT ATC ATG TCT CCC TTG TTG CT-3′ | 5′-CCA TGA CCC AAA TTA ACG CCC A-3′ |
| 5 | 159 | 5′-CTT TAG AAT CTC AAT CGC CTG C-3′ | 5′-CCA CAT ATC TGC TAT GTC TTC CT-3′ |
| 6 | 261 | 5′-GTG ACT ATC TCC CTG GGT GTA-3′ | 5′-CCC CAT AAA GGA ATG TAA ACC AAC-3′ |
| 7 | 180 | 5′-GAT TTG GTG CCC ACC CGC TC-3′ | 5′-ACA CAA GGA GCT CAG AGA GGT T-3′ |
| 8 | 187 | 5′-GTA GCT GTT CTT ATT GGA TCC AC-3′ | 5′-CAT CTG CAG TAC ACA CAT GTC C-3′ |
| 9 | 256 | 5′-GTG TGG TTG CGC ATT TGT GGA A-3′ | 5′-CAC AAG ATG TCA CTC TGA TAT CC-3′ |
| 10 | 230 | 5′-TGG GCC AGT AGG CAG TGA AG-3′ | 5′-AGG ACT GAC CAC ACA GTG ACA-3′ |
| 11 | 214 | 5′-CTC GAG CCC TGT GAT TCA ATG-3′ | 5′-AGT CCC CAA GTA GCC TCC TG-3′ |
| 12 | 286 | 5′-CCC ACT TCA GCT AAG AGC ACT-3′ | 5′-CTC CTC GCC AGT CTG GTG C-3′ |
| 13 | 203 | 5′-ACC TGC CCT CTT CTG TGA AGC-3′ | 5′-AGA ACA TCA CCA GGA CTA AGG C-3′ |
| 14 | 213 | 5′-TGA CCC AAG CTC CTA ATC CGA-3′ | 5′-AGT CTA GTT CAC AGC TGC CCA-3′ |
| 15 | 254 | 5′-GTC TCA CTG TCT GCC CAA GC-3′ | 5′-GGT CCT GAT CAG CAA AAT ACA AG-3′ |
| 16 | 188 | 5′-CAT TTT GCA ATG GCA CTT ATG GC-3′ | 5′-CGG GTT AGT ATC ACA GAG GGC-3′ |
| 17 | 268 | 5′-CTC TCA GCT TCT TCT CTG CTT T-3′ | 5′-ACA GGG TCG TAG TTC AAG GCA-3′ |
Note.— Annealing temperature was 54°C.
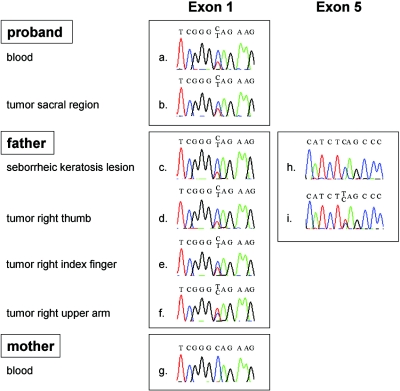
To search for a constitutional mutation in the INI1 gene, the nine exons of this gene were sequenced. As shown in figure 1, a heterozygous C→T mutation in codon 12 of exon 1 (c.34C→T in the mRNA [GenBank accession number U04847]) was identified in blood DNA of the proband and in DNA of the seborrheic keratosis lesion of her father (which was used as a source of constitutional DNA because blood of the deceased father was not available), as well as in DNA of all four schwannomas that were available for further analysis (one from the proband and three from her father), but not in blood DNA of the clinically unaffected mother. This mutation converts a glutamine codon into a stop codon (p.Q12X), resulting in premature termination of translation of the INI1 protein at that position. Additionally, DNA extracted from the right-upper-arm lesion of the father revealed a strongly decreased peak height for the wild-type C allele, suggesting partial loss of the normal INI1 allele, whereas, in DNA from the tumor of his right thumb, an additional, heterozygous C→T mutation in codon 182 of exon 5 was found (c.544C→T), which also converts a glutamine codon into a stop codon (p.Q182X). No additional mutations or clear losses were found in the third schwannoma of the father nor in the tumor of the proband, although the small decrease in peak height for the wild-type C allele may suggest some loss of the normal INI1 allele in both tumors. Since somatically acquired mutations have been reported to accumulate in the NF2 gene in schwannomas of patients with schwannomatosis,2–4 we determined the nucleotide sequence of the 17 exons of this gene in DNA extracted from the tumors of both the proband and the father. We found no NF2 mutations in these tumors.
Figure 1. .
Sequence analysis of parts of INI1 in blood and tissues of proband and parents. The C→T mutation in exon 1 of INI1 was detected in DNA from blood and from the schwannoma of the sacral region of the proband (a and b). It was also detected in DNA from the seborrheic keratosis lesion and from the schwannomas of the right thumb, right index finger, and right upper arm of the father (c–f) but not in blood DNA of the mother (g). An additional C→T mutation in exon 5 of INI1 was detected in DNA of the right thumb tumor of the father (i) but not in DNA of his seborrheic keratosis lesion (h).
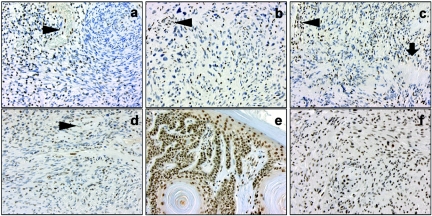
The truncating mutations in exons 1 and 5 of INI1 are both predicted to cause mRNA decay and loss of INI1 protein expression by the mutated allele.16 We studied the effect of these mutations on the INI1 protein level by immunohistochemical analysis performed using an INI1/BAF47 antibody (BD Transduction Laboratories). For this purpose, 5-μm histological sections of formalin-fixed, paraffin-embedded tumor-tissue sections were dewaxed in xylol and rehydrated through alcohol. Endogenous peroxidase was blocked with 3% hydrogen peroxide. The sections were pretreated by microwave heating, cooled down to room temperature, and incubated for 60 min with the INI1/BAF47 antibody. After incubation with the secondary antibody (poly-HRP-GAM/R/R IgG [Immunologic]), the staining procedure was finished using DAB. The sections were counterstained with hematoxylin (1 min), dehydrated, and cover slipped. All four schwannomas from this family showed a consistent mosaic staining pattern for INI1, with a mixture of negative and positive cell nuclei (fig. 2a–d). This pattern was maintained after a fourfold concentrated INI1-antibody solution was applied to the sections (dilution 1:60 instead of 1:250 [data not shown]). The seborrheic keratosis lesion of the father (fig. 2e) and the schwannomas of five different patients unaffected with schwannomatosis—including vestibular and soft-tissue schwannomas and a schwannoma from a patient with multiple schwannomas (fig. 2f)—demonstrated abundant and uniform nuclear staining in all cells.
Figure 2. .
Immunohistochemical INI1/BAF47 staining of schwannomas of the proband (a, sacral region) and her father (b, right index finger; c, right thumb; d, right upper arm), as well as of a seborrheic keratosis lesion of the father (e) and of a schwannoma of a patient not affected with schwannomatosis (f). Note the mosaic staining of cell nuclei in schwannomas of both the proband and her father. In contrast, the nuclei of cells in the tumor vessels in panels a–d (arrowheads), as well as the nuclei in a seborrheic keratosis lesion of the father and in a schwannoma in a patient unaffected with schwannomatosis, all show unequivocal staining. The arrow in panel c indicates palisading of tumor cell nuclei in a Verocay body. Original magnification ×200.
Our findings suggest that INI1 germline mutations predispose individuals to familial schwannomatosis. Earlier linkage studies by others in families affected with schwannomatosis excluded NF2 as the schwannomatosis gene and pointed to the location of this presumptive gene in a region nearby marker D22S1174 (multipoint LOD score of 6.60), which is centromeric to NF2 on chromosome 22.4 We considered INI1 an attractive candidate for representing the familial schwannomatosis gene, because it is a tumor-suppressor gene and it is located within short distance of D22S1174 (UCSC Genome Browser). We therefore sequenced this gene in constitutional and tumor DNA of the proband and her father, who both suffered from clinically proven schwannomatosis. Because an inactivating C→T mutation was present in exon 1 of the INI1 gene, not only in all schwannomas of these patients but also in blood of the proband and in a seborrheic keratosis lesion of her father, we conclude that this mutation represents a germline mutation.
According to Knudson’s two-hit hypothesis for the oncogenesis of tumors, this germline mutation may very well represent the first hit in tumor development.17 The second hit that leads to the formation of schwannomas should then involve the wild-type INI1 allele and could theoretically consist of somatic mutation, loss of heterozygosity (LOH), or silencing by other means, such as epigenetic modification. Indeed, using sequence analysis, we detected an additional truncating C→T mutation in exon 5 in part of the cells in one schwannoma and partial loss of the wild-type allele in another tumor of the father. In accordance with these observations, these schwannomas showed a mosaic INI1 staining pattern, with a mixture of negative and positive cell nuclei. This pattern was also present in the two schwannomas without an easily detectable second hit, suggesting that silencing of the wild-type INI1 allele occurred by other mechanisms, as has been suggested for rhabdoid tumors without INI1 mutations.10 The mosaic staining pattern seems to be characteristic of schwannomas of patients with schwannomatosis, as we noted uniform and unequivocal nuclear staining in the seborrheic keratosis lesion of the father and in various schwannomas of patients without schwannomatosis. Clearly, our immunohistochemical findings corroborate the involvement of INI1 in the oncogenesis of schwannomas in our patients with schwannomatosis.
Further elucidation of the exact oncogenetic role of INI1 is warranted. It is unknown at present how INI1 germline mutations can predispose infants to malignant rhabdoid tumors10–12 and patients with familial schwannomatosis to schwannomas. Since, to our knowledge, INI1 germline mutations in exon 1 have not been reported before, one possible explanation is that different INI1 mutations are associated with different tumor types. Furthermore, as suggested by others,11 individuals with a germline INI1 mutation may have an increased risk for developing a malignant rhabdoid tumor especially in early childhood. Later in life, this risk may decrease, but germline INI1 mutations may then predispose individuals to the development of other tumors, such as schwannomas. Finally, additional somatic inactivation of NF2 or other genes may play a role in the development of schwannomas of patients with schwannomatosis. Although we were unable to detect NF2 aberrations in our patients, others found NF2 mutation and loss in a considerable fraction of the investigated tumors.2–4 In contrast with malignant rhabdoid tumors and the proximal type of epithelioid sarcomas that generally show complete loss of nuclear INI1 staining,9,10,13 the schwannomas of our patients with schwannomatosis showed a mosaic staining pattern of cell nuclei, indicating complete loss of INI1 protein expression in part of the cells. Additional immunohistochemical INI1 and S100 stainings on serial sections of the tumors showed that the majority of cells are S100 positive, corroborating the Schwann cell nature of these cells (data not shown). As has been described for neurofibromas,18,19 the tumors in our patients may be comprised of different (Schwann) cell populations, the cells that completely lost INI1 protein expression being the true neoplastic cells.
In conclusion, this is the first report of a germline mutation that predisposes individuals to familial schwannomatosis. The mutation is located in the INI1 gene, a tumor-suppressor gene that is also involved in the oncogenesis of tumors with a completely different histology and prognosis. Further studies are needed to determine whether the inactivation of the INI1 gene is the cause of tumor formation in other cases of familial and sporadic schwannomatosis as well and to elucidate the exact oncogenetic mechanisms and functional consequences of INI1 inactivation in these and other tumors.
Acknowledgments
This study was supported by Dutch Cancer Society grant UVA 2001–2561. We express our gratitude to Dr. P. R. Schuurman (Department of Neurosurgery, Academic Medical Center, Amsterdam), who brought the family to our attention. We thank Dr. E. Bijlsma (Department of Clinical Genetics, Leiden University Medical Center, Leiden) for her help in identifying patients with schwannomatosis in the Netherlands. We are indebted to Dr. D. Troost (Neuropathology Department, Academic Medical Center, Amsterdam), Dr. J. Westerga (Pathology Department, Slotervaart Hospital, Amsterdam), and Dr. B. van de Wiel (Pathology Department, Sint Lucas Andreas Hospital, Amsterdam) for providing tumor materials; to Dr. M. Mannens (DNA-Diagnostics Laboratory, Clinical Genetics Department, Academic Medical Center, Amsterdam) for donating the peripheral blood DNA of the proband; and to A. Gemmink (Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen) for performing the immunohistochemical stainings.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for INI1 mRNA [accession number U04847])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for schwannomatosis and NF2)
- UCSC Genome Browser, http://genome.ucsc.edu/ (March 2006 build)
References
- 1.MacCollin M, Woodfin W, Kronn D, Short MP (1996) Schwannomatosis: a clinical and pathologic study. Neurology 46:1072–1079 [DOI] [PubMed] [Google Scholar]
- 2.Jacoby LB, Jones D, Davis K, Kronn D, Short MP, Gusella J, MacCollin M (1997) Molecular analysis of the NF2 tumor-suppressor gene in schwannomatosis. Am J Hum Genet 61:1293–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman DL, Heinrich BS, Willett C, Perry A, Finseth F, Sobel RA, McCollin M (2003) Somatic instability of the NF2 gene in schwannomatosis. Arch Neurol 60:1317–1320 10.1001/archneur.60.9.1317 [DOI] [PubMed] [Google Scholar]
- 4.MacCollin M, Willett C, Heinrich B, Jacoby LB, Acierno JS Jr, Perry A, Louis DN (2003) Familial schwannomatosis: exclusion of the NF2 locus as the germline event. Neurology 60:1968–1974 [DOI] [PubMed] [Google Scholar]
- 5.Buckley PG, Mantripragada KK, Diaz de Stahl T, Piotrowski A, Hansson CM, Kiss H, Vetrie D, Ernberg IT, Nordenskjold M, Bolund L, et al (2005) Identification of genetic aberrations on chromosome 22 outside the NF2 locus in schwannomatosis and neurofibromatosis type 2. Hum Mutat 26:540–549 10.1002/humu.20255 [DOI] [PubMed] [Google Scholar]
- 6.Roberts CW, Orkin SH (2004) The SWI/SNF complex—chromatin and cancer. Nat Rev Cancer 4:133–142 [DOI] [PubMed] [Google Scholar]
- 7.Bruder CE, Dumanski JP, Kedra D (1999) The mouse ortholog of the human SMARCB1 gene encodes two splice forms. Biochem Biophys Res Commun 257:886–890 10.1006/bbrc.1999.0563 [DOI] [PubMed] [Google Scholar]
- 8.Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O (1998) Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394:203–206 10.1038/28212 [DOI] [PubMed] [Google Scholar]
- 9.Judkins AR, Mauger J, Rorke LB, Biegel JA (2004) Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol 28:644–650 [DOI] [PubMed] [Google Scholar]
- 10.Biegel JA (2006) Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg Focus 20:E11 [DOI] [PubMed] [Google Scholar]
- 11.Janson K, Nedzi LA, David O, Schorin M, Walsh JW, Bhattacharjee M, Pridjian G, Tan L, Judkins AR, Biegel JA (2006) Predisposition to atypical teratoid/rhabdoid tumor due to an inherited INI1 mutation. Pediatr Blood Cancer 47:279–284 10.1002/pbc.20622 [DOI] [PubMed] [Google Scholar]
- 12.Taylor MD, Gokgoz N, Andrulis IL, Mainprize TG, Drake JM, Rutka JT (2000) Familial posterior fossa brain tumors of infancy secondary to germline mutation of the hSNF5 gene. Am J Hum Genet 66:1403–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modena P, Lualdi E, Facchinetti F, Galli L, Teixeira MR, Pilotti S, Sozzi G (2005) SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 65:4012–4019 10.1158/0008-5472.CAN-04-3050 [DOI] [PubMed] [Google Scholar]
- 14.MacCollin M, Chiocca EA, Evans DG, Friedman JM, Horvitz R, Jaramillo D, Lev M, Mautner VF, Niimura M, Plotkin SR, et al (2005) Diagnostic criteria for schwannomatosis. Neurology 64:1838–1845 10.1212/01.WNL.0000163982.78900.AD [DOI] [PubMed] [Google Scholar]
- 15.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B (1999) Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59:74–79 [PubMed] [Google Scholar]
- 16.Nagy E, Maquat LE (1998) A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci 23:198–199 10.1016/S0968-0004(98)01208-0 [DOI] [PubMed] [Google Scholar]
- 17.Knudson AG Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68:820–823 10.1073/pnas.68.4.820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluwe L, Friedrich R, Mautner VF (1999) Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer 24:283–285 [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski JL, Wu K, Gutmann DH, Boyer PJ, Legius E (2000) Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1. Hum Mol Genet 9:1059–1066 10.1093/hmg/9.7.1059 [DOI] [PubMed] [Google Scholar]