Abstract
Crisponi syndrome is a severe autosomal recessive condition that is phenotypically characterized by abnormal, paroxysmal muscular contractions resembling neonatal tetanus, large face, broad nose, anteverted nares, camptodactyly, hyperthermia, and sudden death in most cases. We performed homozygosity mapping in five Sardinian and three Turkish families with Crisponi syndrome, using high-density single-nucleotide polymorphism arrays, and identified a critical region on chromosome 19p12-13.1. The most prominent candidate gene was CRLF1, recently found to be involved in the pathogenesis of cold-induced sweating syndrome type 1 (CISS1). CISS1 belongs to a group of conditions with overlapping phenotypes, also including cold-induced sweating syndrome type 2 and Stüve-Wiedemann syndrome. All these syndromes are caused by mutations of genes of the ciliary neurotrophic factor (CNTF)–receptor pathway. Here, we describe the identification of four different CRLF1 mutations in eight different Crisponi-affected families, including a missense mutation, a single-nucleotide insertion, and a nonsense and an insertion/deletion (indel) mutation, all segregating with the disease trait in the families. Comparison of the mutation spectra of Crisponi syndrome and CISS1 suggests that neither the type nor the location of the CRLF1 mutations points to a phenotype/genotype correlation that would account for the most severe phenotype in Crisponi syndrome. Other, still-unknown molecular factors may be responsible for the variable phenotypic expression of the CRLF1 mutations. We suggest that the syndromes can comprise a family of “CNTF-receptor–related disorders,” of which Crisponi syndrome would be the newest member and allelic to CISS1.
Crisponi syndrome (MIM 601378) is a severe autosomal recessive condition described for the first time in 1996 by Giangiorgio Crisponi.1 Recently, Accorsi et al.2 and Nannenberg et al.3 each reported a further case with the same phenotype and suggested that the syndrome be named after the author of the first report. The disorder is evident at birth and is characterized by marked contraction of the facial muscles in response to tactile stimuli or during crying, with trismus and abundant salivation simulating a tetanic spasm. Contractions of the oropharyngeal muscles associated with absence of the swallowing reflex are also observed. These contractions slowly disappear as the infant calms. All patients described to date present facial anomalies, including large face, chubby cheeks, broad nose with anteverted nostrils, and bilateral camptodactyly (fig. 1). The clinical course is characterized by marked sucking difficulties requiring nasogastric tube feeding. Moreover, patients become hyperthermic, with core temperatures of ∼38°C accompanied by irregular febrile episodes of >42°C, with age at onset ranging from birth to a few weeks. In some patients, generalized seizures occur. Most patients die within the first months of life, during hyperthermic episodes. In two of these cases, reduced levels of γ-aminobutyric acid (GABA) were noted in cerebrospinal fluid,1,4 but the pathogenic relevance of this finding is unclear. Surviving patients usually develop a severe progressive kyphoscoliosis (fig. 1E) requiring corset therapy or corrective surgery. We have also observed paradoxical sweating after exposure to low ambient temperature in some affected adolescents. On the basis of the appearance of multiple affected siblings of both sexes in one family, autosomal recessive inheritance is most likely. Phenotypic overlap of Crisponi syndrome with Stüve-Wiedemann syndrome (SWS)/ Schwartz-Jampel type 2 syndrome (SJS2) (MIM 601559)5–7 was discussed by Nannenberg et al.3 This syndrome was recently linked to mutations in the LIFR gene.8 However, bowing of the long bones, the most prominent feature of SWS, was never observed in patients with Crisponi syndrome. Therefore, having excluded linkage to LIFR by microsatellite-marker studies (data not shown), we started a positional cloning effort in eight unrelated families from Sardinia and Turkey, to identify the causative gene for Crisponi syndrome (fig. 2A and 2B). The study was approved by the Research Ethics Committee of the Azienda Sanitaria Locale 8 (ASL8), Cagliari, and by the Research Ethics Committee of Münster University Hospital.
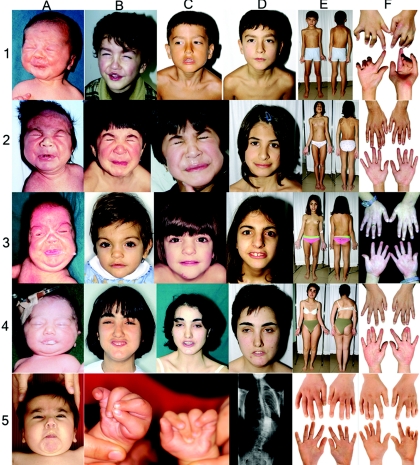
Figure 1. .
Typical clinical features of Crisponi syndrome. 1, Subject CS07. 2, Subject CS10. 3, Subject CS14. 4, Subject CS03. A–D, Facial expression at different ages, from birth to present. E, Front and back views, at the present time. Note scoliosis and limited extension of elbows. F, Subjects' hands. Note consistent camptodactyly. 5, Subject CS37, aged 14 mo. Note perioral muscular contractions (A) and camptodactyly (B and C). 5D and 5E, Subject CS42, aged 13 years. Note thorakolumbal scoliosis (D) and camptodactyly (E). 5F, Subject CS41, aged 14 years. Note camptodactyly.
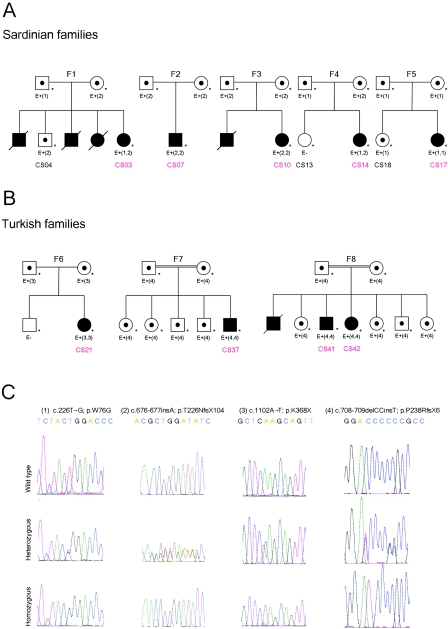
Figure 2. .
A and B, Pedigrees of the five Sardinian and three Turkish families. A blackened symbol indicates an affected individual with Crisponi syndrome; an unblackened symbol indicates an unaffected individual; a dot in the center of a symbol indicates an obligate carrier; a blackened symbol with slash indicates a deceased affected individual; and an asterisk (*) indicates that a sample from the subject was analyzed in the present study. E+ and E− indicate the presence or absence, respectively, of mutations, which are indicated by number(s) in parentheses and are described in panel C. In families F1 and F4, the probands CS03 and CS14 are compound heterozygous. C, CRLF1 sequence analysis. Electropherograms are shown for each of the four mutations found in the present study: heterozygous (middle) and homozygous (bottom) compared with wild type (top).
The phenotype and the clinical course of Crisponi syndrome is exemplified by the following case report: subject CS07 (fig. 1 rows 1A–F), the male proband of family 2 (F2) (fig. 2), was born at 38 wk of gestation as the first child of nonconsanguineous Sardinian parents. Birth weight was 3,130 g (50th percentile) and length was 48 cm (50th percentile). Right after birth, he was unable to suck and had massive spasms of the mimic muscles during crying, with abundant salivation. Minor anomalies included camptodactyly and a broad nose with anteverted nostrils. An exaggerated response to noise and tactile stimuli, with spasmodic contractions of the face, jaw, and neck muscles and opisthotonus, was noted. Frequent contractions of the oropharyngeal muscles associated with absence of the swallowing reflex required nasogastric tube feeding. Early in the neonatal period, the patient developed continuous hyperthermia unrelated to infectious agents, dyspnea, apnea spells, and cyanosis during crying. Febrile episodes disappeared after the 1st year of life, whereas feeding difficulties persisted. At age 6 years, episodic sweating began. The sweating commenced at the upper lip and eyebrows and spread to the thorax and the palms of both hands, which then became cold and cyanotic. Sweating was provoked by low ambient temperature and by increased humidity. The child could not tolerate heat, lived in a house with air conditioning, and walked barefoot. During a sweat test, at 22°C ambient temperature, localized sweating around the eyebrows and the lips was noted, followed by mild sweating on the shoulders.
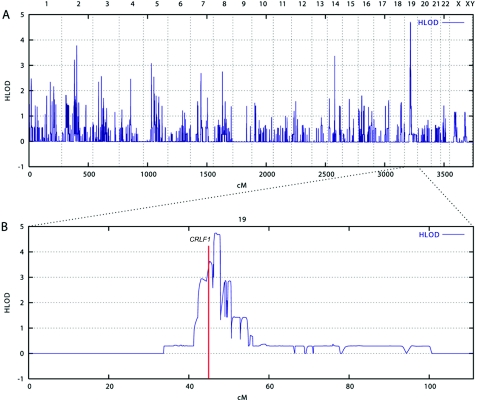
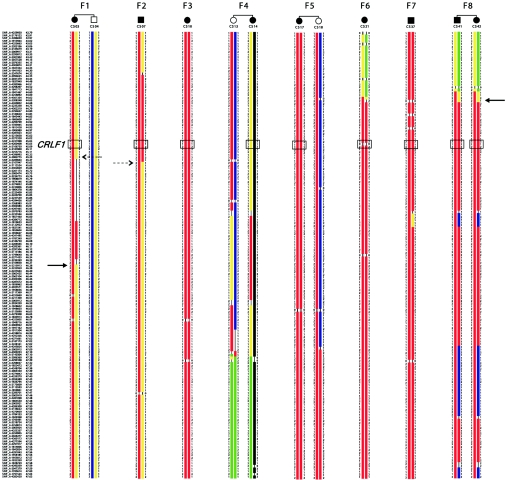
The phenotypes of the patients in families 1 and 4 were summarized in the original article by Crisponi, and “family 1” and “family 4” in the present study refer to family E and family K, respectively, in the work of Crisponi.1 We noted that the Sardinian family 4 described in our report is also identical to family 1 in the recent report by Dagoneau et al.9(in this issue) We include an overview of the phenotype and follow-up data from these patients and from an additional eight patients of six new families with Crisponi syndrome (table 1). To identify the underlying disease gene, we performed a genome scan to identify homozygous regions shared by the probands of the different families. The parents of the Sardinian patients were not known to be related, but several grandparents had been born in the same rural area, raising the possibility of a distant consanguineous loop. Moreover, the relatively high incidence of Crisponi syndrome among Sardinians strongly suggested a founder effect in this isolated population. Therefore, we introduced consanguineous loops into all eight families (conservatively assigned as second-cousin parents for those of unknown consanguinity), with either one or two offspring, when performing the linkage analysis. We have used this approach successfully in previous positional cloning projects in which also a high proportion of consanguineous families was only assumed but not proven a priori.10,11 Here, we genotyped DNA samples from nine affected and three unaffected children of known and suspected consanguineous unions, using the Affymetrix GeneChip Human Mapping 250K Sty Array. For this array, the StyI restriction enzyme was used as an initial step in sample preparation (Affymetrix protocol). Up to ∼238,000 SNPs with an average spacing of ∼12 kb were interrogated. Genotypes were called using the GeneChip DNA Analysis Software (GDAS v. 3.0 [Affymetrix]). We verified sample sexes by counting heterozygous SNPs on the X chromosome. Relationship errors were evaluated with the help of the program Graphical Relationship Representation.12 Non-Mendelian errors were identified by using the program MERLIN,13 and unlikely genotypes for related samples were deleted. Parametric linkage analysis was performed with the programs ALLEGRO14 and a new version of MERLIN that allows for marker clustering to compensate for linkage disequilibrium (LD) between adjacent markers.13,15 Haplotypes were reconstructed by a modified version of the program GENEHUNTER 2.116,17 and were presented graphically with HaploPainter.18 All data handling was performed using the graphical user interface ALOHOMORA.19 Linkage analysis under the assumption of locus heterogeneity (i.e., heterogeneity LOD [HLOD] score) revealed a single maximum LOD score of 4.74 for the region 19p12-13.1 (fig. 3). On the basis of the haplotype data from patients with extended homozygosity, this interval is delimited by SNP markers rs2023878 and rs2023414 and comprises ∼1.5 Mb, with CRLF1 (cytokine receptor–like factor 1) as the most promising positional candidate (fig. 4). CRLF1 was still located in the critical interval of ∼700 kb when a less conservative definition was used and the bottom end of the short homozygous segment of patient CS07 of F2 was considered for border determination. Only two patients (from families F1 and F4) did not show a homozygous segment in this narrow interval, suggesting either a compound heterozygous status or locus heterogeneity.
Table 1. .
Overview of the Phenotype of Patients Investigated for CRLF1 Mutations[Note]
| Abnormal |
|||||||||||
| Family (Consanguinity), Geographic Origin, and Patient |
Born | Sex | Age at Death | Scoliosis | Seizures | Age at Onset of Paradoxical Sweating (years) |
EEGa | Karyotype | Clinical Course | DNA Sequence Variant | Amino Acid Change |
| 1 (−): | |||||||||||
| Sardinia: | |||||||||||
| Brother | 1971 | M | 5 wk | − | + | − | NE | NE | NE | NE | |
| Brother | 1973 | M | 12 wk | − | + | − | NE | − | NE | NE | |
| Sister | 1975 | F | 8 wk | − | − | − | NE | − | NE | NE | |
| CS03 | 1979 | F | Alive | + | − | Puberty | − | − | High-arched palate, developmental delay, and nasogastric tube feeding for 3 years | c.226T→G c.676-677insA | p.Trp76Gly p.Thr226AsnfsX104 |
| 2 (−): | |||||||||||
| Sardinia: | |||||||||||
| CS07 | 1998 | M | Alive | + | − | 6 | − | NE | Heat intolerance | c.676-677insA | p.Thr226AsnfsX104 |
| 3 (−): | |||||||||||
| Sardinia: | |||||||||||
| Brother | 1993 | M | 12 wk | − | + | − | + | − | NE | NE | |
| CS10 | 1995 | F | Alive | + | − | 9 | − | − | Heat intolerance and erosive keratitis | c.676-677insA | p.Thr226AsnfsX104 |
| 4 (−): | |||||||||||
| Sardinia: | |||||||||||
| CS14 | 1993 | F | Alive | + | + | 9 | + | NE | Suspected anhydrotic ectodermal dysplasia and erosive keratitis | c.226T→G c.676-677insA | p.Trp76Gly p.Thr226AsnfsX104 |
| 5 (−): | |||||||||||
| Sardinia: | |||||||||||
| CS17 | 2004 | F | Alive | + | − | − | − | − | Heat intolerance; camptodactyly resolved at age 2 years | c.226T→G | p.Trp76Gly |
| 6 (−): | |||||||||||
| Turkey: | |||||||||||
| CS21 | 2002 | F | Alive | − | − | − | − | − | Speech delay, erosive keratitis, and gastrostomy tube feeding for 18 mo | c.1102A→T | p.Lys368X |
| 7 (+): | |||||||||||
| Turkey: | |||||||||||
| CS37 | 2003 | M | 30 mo | − | − | − | − | − | High-arched palate, gastroesophageal reflux, low CSF GABA levels,b and gastrostomy tube feeding for 2 years | c.708-709delCCinsT | p.Pro238ArgfsX6 |
| 8 (+): | |||||||||||
| Turkey: | |||||||||||
| CS41 | 1990 | M | Alive | + | − | 10 | − | − | Nasogastric tube feeding for 10 mo and speech delay | c.708-709delCCinsT | p.Pro238ArgfsX6 |
| CS42 | 1991 | F | Alive | + | − | 7 | − | − | Nasogastric tube feeding for 12 mo and speech delay | c.708-709delCCinsT | p.Pro238ArgfsX6 |
Note.— All subjects had perioral contractions, camptodactyly, hyperthermia, and feeding difficulties. NE=not examined; a minus sign (−)=unaffected or normal result.
Electroencephalogram.
Levels of GABA in cerebrospinal fluid (CSF) at age 17 mo: total GABA 3.1 μmol/liter (normal range 4.2–13.4 μmol/liter), free GABA 0.011 μmol/liter (normal range .017–.067 μmol/liter).
Figure 3. .
Mapping of a locus for Crisponi syndrome on 19p12-13.1. Multipoint linkage analysis was performed using 250K SNP genotype data from nine affected and three unaffected children. The program MERLIN was used with the recently implemented LD-modeling option, to avoid inflated LOD scores.13 A, Under the assumption of locus heterogeneity (i.e., HLOD calculation), the highest LOD scores were obtained for chromosome 19 markers, with a maximum LOD score of 4.74 at 46.77 cM, according to the deCODE map. B, Magnification of chromosome 19. The red line indicates the position of the CRLF1 gene.
Figure 4. .
Haplotype analyses on chromosome 19p12-13.1. Homozygous regions that are supposed to be identical by descent in each family are shown in red. The position of the CRLF1 gene is indicated by a boxed SNP trio representing an intragenic SNP (rs8107912) and the two nearest flanking SNPs (rs7258589 and rs7256751) that are tiled on the 250K Sty array from Affymetrix. A critical interval of 1.45 Mb may be defined by the overlap of homozygous regions (solid arrows). The broken-line arrows define less reliable borders if it is decided beforehand that either F1 or F2 is homozygous at the disease-gene locus.
CRLF1 was considered the most prominent candidate gene because it was recently found to be involved in the pathogenesis of cold-induced sweating syndrome type 1 (CISS1 [MIM 272430]), which shows some overlapping features with Crisponi syndrome as well as with SWS/SJS2.20,21 CISS1 was first described by Sohar et al.22 in two Israeli sisters, and three other cases were subsequently reported by Knappskog et al.20 and by Hahn et al.21 CISS1 is characterized by feeding difficulties during infancy, dysmorphic features such as high-arched palate, low-set rotated ears, depressed nasal bridge, flexion deformities of elbows and fingers, and kyphoscoliosis. In addition, affected patients experienced profuse sweating, induced by cold ambient temperature, on their back and chest. These features are also present in a similar although variable manner in those patients affected by Crisponi syndrome who survived to adolescence (see table 1).
Another form of cold-induced sweating syndrome (i.e., CISS2 [MIM 610313]) is known to be caused by mutations in the CLCF1 (cardiotrophin-like cytokine factor 1) gene on chromosome 11q13.3. Only one case has been reported in the literature thus far, by Rousseau et al.23 The clinical phenotype of both CISS1 and CISS2 is extremely similar, although variable.
Using specific primers (see table 2), we performed direct sequencing of the nine coding exons and surrounding intronic regions of CRLF1 (GenBank accession number NM_004750) in all our patients. We identified homozygous and compound heterozygous mutations in the CRLF1 gene in all affected individuals from all families, and four mutations accounted for all mutant alleles. Mutation types included one missense mutation, a single-nucleotide insertion, a nonsense mutation, and an indel mutation (see fig. 2C and table 1 for details). By sequencing the mutated exons in all the available family members, we confirmed that the mutations cosegregated with the disease trait (fig. 2A and 2B).
Table 2. .
CRLF1 Primers and Conditions for Genomic PCR[Note]
| Primer Sequence(5′→3′) |
||||
| Exon | Forward | Reverse | Size (bp) |
Temperature (°C) |
| 1a | TTAGCGCCTTGTCAATTCGGC | TGTTCCCCGGCCGTCCAGG | 394 | 60 |
| 2 | GACAATCATTAACAGCGTC | AGTGTGCCCACAGCTCATCC | 507 | 54 |
| 3 | GGAGATCGAGTCACCAGCCTC | GGCAGCCTCAGGGTGCAGAC | 441 | 68 |
| 4 | CTTGACCAACGCGGACCCT | ACTTACCTACCTTCCCTCTG | 456 | 60 |
| 5 | ACAGAGGCAGGTTCCATC | CAGGAGGTCTGGTTGCTCAC | 250 | 60 |
| 6 | CTACCGAGTGGAGGACAGTG | TATGCGACAGAATGAGGCCG | 421 | 60 |
| 7a | TCGGTCCTTGAGAAACGGG | TTGGAGCAGTACGCGTGC | 252 | 60 |
| 8a | AGCTCAAGCAGTTCCTGG | GGGTGTGAACAAGACCTGC | 377 | 56 |
| 9 | GGACAGGACACGAATGAAGC | CATTAAGACGCCTCACATTCCC | 518 | 62 |
Note.— PCR was performed in 25-μl final volume, with 6 ng of genomic DNA, .64 mM dinucleotide triphosphate, .16 pmol of each primer, and .75 U Taq polymerase (Invitrogen).
Requires 1M betaine (Sigma-Aldrich).
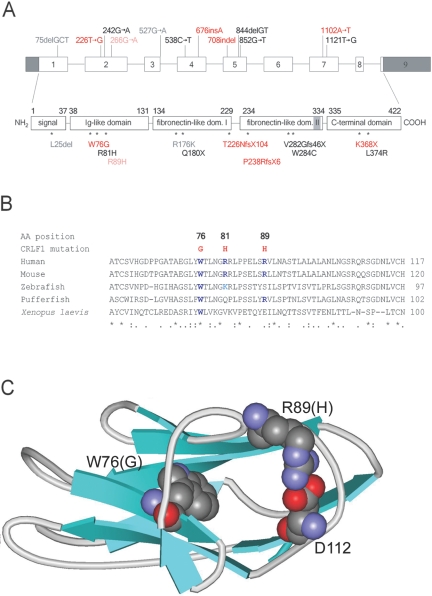
The CRLF1 protein (GenBank accession number NP_004741) has a domain structure that includes a signal sequence (positions 1–37) followed by an Ig-like C2-type N-terminal domain (positions 38–131), two consecutive fibronectin III–like domains (positions 134–229 and 234–334), and a C-terminal domain (positions 335–422). For the first three domains, homologous proteins have known structures: for the Ig-like domain, the highest sequence homology is found with a corresponding domain of the neural cell adhesion molecule (NCAM [Protein Data Bank {PDB} code 1EPF]); for the two fibronectin domains, high homology is seen with human prolactin receptor (PDB code 1BP3). The C-terminus shows no homology to known functional domains.24 The WSEWS motif at position 327 is probably needed for correct folding and domain orientation of the protein.25
In all Sardinian families except family 5, the mutation c.676-677insA was found. This mutation results in a threonine→asparagine change at position 226, followed by a frameshift, which leads to the deletion of a complete fibronectin domain as well as the C-terminal domain (p.T226NfsX104) (fig. 5A). Subjects CS07 and CS10 were both homozygous for this mutation, whereas subjects CS03 and CS14 were compound heterozygotes for p.T226NfsX104 and the missense mutation c.226T→G. This mutation results in a tryptophan→glycine substitution at position 76 of the Ig-like domain (p.W76G) (fig. 5A). An inspection of the corresponding positions within the structure of NCAM shows that tryptophan 76 is likely buried within the molecule (fig. 5C), and a substitution by glycine would be expected to result in a loss of tight internal side-chain arrangement and, thus, in a considerable decrease in stability. The W76 is strictly conserved within CRLF1 homologous proteins from different organisms, as seen from a sequence alignment for the homologous sequences from mouse, zebrafish, pufferfish, and Xenopus laevis (fig. 5B). The p.W76G missense mutation was present homozygously in subject CS17 and was associated with a severe phenotype as severe as observed with other truncating mutations found in Crisponi syndrome. Thus, two pathogenic CRLF1 mutations were identified in the Sardinian families, suggesting potential founder effects.
Figure 5. .
Positions of mutations in the CRLF1 gene and the CRLF1 protein. A, Intron/exon arrangement of the gene (dark shaded areas are the 3′ and 5′ UTRs) and domain (dom.) arrangement of the protein (light shaded area: WSXWS consensus sequence of the type I cytokine receptor family); red indicates mutations described in the present report; black indicates mutations described by Knappskog et al.20 and Hahn et al.,21 gray indicates known polymorphisms, and pale red indicates the nonpathogenic variant described in the present report. B, Multiple-sequence alignment for CRLF1 homologues and position of missense mutations within the Ig-like domain. Conserved residues at the mutated positions are indicated in blue. Mouse (Mus musculus): Crlf1 (Q9JM58); zebrafish (Brachydanio rerio): protein Zgc:91992 (Q6D628); pufferfish (Tetraodon nigriviridis): class I helical cytokine receptor number 1 (Q6UAQ5); X. laevis (African clawed frog): GP130p1 (O57519). C, Schematic representation of the CRLF1 Ig-like domain homologous structure from NCAM. Equivalent positions of the mutated residues (W76 and R89 in CRLF1, together with D112, most probably involved in a salt bridge with R89) are shown in space-filling representation. The figure was drawn with ViewerPro 4.2 (ACCELRYS).
In the father and the unaffected child of family 4, we detected the nucleotide change c.266G→A on one allele, which predicts the amino acid change p.R89H. This alteration was found in trans with the deleterious mutation c.226T→G/p.T226Nfs104X in the father. Because the father did not present any symptoms of Crisponi syndrome, we assumed that p.R89H was nonpathogenic. Arginine 89 may form a salt bridge with aspartate 112, as inferred from the NCAM structure (fig. 5C), but this salt bridge is exposed to the solvent and is also not completely conserved in the four CRLF homologous sequences (fig. 5B); it is thus probably less important for a stable fold. The nucleotide change c.266G→A was not reported elsewhere as a sequence variant, but it was also present in heterozygous form in two of 192 unrelated Sardinian controls.
In the Turkish families, we detected a homozygous indel mutation c.708-709delCCinsT in subjects CS37 (family 7) and CS41 and CS42 (family 8), which leads to a frameshift in the second fibronectin type III domain (p.P238RfsX6) (fig. 5A). The fathers of the affected probands from these two families originated from the same town in eastern Turkey, again suggesting a common founder effect in this instance. In family 6, the homozygous nonsense mutation c.1102A→T was detected in subject CS21, resulting in a prematurely terminated, truncated protein (p.K368X) (fig. 5A). Parental heterozygosity was proved for the respective mutations in all the families. All four identified mutations were screened in two panels of ethnically matched control individuals (192 Sardinian and 152 Turkish). We found one heterozygous carrier for p.W76G, one heterozygote for p.T226Nfs104X, and two heterozygotes for p.R89H. This carrier frequency is in line with the relatively high incidence of Crisponi syndrome in the Sardinian population.
Whereas the expression of the phenotype was quite similar in the Sardinian families presenting with identical CRLF1 mutations (mutations in family 1 identical to that in family 4 and mutation in family 2 identical to that in family 3), we observed variable expression of the phenotype in subject CS37 from family 7 and subjects CS41 and CS42 from family 8, all carrying the same nonsense mutation. Whereas subject CS37 of family 7 had severe dystonia, extreme feeding difficulties, gastroesophageal reflux, persistent hyperthermia, and recurrent attacks of periorbicular spasms before he died at age 30 mo, the clinical course of the affected siblings of family 8 (subjects CS41 and CS42) was relatively mild. Their initial symptoms gradually resolved during their 2nd year, and scoliosis and paradoxical sweating started only after several years.
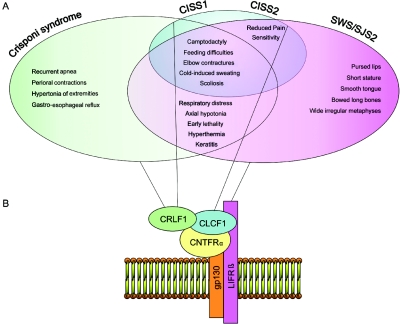
By demonstrating mutations in CRLF1 in patients with Crisponi syndrome, we were able to prove allelism to CISS1. Although present in the same gene, the different mutations associated with CISS1 have been reported to be associated with a phenotype less severe than that of Crisponi syndrome. In a more recent study, Dagoneau et al.9(in this issue) reported independently that CRLF1 mutations account for both Crisponi syndrome and CISS1. Dagoneau et al.9 suggested that nonsense mutations are associated with the severe neonatal manifestations of Crisponi syndrome and that missense mutations are associated with late-onset CISS1.9 In contrast to their observations, we found no obvious genotype/phenotype correlation for type or site of action of CRLF1 mutations in patients with Crisponi syndrome or CISS1. In particular, we found a homozygous missense mutation—namely, c.226T→G—in the proband of family 5, which was associated with the most severe phenotype of Crisponi syndrome. Distinctions are further complicated by the frequent presence of combinations of different mutations in some cases of each syndrome, with a considerable overlap of resulting symptoms between CISS1 and Crisponi syndrome (fig. 6), including feeding difficulties in infancy, cold-induced sweating, and scoliosis in adolescence.
Figure 6. .
Family of CNTF-receptor–pathway related disorders. A, Phenotypic overlap of Crisponi syndrome, CISS1, CISS2, and SWS/SJS2. B, CNTF-receptor complex, composed of gp130, LIFRβ, and CNTFRα, required for binding CRLF1/CLCF1, which activates downstream signal–transduction events, including phosphorylation of STAT3. Black lines link each protein to the corresponding related disorder.1–8,20–22 (See also the London Medical Databases Web site.)
CRLF1 protein is involved in the ciliary neurotrophic factor (CNTF)–receptor pathway, which is important for both development and maintenance of the nervous system.26 The stable secreted complex of CRLF1 and CLCF1 forms a ligand for CNTFRα, which, along with gp130 and LIFR, compose the CNTF-receptor complex.27 CRLF1/CLCF1 binding to CNTFRα leads to dimerization of gp130/LIFR, which in turn induces downstream signaling events, including activation of the Janus kinase 1/STAT3 pathway.28 In a mouse model, ablation of the CRLF1 gene suggestively leads to inability to suck, and the mice die of starvation shortly after birth, with their stomachs devoid of milk.29 CRLF1 and CLCF1 are cytokines expressed in sweat-gland tissue, and, currently, this complex is one of the most likely candidates for the target derived sweat-gland cholinergic differentiation factor.30 Therefore, it is likely that, in Crisponi syndrome as well as in CISS1 and CISS2, peripheral sudomotor functions are affected in a comparable way, to cause the observed paradoxical sweating. In addition to sweat glands, there are at least two different targets that are innervated by cholinergic sympathetic neurons: the vasculature in skeletal muscle and the periosteum.31 Asmus et al.32 demonstrated that immature osteoblasts release soluble factor(s) of the neuropoietic cytokine family that induce cholinergic differentiation in sympathetic neurons. Both the sweat-gland factor and the periosteal factor(s) signal through LIFRβ and are members of the neuropoietic cytokine family that is still incompletely defined. It would be critical to determine whether CRLF1 is the long-sought cholinergic differentiation factor that alters the transmitter properties of sympathetic innervation of sweat glands and the periosteum during development. Moreover, because the CNTF receptor has different ligands but a common downstream signaling pathway, the phenotypic overlap of CISS1/Crisponi syndrome, CISS2, and SWS/SJS can be rationalized (fig. 6). We have shown allelism between Crisponi syndrome and CISS1, although it remains open whether these are distinct clinical entities or a single disorder with variable degree of phenotypic expression. In the present study, no obvious genotype/phenotype correlation was observed for type or localization of CRLF1 mutations in patients with Crisponi syndrome or CISS1. Consequently, we suggest that other, still-unknown molecular defects or variants could be responsible for the variable phenotypic expression of the CRLF1 mutations.
In summary, we identified the molecular basis of Crisponi syndrome and found four different disease-causing mutations in CRLF1 in five Sardinian and three Turkish families. Furthermore, our findings demonstrate allelism of Crisponi syndrome and CISS1. Although differences in the severity of the two disorders exist, there is considerable phenotypic overlap between both syndromes and similarity to other syndromes related to the CNTF receptor (i.e., CISS2 and SWS/SJS2). We therefore propose a new syndrome family, called “CNTF receptor–related disorders,” of which Crisponi syndrome would be the newest member.
Acknowledgments
We are grateful to all the family members, who, with their enthusiastic, continuous and generous participation, made this study possible. This work was supported by the Associazione Sindrome di Crisponi e Malattie Rare ONLUS, by the Regione Autonoma della Sardegna, and by German Federal Ministry of Sciences and Education grant 01GR0416 (to P.N.) through the National Genome Research Network. We thank Elisabeth Kirst and Mario Lovicu, for expert technical assistance; Dr. David Schlessinger, for the critical reading of the manuscript, and Peter Gutdeutsch and Karl Ernst von Muehlendahl, for providing patient material.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CRLF1 [accession number NM_004750] and CRLF1 [accession number NP_004741])
- London Medical Databases, http://www.lmdatabases.com/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Crisponi syndrome, SWS/SJS2, CISS1, and CISS2)
- PDB, http://www.rcsb.org/pdb/ (for NCAM [code 1EPF] and human prolactin receptor [code 1BP3])
References
- 1.Crisponi G (1996) Autosomal recessive disorder with muscle contractions resembling neonatal tetanus, characteristic face, camptodactyly, hyperthermia, and sudden death: a new syndrome? Am J Med Genet 62:365–371 [DOI] [PubMed] [Google Scholar]
- 2.Accorsi P, Giordano L, Faravelli F (2003) Crisponi syndrome: report of a further patient. Am J Med Genet A 123:183–185 10.1002/ajmg.a.20292 [DOI] [PubMed] [Google Scholar]
- 3.Nannenberg EA, Bijlmer R, Van Geel BM, Hennekam RC (2005) Neonatal paroxysmal trismus and camptodactyly: the Crisponi syndrome. Am J Med Genet A 133:90–92 [DOI] [PubMed] [Google Scholar]
- 4.Rutsch F, Strasser F, Gutdeutsch P, Reese T, Debus O, Marquardt T, Kurlemann G, Jakobs C (2006) Low GABA concentrations in CSF in patients with Crisponi syndrome: a sign of poor prognosis? Eur J Hum Gen Suppl 14:119 [Google Scholar]
- 5.Cormier-Daire V, Superti-Furga A, Munnich A, Lyonnet S, Rustin P, Delezoide AL, De Lonlay P, Giedion A, Maroteaux P, Le Merrer M (1998) Clinical homogeneity of the Stuve-Wiedemann syndrome and overlap with the Schwartz-Jampel syndrome type 2. Am J Med Genet 78:146–149 [DOI] [PubMed] [Google Scholar]
- 6.Di Rocco M, Stella G, Bruno C, Doria Lamba L, Bado M, Superti-Furga A (2003) Long-term survival in Stuve-Wiedemann syndrome: a neuro-myo-skeletal disorder with manifestations of dysautonomia. Am J Med Genet A 118:362–368 10.1002/ajmg.a.10242 [DOI] [PubMed] [Google Scholar]
- 7.Al-Gazali LI, Ravenscroft A, Feng A, Shubbar A, Al-Saggaf A, Haas D (2003) Stuve-Wiedemann syndrome in children surviving infancy: clinical and radiological features. Clin Dysmorphol 12:1–8 10.1097/00019605-200301000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Dagoneau N, Scheffer D, Huber C, Al-Gazali LI, Di Rocco M, Godard A, Martinovic J, Raas-Rothschild A, Sigaudy S, Unger S, et al (2004) Null leukemia inhibitory factor receptor (LIFR) mutations in Stüve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am J Hum Genet 74:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagoneau N, Bellais S, Blanchet P, Sarda P, Al-Gazali LI, Di Rocco M, Huber C, Djouadi F, Le Goff C, Munnich A, et al (2007) Mutations in cytokine receptor-like factor 1 (CRLF1) account for both Crisponi and cold-induced sweating syndromes. Am J Hum Genet 80:966–977 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, et al (2006) Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79:949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifiova D, Mathijssen IMJ, Morton JEV, et al RAB23 mutations in Carpenter syndrome imply an unexpected role for Hedgehog signaling in cranial suture development and obesity. Am J Hum Genet (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2001) GRR: graphical representation of relationship errors. Bioinformatics 17:742–743 10.1093/bioinformatics/17.8.742 [DOI] [PubMed] [Google Scholar]
- 13.Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101 10.1038/ng786 [DOI] [PubMed] [Google Scholar]
- 14.Gudbjartsson DF, Jonasson K, Frigge ML, Kong A (2000) Allegro, a new computer program for multipoint linkage analysis. Nat Genet 25:12–13 10.1038/75514 [DOI] [PubMed] [Google Scholar]
- 15.Abecasis GR, Wigginton JE (2005) Handling marker-marker linkage disequilibrium: pedigree analysis with clustered markers. Am J Hum Genet 77:754–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- 17.Strauch K, Fimmers R, Kurz T, Deichmann KA, Wienker TF, Baur MP (2000) Parametric and nonparametric multipoint linkage analysis with imprinting and two-locus–trait models: application to mite sensitization. Am J Hum Genet 66:1945–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiele H, Nürnberg P (2005) HaploPainter: a tool for drawing pedigrees with complex haplotypes. Bioinformatics 21:1730–1732 10.1093/bioinformatics/bth488 [DOI] [PubMed] [Google Scholar]
- 19.Rüschendorf F, Nürnberg P (2005) ALOHOMORA: a tool for linkage analysis using 10K SNP array data. Bioinformatics 21:2123–2125 10.1093/bioinformatics/bti264 [DOI] [PubMed] [Google Scholar]
- 20.Knappskog PM, Majewski J, Livneh A, Nilsen PTE, Bringsli JS, Ott J, Boman H (2003) Cold-induced sweating syndrome is caused by mutations in the CRLF1 gene. Am J Hum Genet 72:375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn AF, Jones DL, Knappskog PM, Boman H, McLeod JG (2006) Cold-induced sweating syndrome: a report of two cases and demonstration of genetic heterogeneity. J Neurol Sci 250:62–70 10.1016/j.jns.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Sohar E, Shoenfeld Y, Udassin R, Magazanik A, Revach M (1978) Cold-induced profuse sweating on back and chest: a new genetic entity? Lancet 2:1073–1074 10.1016/S0140-6736(78)91805-6 [DOI] [PubMed] [Google Scholar]
- 23.Rousseau F, Gauchat JF, McLeod JG, Chevalier S, Guillet C, Guilhot F, Cognet I, Froger J, Hahn AF, Knappskog PM, et al (2006) Inactivation of cardiotrophin-like cytokine, a second ligand for ciliary neurotrophic factor receptor, leads to cold-induced sweating syndrome in a patient. Proc Natl Acad Sci USA 103:10068–10073 10.1073/pnas.0509598103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elson GCA, Graber P, Losberger C, Herren S, Gretener D, Menoud LN, Wells TNC, Kosco-Vilbois MH, Gauchat J-F (1998) Cytokine-like factor-1, a novel soluble protein, shares homology with members of the cytokine type I receptor family. J Immunol 161:1371–1379 [PubMed] [Google Scholar]
- 25.Bazan J F (1990) Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA 87:6934–6938 10.1073/pnas.87.18.6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N, et al (1995) Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 83:313–322 10.1016/0092-8674(95)90172-8 [DOI] [PubMed] [Google Scholar]
- 27.Elson GC, Lelievre E, Guillet C, Chevalier S, Plun-Favreau H, Froger J, Suard I, de Coignac AB, Delneste Y, Bonnefoy JY, et al (2000) CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci 3:867–872 10.1038/78765 [DOI] [PubMed] [Google Scholar]
- 28.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334:297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexander WS, Rakar S, Robb L, Farley A, Willson TA, Zhang JG, Hartley L, Kikuchi Y, Kojima T, Nomura H, et al (1999) Suckling defect in mice lacking the soluble haemopoietin receptor NR6. Curr Biol 9:605–608 10.1016/S0960-9822(99)80266-8 [DOI] [PubMed] [Google Scholar]
- 30.Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, et al (2006) Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development 133:141–150 10.1242/dev.02189 [DOI] [PubMed] [Google Scholar]
- 31.Francis NJ, Landis SC (1999) Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci 22:541–566 10.1146/annurev.neuro.22.1.541 [DOI] [PubMed] [Google Scholar]
- 32.Asmus SE, Tian H (2001) Induction of cholinergic function in cultured sympathetic neurons by periosteal cells: cellular mechanisms. Dev Biol 235:1–11 10.1006/dbio.2001.0282 [DOI] [PubMed] [Google Scholar]