Abstract
Folates are carriers of one-carbon units and are metabolized by 5,10-methylenetetrahydrofolate reductase (MTHFR) and other enzymes that use riboflavin, cobalamin, or vitamin B6 as cofactors. These B vitamins are essential for the remethylation and transsulfuration of homocysteine, which is an important intermediate in one-carbon metabolism. We studied the MTHFR 677C→T polymorphism and B vitamins as modulators of one-carbon metabolism in 10,601 adults from the Norwegian Colorectal Cancer Prevention (NORCCAP) cohort, using plasma total homocysteine (tHcy) as the main outcome measure. Mean concentrations of plasma tHcy were 10.4 μmol/liter, 10.9 μmol/liter, and 13.3 μmol/liter in subjects with the CC (51%), CT (41%), and TT (8%) genotypes, respectively. The MTHFR 677C→T polymorphism, folate, riboflavin, cobalamin, and vitamin B6 were independent predictors of tHcy in multivariate models (P<.001), and genotype effects were strongest when B vitamins were low (P⩽.006). Conversely, the MTHFR polymorphism influenced B vitamin effects, which were strongest in the TT group, in which the estimated tHcy difference between subjects with vitamin concentrations in the lowest compared with the highest quartile was 5.4 μmol/liter for folate, 4.1 μmol/liter for riboflavin, 3.2 μmol/liter for cobalamin, and 2.1 μmol/liter for vitamin B6. Furthermore, interactions between B vitamins were observed, and B vitamins were more strongly related to plasma tHcy when concentrations of other B vitamins were low. The study provides comprehensive data on the MTHFR–B vitamin network, which has major effects on the transfer of one-carbon units. Individuals with the TT genotype were particularly sensitive to the status of several B vitamins and might be candidates for personalized nutritional recommendations.
The flavoenzyme 5,10-methylenetetrahydrofolate reductase (MTHFR [MIM 236250]) regulates the flow of folates between the production of nucleotides and the supply of methyl groups for methionine synthesis1,2 and has major effects on the distribution of intracellular folates.3 The MTHFR substrate is 5,10-methylenetetrahydrofolate, which is formed from serine and tetrahydrofolate by vitamin B6–dependent serine hydroxymethyltransferase.2,4 The product of the MTHFR reaction is 5-methyltetrahydrofolate, which is the methyl donor in the conversion of homocysteine to methionine catalyzed by cobalamin-dependent methionine synthase.2,4 Methionine may be incorporated into proteins or may serve as the precursor of S-adenosylmethionine, a universal methyl group donor, which is converted to homocysteine after demethylation.2,5 Homocysteine is metabolized through two vitamin B–dependent pathways and may be either remethylated and recycled as methionine or removed from the remethylation cycle by undergoing irreversible B6-dependent transsulfuration to form cysteine.5 This makes homocysteine a key intermediate in one-carbon metabolism and explains why B vitamins involved in the transfer of one-carbon units are related to plasma concentrations of total homocysteine (tHcy).6
The 677C→T transition in the MTHFR gene (dbSNP accession number rs1801133) results in an Ala222Val substitution in the polypeptide chain,7 which is associated with a thermolabile8 enzyme. MTHFR in lymphocytes from subjects with the TT genotype has ∼30% of the catalytic activity of the wild type, whereas the CT genotype has 65% catalytic activity.7 Lower catalytic activity is associated with a redistribution of one-carbon substituted folates away from 5-methyltetrahydrofolate toward more oxidized forms, which may be used for DNA synthesis and repair.3,4 The MTHFR polymorphism is associated with clinical endpoints; an increased risk of cardiovascular disease9,10 and neural tube defects (MIM 601634)11,12 and a lower risk of colorectal cancer (MIM 114500)13–15 are found in subjects with the variant compared with the wild-type enzyme. The MTHFR 677C→T polymorphism is the single most important genetic determinant of plasma tHcy.16,17 Much of the interest in this polymorphism stems from its association with moderate hyperhomocysteinemia (MIM 603174), which is a risk factor for occlusive arterial disease,18 cognitive decline,19 and osteoporosis.20,21 It is still not clear whether these conditions are caused by homocysteine toxicity22 or if an elevated concentration of plasma tHcy is mainly an epiphenomenon.23–25
Most published work on B vitamins and homocysteine has focused on folate and cobalamin in smaller studies, which do not allow a comprehensive investigation of the various components of the metabolic network related to one-carbon metabolism. The present study included 10,601 middle-aged or elderly men and women from a population-based cohort, and our aim was to assess the MTHFR polymorphism and B vitamins as modulators of one-carbon metabolism, with use of plasma tHcy as the main outcome measure.
Subjects and Methods
Subjects and Study Design
The Norwegian Colorectal Cancer Prevention (NORCCAP) cohort was established to study the utility of sigmoidoscopy and occult blood testing as screening modalities for early detection of colorectal cancer in men and women aged 50–64 years.26 Study subjects were randomly selected from the population registries in the city of Oslo and the county of Telemark and were included from 1999 to 2001. The study was approved by the Regional Ethics Committee and the Data Inspectorate. The procedures followed were in accordance with the Helsinki Declaration, and written informed consent was obtained from all participants.
Biochemical Analyses
Whole blood collected into EDTA tubes was immediately put on ice and was centrifuged at 11,000 g for 10 min to obtain plasma. Blood samples collected into serum tubes without additives were allowed to clot at room temperature and were centrifuged within 1 h. Plasma and serum samples were stored at −80°C until analysis. tHcy was analyzed in plasma by gas chromatography–mass spectrometry,27 whereas riboflavin, vitamin B6 (pyridoxal-5′-phosphate), and creatinine were analyzed in plasma by liquid chromatography–tandem mass spectrometry.28 Folate29 and cobalamin30 were determined in serum by microbiological methods. MTHFR 677C→T genotyping was performed by real-time PCR with 5V exonuclease (Taqman) probes.31
Statistical Methods
Means and medians with percentiles were used for descriptive statistics. Correlation analyses were performed using partial Spearman correlation coefficients adjusted for sex, age, and study center. The χ2 test was used to compare proportions.
We used simple linear regression models and models with multiple adjustments to identify predictors of tHcy. Independent variables were represented in the model as indicator variables denoting membership in two or more categories for sex, age, the MTHFR 677C→T polymorphism, folate, riboflavin, cobalamin, vitamin B6, and creatinine. Thus, the regression coefficients estimated the difference in mean tHcy between the chosen reference category and the other categories for each factor. Concentrations of tHcy across categories of each factor were tested for linear trend.
We investigated the possible interaction between MTHFR 677C→T genotype and other tHcy predictors, by including product terms between genotype and each predictor in a multiple linear regression model with tHcy as the dependent variable, retaining the main effects of all variables in the model. Furthermore, the data were presented according to MTHFR genotype and B vitamin concentrations, and the regression analyses were performed separately for the CC, CT, and TT genotypes, at concentrations of folate, riboflavin, cobalamin, and vitamin B6 below and above the median. All statistical analyses were performed by SPSS version 11.0. Tests were two-tailed, and P values <.05 were considered statistically significant.
Results
Population Characteristics and Blood Indices
The study population (n=10,601, 49% male) was predominantly white (>98%) and had a mean age of 56 years (table 1). MTHFR 677C→T genotype frequencies were 50.3% (CC), 41.3% (CT), and 8.3% (TT) for men and 52.5% (CC), 39.8% (CT), and 7.7% (TT) for women. The genotype distribution was in Hardy-Weinberg equilibrium for each sex and for the whole study group (P⩾.69). Mean concentrations of metabolites and vitamins measured for all genotypes combined were 10.8 μmol/liter for plasma tHcy, 17.1 nmol/liter for serum folate, 18.1 nmol/liter for plasma riboflavin, 331 pmol/liter for serum cobalamin, 62.8 nmol/liter for plasma vitamin B6, and 70 μmol/liter for plasma creatinine (table 1). Plasma tHcy was higher in subjects with the T allele as compared with the wild type. In subjects with plasma tHcy ⩾20 μmol/liter (n=209), the prevalence of the TT genotype was 48% (data not shown). Serum folate decreased according to the number of T alleles. Concentrations of riboflavin, cobalamin, and vitamin B6 were independent of genotype (table 1).
Table 1. .
Characteristics of the Study Population
| All |
MTHFR 677C→T Genotype(Median [1st–99th Percentile]) |
|||||
| Characteristic | Mean | Median (1st–99th Percentile) | CC (n=5,452) |
CT (n=4,299) |
TT (n=850) |
P for trend |
| Age, years | 56 | 55 (50–64) | 55 (50–64) | 55 (50–64) | 55 (50–64) | .11 |
| Plasma tHcy, μmol/liter | 10.8 | 10.2 (5.8–24.2) | 9.9 (5.7–19.2) | 10.4 (5.9–22.8) | 11.2 (5.7–43.3) | <.001 |
| Serum folate, nmol/liter | 17.1 | 13.7 (4.7–59.8) | 14.5 (5.2–60.8) | 13.4 (4.8–59.9) | 10.5 (3.5–50.1) | <.001 |
| Plasma riboflavin, nmol/liter | 18.1 | 10.4 (3.0–135) | 10.4 (3.0–150) | 10.4 (2.8–135) | 10.7 (2.8–119) | .06 |
| Serum cobalamin, pmol/liter | 331 | 307 (128–732) | 308 (127–734) | 308 (130–730) | 300 (113–766) | .19 |
| Plasma vitamin B6, nmol/liter | 62.8 | 48.0 (12.7–295) | 47.9 (13.3–280) | 49.0 (12.6–336) | 44.4 (11.9–286) | .34 |
| Plasma creatinine, μmol/liter | 70 | 69 (44–105) | 69 (44–106) | 70 (45–104) | 66 (45–101) | .01 |
Correlations
Simple relationships between variables were calculated as nonparametric Spearman correlation coefficients, which were adjusted for sex, age, and study center. Concentrations of several B vitamins were positively related (table 2). The riboflavin-folate and the vitamin B6–folate relationships were modified by MTHFR genotype. Plasma tHcy was inversely related to all B vitamins, and the tHcy–B vitamin relationships were modified by the MTHFR polymorphism and were strongest in subjects with the TT genotype, particularly for folate and riboflavin (table 2). A positive relationship (r=0.21) was observed between tHcy and creatinine.
Table 2. .
Spearman Correlation Coefficients[Note]
| Variable and Genotype |
tHcy | Folate | Riboflavin | Cobalamin |
| Folate: | −.44 | |||
| CC | −.38 | |||
| CT | −.45 | |||
| TT | −.58 | |||
| Riboflavin: | −.18 | .26 | ||
| CC | −.15 | .23 | ||
| CT | −.19 | .28 | ||
| TT | −.38 | .35 | ||
| Cobalamin: | −.24 | .16 | .20 | |
| CC | −.23 | .14 | .19 | |
| CT | −.25 | .19 | .22 | |
| TT | −.28 | .16 | .17 | |
| Vitamin B6: | −.24 | .39 | .35 | .18 |
| CC | −.20 | .35 | .34 | .16 |
| CT | −.24 | .41 | .36 | .20 |
| TT | −.38 | .47 | .43 | .17 |
Note.— Nonparametric Spearman correlation coefficients adjusted for sex, age, and study center are shown for the entire population (n=10,570) and separately for the MTHFR 677CC (n=5,433), 677CT (n=4,282), and 677TT (n=843) genotypes. All correlations were highly significant (P<.001).
The MTHFR 677C→T Polymorphism and B Vitamins as tHcy Predictors
The MTHFR polymorphism, folate, riboflavin, cobalamin, vitamin B6, and creatinine were independently related to plasma tHcy in regression models adjusted for sex, age, and study center (P<.001; data not shown) and in models with multiple adjustments (P<.001) (table 3).
Table 3. .
Determinants of Plasma tHcy according to MTHFR 677C→T Genotype[Note]
|
MTHFR Genotype |
|||||||||
| All |
CC(n=5,452) |
CT(n=4,299) |
TT(n=850) |
||||||
| Variable | Estimated tHcy Differencea |
P for trend | Estimated tHcy Differencea |
P for trend | Estimated tHcy Differencea |
P for trend | Estimated tHcy Differencea |
P for trend | Interaction Term (P)b |
| Sex (vs. female; n=5,386): | |||||||||
| Male (n=5,213) | .4 (.2–.5) | <.001 | .3 (.1–.5) | <.001 | .4 (.2–.6) | <.001 | 1.0 (−.1–2.0) | .13 | .04 |
| Age, years (vs. 50–53; n=2,540): | |||||||||
| 54–55 (n=3,052) | .5 (.3–.7) | .5 (.4–.7) | .4 (.1–.6) | 1.0 (−.2–2.2) | |||||
| 56–59 (n=2,637) | .7 (.5–.9) | <.001 | .7 (.5–.9) | <.001 | .7 (.4–1.0) | <.001 | .5 (−.7–1.7) | .16 | .34 |
| 60–64 (n=2,370) | 1.2 (1.0–1.3) | 1.2 (1.0–1.4) | 1.0 (.8–1.3) | 1.5 (.2–2.7) | |||||
| Serum folate, nmol/liter (vs. 20.2–151; n=2,647): | |||||||||
| 13.7–20.2 (n=2,647) | .8 (.6–1.0) | .8 (.6–1.0) | .9 (.6–1.1) | 1.3 (−.2–2.9) | |||||
| 10.1–13.7 (n=2,646) | 1.2 (1.0–1.4) | <.001 | 1.3 (1.1–1.5) | < .001 | 1.3 (1.0–1.6) | <.001 | 1.1 (−.4–2.7) | <.001 | <.001 |
| 1.5–10.1 (n=2,651) | 3.0 (2.8–3.2) | 2.3 (2.1–2.5) | 3.0 (2.7–3.3) | 5.4 (4.0–6.8) | |||||
| Plasma riboflavin, nmol/liter (vs. 18.1–596; n=2,644): | |||||||||
| 10.4–18.1 (n=2,656) | .1 (−.1–.3) | .1 (−.1–.3) | .0 (−.3–.3) | −.1 (−1.4–1.1) | |||||
| 6.8–10.4 (n=2,649) | .2 (.0–.4) | <.001 | .1 (−.1–.3) | < .001 | .2 (−.1–.5) | <.001 | 1.0 (−.2–2.3) | <.001 | <.001 |
| .9–6.8 (n=2,652) | .8 (.6–1.0) | .4 (.2–.6) | .7 (.4–.9) | 4.1 (2.7–5.5) | |||||
| Serum cobalamin, pmol/liter (vs. 380–6,500; n=2,644): | |||||||||
| 307–380 (n=2,650) | .3 (.1–.5) | .2 (.0–.4) | .5 (.2–.7) | .6 (−.7–1.8) | |||||
| 245–307 (n=2,646) | .5 (.3–.7) | <.001 | .4 (.2–.5) | <.001 | .6 (.3–.8) | <.001 | 1.4 (.1–2.6) | <.001 | <.001 |
| 34–245 (n=2,645) | 1.5 (1.3–1.6) | 1.2 (1.0–1.4) | 1.5 (1.3–1.8) | 3.2 (2.0–4.5) | |||||
| Plasma vitamin B6, nmol/liter (vs. 73.2–1,093; n=2,651): | |||||||||
| 48.0–73.1 (n=2,655) | .1 (−.1–.3) | .2 (.0–.4) | .1 (−.2–.4) | −.5 (−1.9–.8) | |||||
| 32.6–47.9 (n=2,642) | .1 (−.1–.3) | <.001 | .2 (.0–.4) | <.001 | .0 (−.3–.3) | <.01 | −.4 (−1.8–1.0) | .01 | .006 |
| 4.3–32.6 (n=2,653) | .7 (.5–.9) | .5 (.3–.8) | .5 (.2–.8) | 2.1 (.6–3.7) | |||||
| Serum creatinine, μmol/liter (vs. 30–61; n=2,650): | |||||||||
| 61–69 (n=2,646) | .3 (.1–.5) | .5 (.3–.7) | .1 (−.1–.4) | .4 (−.8–1.5) | |||||
| 69–78 (n=2,659) | .8 (.7–1.0) | <.001 | 1.0 (.8–1.2) | <.001 | .5 (.2–.8) | <.001 | 1.5 (.2–2.9) | .003 | .29 |
| 78–571 (n=2,645) | 1.7 (1.5–1.9) | 1.8 (1.6–2.0) | 1.5 (1.2–1.8) | 2.5 (1.0–4.0) | |||||
| MTHFR 667C→T genotype (vs. CC; n=5,254): | |||||||||
| CT (n=4,299) | .4 (.3–.5) | <.001 | |||||||
| TT (n=850) | 2.4 (2.2–2.6) | ||||||||
Note.— Data were analyzed by multiple regression with tHcy as the dependent variable. All variables in the table and study center were included in the model.
Values are given as means (95% CIs), in μmol/liter.
P for the product term between MTHFR genotype and the various tHcy predictors.
The estimated difference in mean plasma tHcy between subjects with the TT genotype compared with the CC genotype was 2.4 μmol/liter (table 3). Folate was a strong tHcy predictor, and tHcy was 3.0 μmol/liter higher in subjects in the lowest compared with the highest quartile of folate concentrations (table 3). MTHFR genotype strongly modified folate effects, and, in TT subjects, plasma tHcy was 5.4 μmol/liter higher in the lowest compared with the highest folate quartile.
Riboflavin was only a weak tHcy predictor in subjects with the CC and CT genotypes, but, in the TT group, riboflavin was the second strongest tHcy predictor, and the difference between extreme riboflavin quartiles was 4.1 μmol/liter of plasma tHcy (table 3).
The cobalamin-tHcy and vitamin B6–tHcy relationships were similarly but less strongly related to genotype. The tHcy difference between extreme vitamin quartiles in subjects with the TT genotype was 3.2 μmol/liter for cobalamin and 2.1 μmol/liter for vitamin B6 (table 3). Sex, but not age or creatinine, interacted with genotype.
Modification of the MTHFR Genotype–tHcy Relationship by B Vitamins
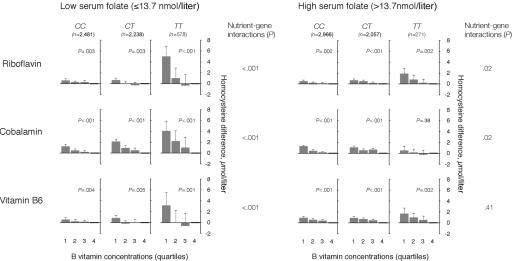
Predictors of plasma tHcy were studied in subjects with the CC, CT, and TT genotypes at folate concentrations below and above the median, with the use of regression models with multiple adjustments (fig. 1). There was an inverse relationship between riboflavin and plasma tHcy, which was modified by MTHFR genotype, both at low and high folate concentrations (fig. 1). The relationship was strongest at low folate levels, however, and, for subjects in the TT group, the estimated tHcy difference between extreme riboflavin quartiles was 5.0 μmol/liter, whereas the corresponding difference was 1.8 μmol/liter at high folate levels. At low folate levels, cobalamin was also strongly and inversely related to tHcy (fig. 1). At high folate concentrations, this relationship was dramatically weakened, particularly in the TT group. Vitamin B6 was related to tHcy both at low and high folate concentrations, but at high folate levels no genotype effect was observed (fig. 1).
Figure 1. .
B vitamins as determinants of plasma tHcy according to folate concentrations and MTHFR 677C→T genotype. The population was stratified according to levels of serum folate (below and above the median) and MTHFR 677C→T genotype. The riboflavin-tHcy, cobalamin-tHcy, and vitamin B6–tHcy relationships were then studied in a regression model, which included these vitamins in addition to sex, age, creatinine, and study center. Means with upper limits of 95% CIs and P for trend across quartiles are shown in each panel. Nutrient-gene interaction terms were calculated as the product between MTHFR genotype and the various B vitamins.
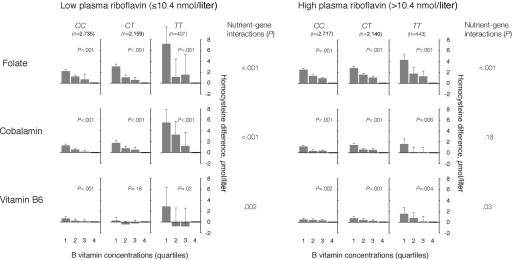
MTHFR genotype and B vitamin effects were similarly studied at concentrations of plasma riboflavin below and above the median (fig. 2). Folate was robustly related to plasma tHcy, both at low and high riboflavin concentrations, but high riboflavin levels weakened the folate-tHcy relationship in subjects with the TT genotype. Cobalamin was related to plasma tHcy at low and high riboflavin levels, but the relationship was much weaker and was not significantly modified by genotype when riboflavin levels were high (fig. 2). Vitamin B6 was moderately related to plasma tHcy at low and high riboflavin levels, particularly in subjects with the variant enzyme, but the genotype dependency of the vitamin B6–tHcy relationship was weaker when riboflavin levels were high.
Figure 2. .
B vitamins as determinants of plasma tHcy according to riboflavin concentrations and MTHFR 677C→T genotype. The population was stratified according to levels of plasma riboflavin (below and above the median) and MTHFR 677C→T genotype. The folate-tHcy, cobalamin-tHcy, and vitamin B6–tHcy relationships were then studied in a regression model, which included these vitamins in addition to sex, age, creatinine, and study center. Means with upper limits of 95% CIs and P for trend across quartiles are shown in each panel. Nutrient-gene interaction terms were calculated as the product between MTHFR genotype and the various B vitamins.
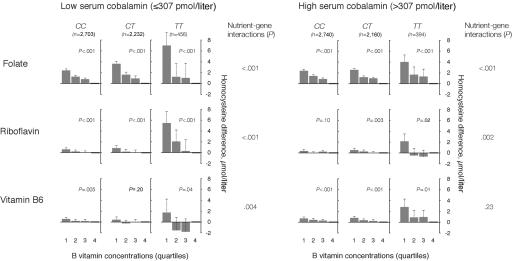
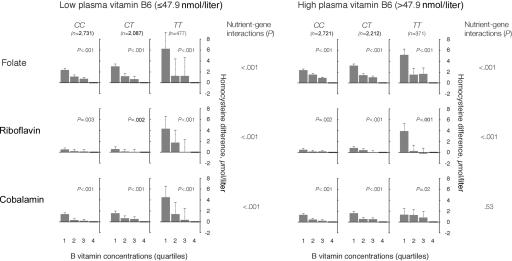
Concentrations of cobalamin below and above the median had a similar but less pronounced effect on the folate-tHcy, riboflavin-tHcy, and vitamin B6–tHcy relationships in subjects with different MTHFR genotypes (fig. 3). High levels of cobalamin weakened the genotype dependency of the B vitamin–tHcy associations. Vitamin B6 had a similar impact on the effects of other B vitamins and the MTHFR genotype (fig. 4). Folate, riboflavin, and cobalamin were determinants of plasma tHcy both at low and high levels of vitamin B6, but high levels of vitamin B6 attenuated the relationships between the other B vitamins and tHcy, and the cobalamin-tHcy relationship showed no genotype dependency at high vitamin B6 levels.
Figure 3. .
B vitamins as determinants of plasma tHcy according to cobalamin concentrations and MTHFR 677C→T genotype. The population was stratified according to levels of serum cobalamin (below and above the median) and MTHFR 677C→T genotype. The folate-tHcy, riboflavin-tHcy, and vitamin B6–tHcy relationships were then studied in a regression model, which included these vitamins in addition to sex, creatinine, and study center. Means with upper limits of 95% CIs and P for trend across quartiles are shown in each panel. Nutrient-gene interaction terms were calculated as the product between MTHFR genotype and the various B vitamins.
Figure 4. .
B vitamins as determinants of plasma tHcy according to vitamin B6 concentrations and MTHFR 677C→T genotype. The population was stratified according to levels of plasma vitamin B6 (below and above the median) and MTHFR 677C→T genotype. The folate-tHcy, riboflavin-tHcy, and cobalamin-tHcy relationships were then studied in a regression model, which included these vitamins in addition to sex, age, creatinine, and study center. Means with upper limits of 95% CIs and P for trend across quartiles are shown in each panel. Nutrient-gene interaction terms were calculated as the product between MTHFR genotype and the various B vitamins.
Discussion
We studied the MTHFR 677C→T polymorphism and several B vitamins that are involved in one-carbon metabolism in a large population-based cohort (n=10,601), using plasma tHcy as the main outcome measure. The MTHFR polymorphism, folate, riboflavin, cobalamin, and vitamin B6 were independently related to plasma tHcy. The MTHFR polymorphism had the strongest impact when B vitamin levels were low. Conversely, MTHFR genotype modified B vitamin–tHcy relationships, which were strongest in subjects with the T allele, particularly for folate and riboflavin. Finally, interactions between B vitamins were observed, and B vitamin–tHcy relationships were stronger when concentrations of other B vitamins were low.
Study Design
The large study population allowed us to investigate subgroups and to obtain precise estimates of MTHFR 677C→T polymorphism and B vitamin effects. The homogeneity of the study population with respect to age and ethnicity reduced the potential for confounding from these variables. B vitamin status was determined in blood. The relationship between the estimated intake of folate and vitamin B6 and blood vitamin concentrations is relatively strong, with an adjusted correlation coefficient in the range 0.5–0.6.32,33 Circulating vitamin B12 shows a somewhat weaker relationship to intake data, which may reflect impaired absorption in the elderly.32
The distribution of MTHFR genotypes may be assumed to be unrelated to diet and lifestyle factors. Therefore, the investigation of phenotypic effects of the MTHFR polymorphism provides a means for reducing the effect of confounders that may distort interpretations of conventional observational studies.34,35 A similar approach was used for the investigation of B vitamin effects. Although B vitamin status may be related to lifestyle,36 the problem of confounding may be reduced by assessing B vitamin effects across subgroups defined by genotype.
MTHFR 677C→T Genotype and Blood Indices
The T allele frequency was ∼0.3, which accords well with previous reports from population-based studies in northern Europe.37 Concentrations of plasma tHcy and B vitamins were comparable to those reported in other studies.28,38,39 The concentration of plasma tHcy increased, and folate decreased, according to the number of T alleles, as has been shown by others.40
B Vitamin Interrelationships
Spearman correlation studies showed that B vitamins were positively related to each other, as has been shown elsewhere.38 Such a positive relationship may be caused by common dietary sources for B vitamins41 or may be explained by the fact that the metabolism of several B vitamins depends on other B vitamins.1,42,43 A novel finding is that the riboflavin-folate relationship was MTHFR genotype–dependent. The variant enzyme associated with the 677C→T transition of the MTHFR gene has lower affinity for its flavin cofactor than the wild-type enzyme.44,45 Thus, the variant enzyme might depend on higher concentrations of riboflavin for sufficient catalytic activity. An inadequate riboflavin status might therefore reduce the formation of 5-methyltetrahydrofolate, the prevailing folate species in serum.11
MTHFR Genotype and B Vitamins as Modulators of One-Carbon Metabolism
The MTHFR 677C→T polymorphism was an independent predictor of plasma tHcy. This association was modified by several B vitamins, particularly folate and riboflavin, and the impact of the T allele was strongest when B vitamins were low. The folate-MTHFR40 and riboflavin-MTHFR38 relationships provide a paradigm of nutrient-gene interactions,46 which may be explained by the role of riboflavin as cofactor for MTHFR,1,45 whereas folate serves as a substrate for MTHFR and stabilizes the enzyme.45
Other B vitamins displayed similar but somewhat weaker interactions with the MTHFR polymorphism than folate and riboflavin. A genotype dependency of the cobalamin-tHcy relationship has been reported in one study.47 Cobalamin serves as the cofactor for methionine synthase, which uses the product of the MTHFR reaction, 5-methyltetrahydrofolate, as a cosubstrate. Vitamin B6 is the cofactor for serine hydroxymethyltransferase, which forms 5,10-methylenetetrahydrofolate, the MTHFR substrate,2,4,5 and is also involved in homocysteine transsulfuration.5 Thus, cobalamin and vitamin B6 might be expected to have an impact on the metabolic network of which MTHFR is a part.
B vitamins not only modify the phenotypic expression of the MTHFR 677C→T polymorphism but are tHcy predictors in their own right. This has consistently been shown in several studies for folate39,48 and cobalamin,39,49 whereas the highly MTHFR genotype–dependent riboflavin-tHcy relationship was demonstrated more recently.38,50
Several studies show that vitamin B6 is a strong determinant of plasma tHcy in human subjects after methionine loading.5,51 However, data on the vitamin B6–tHcy relationship in subjects who have not undergone methionine loading are less consistent; some,52 but not all,53,54 studies report that vitamin B6 is an independent determinant of plasma tHcy. Our study shows that the vitamin B6–tHcy relationship is largely driven by the TT group, which comprises only 10% of most populations of Asian or European descent,37 and this may explain why a relationship between vitamin B6 and plasma tHcy has not been observed in smaller studies. In addition, interactions between vitamin B6 and other B vitamins might explain apparent inconsistencies between studies on vitamin B6 and tHcy, because B vitamin status may vary between study populations.
Implications
At present, plasma tHcy is an established marker of folate and cobalamin status and may be used for the diagnosis and follow-up of deficiency states.39,48,49 Our data indicate that elevated tHcy may also reflect impaired riboflavin or vitamin B6 status, particularly in individuals with the TT genotype. Because a large fraction of hyperhomocysteinemic subjects have the TT genotype,55 deficiencies of these vitamins may be an important cause of moderate hyperhomocysteinemia.
Our results indicate the existence of a functional metabolic network including the MTHFR enzyme and several B vitamins, which suggests that physiological and balanced regimes may be needed for the treatment of B vitamin deficiencies and for B vitamin intervention trials. In pernicious anemia, folate treatment may effectively reduce plasma tHcy even though underlying metabolic derangements may progress if cobalamin is not also given.56 B vitamin intervention trials designed to assess the impact of plasma tHcy–lowering treatment on cardiovascular disease risk have generally been negative.25,57 Most studies have included only folic acid and vitamin B6, however, and more balanced regimens including several B vitamins at physiological doses may be needed to optimize such trials.58
We found that the MTHFR 677C→T polymorphism and B vitamins were interactive modulators of the metabolism of homocysteine, which is an important intermediate in the transfer of one-carbon units. Diseases that might be caused by derangements of one-carbon metabolism might similarly depend on MTHFR genotype and B vitamins. Several studies support this idea, and low blood concentrations of folate strengthen the association between the MTHFR polymorphism and the risk of cardiovascular disease.10 The MTHFR polymorphism is apparently a weaker predictor of coronary heart disease in American than in European populations,10,59 and this might be explained by mandatory B vitamin fortification of food items in the United States,60 which predictably increases the dietary intake of riboflavin and folate.
Blood concentrations of folate also modulate the impact of the MTHFR polymorphism, with respect to the risk of colorectal cancer.13,15 The relationship between MTHFR genotype and colorectal cancer may also be modified by cobalamin15 and vitamin B6.15,61 Several studies report an interaction between the MTHFR polymorphism and folate as risk factors of colorectal adenoma.62,63 Moreover, interactions between MTHFR genotype and B vitamins have been observed in studies on intermediary endpoints that may be related to the development of cancer. Some studies show that the MTHFR 677C→T polymorphism and folate status are interactive determinants of DNA methylation in human leukocytes64–66 and of the incorporation of one-carbon units into monocyte DNA.67
Conclusions
The present study provides comprehensive metabolic data from a population-based cohort and has sufficient statistical power to give a detailed record of the relationship between MTHFR and several B vitamins, which form a network with major effects on the transfer of one-carbon units. B vitamins strongly modified genotype effects and vice versa. Individuals with the TT genotype were particularly sensitive to the status of several B vitamins and might be candidates for personalized nutritional recommendations. Our results corroborate and extend observations from in vitro studies and shed light on published data on the MTHFR polymorphism and B vitamins as determinants of plasma tHcy and chronic diseases. Awareness of the cooperativity between B vitamins involved in one-carbon metabolism should provide guidance on the design of B vitamin regimens to be used in future intervention trials.
Acknowledgments
This study received financial support from the Norwegian Cancer Society, the Norwegian Department of Health and Social Affairs, and the Foundation to Promote Research into Functional Vitamin B12 Deficiency.
Web Resources
The URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/ (for MTHFR 677C→T)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MTHFR, neural tube defects, colorectal cancer, and hyperhomocysteinemia)
References
- 1.Kutzbach C, Stokstad EL (1971) Mammalian methylenetetrahydrofolate reductase: partial purification, properties, and inhibition by S-adenosylmethionine. Biochim Biophys Acta 250:459–477 [DOI] [PubMed] [Google Scholar]
- 2.Selhub J (1999) Homocysteine metabolism. Annu Rev Nutr 19:217–246 10.1146/annurev.nutr.19.1.217 [DOI] [PubMed] [Google Scholar]
- 3.Bagley PJ, Selhub J (1998) A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA 95:13217–13220 10.1073/pnas.95.22.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucock M (2000) Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab 71:121–138 10.1006/mgme.2000.3027 [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JD (2001) Regulation of homocysteine metabolism. In: Carmel R, Jacobsen DW (eds) Homocysteine in health and disease. Cambridge University Press, Cambridge, United Kingdom, pp 92–99 [Google Scholar]
- 6.Vollset SE, Refsum H, Ueland PM (2001) Population determinants of homocysteine. Am J Clin Nutr 73:499–500 [DOI] [PubMed] [Google Scholar]
- 7.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GHJ, den Heijer M, Kluijtmans LAJ, van den Heuvel LP, et al (1995) A candidate genetic risk factor for vascular disease: a common mutation at the methylenetetrahydrofolate reductase. Nat Genet 10:111–113 10.1038/ng0595-111 [DOI] [PubMed] [Google Scholar]
- 8.Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G (1988) Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet 43:414–421 [PMC free article] [PubMed] [Google Scholar]
- 9.Kluijtmans LA, van den Heuvel LP, Boers GH, Frosst P, Stevens EM, van Oost BA, den Heijer M, Trijbels FJ, Rozen R, Blom HJ (1996) Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet 58:35–41 [PMC free article] [PubMed] [Google Scholar]
- 10.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG (2002) MTHFR 677C→T polymorphism and risk of coronary heart disease—a meta-analysis. JAMA 288:2023–2031 10.1001/jama.288.16.2023 [DOI] [PubMed] [Google Scholar]
- 11.van der Put NM, Steegers-Theunissen RP, Frosst P, Trijbels FJ, Eskes TK, van den Heuvel LP, Mariman EC, den Heyer M, Rozen R, Blom HJ (1995) Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 346:1070–1071 10.1016/S0140-6736(95)91743-8 [DOI] [PubMed] [Google Scholar]
- 12.Shields DC, Kirke PN, Mills JL, Ramsbottom D, Molloy AM, Burke H, Weir DG, Scott JM, Whitehead AS (1999) The “thermolabile” variant of methylenetetrahydrofolate reductase and neural tube defects: an evaluation of genetic risk and the relative importance of the genotypes of the embryo and the mother. Am J Hum Genet 64:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Stampfer MJ, Giovannucci E, Artigas C, Hunter DJ, Fuchs C, Willett WC, Selhub J, Hennekens CH, Rozen R (1997) Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res 57:1098–1102 [PubMed] [Google Scholar]
- 14.Ulvik A, Vollset SE, Hansen S, Gislefoss R, Jellum E, Ueland PM (2004) Colorectal cancer and the methylenetetrahydrofolate reductase 677C→T and methionine synthase 2756A→G polymorphisms: a study of 2,168 case-control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev 13:2175–2180 [PubMed] [Google Scholar]
- 15.Kono S, Chen K (2005) Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci 96:535–542 10.1111/j.1349-7006.2005.00090.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozen R (2001) Polymorphisms of folate and cobalamin metabolism. In: Carmel R, Jacobsen DW (eds) Homocysteine in health and disease. Cambridge University Press, Cambridge, United Kingdom, pp 259–269 [Google Scholar]
- 17.Bathum L, Pedersen I, Christiansen L, Konieczna A, Sørensen TIA, Kyvik KO. Genetic and environmental influences on plasma homocysteine: results from a Danish twin study. Clin Chem (in press) [DOI] [PubMed] [Google Scholar]
- 18.Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE (1997) Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 337:230–236 10.1056/NEJM199707243370403 [DOI] [PubMed] [Google Scholar]
- 19.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ (2002) Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr 75:908–913 [DOI] [PubMed] [Google Scholar]
- 20.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP (2004) Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350:2042–2049 10.1056/NEJMoa032739 [DOI] [PubMed] [Google Scholar]
- 21.Herrmann M, Widmann T, Colaianni G, Colucci S, Zallone A, Herrmann W (2005) Increased osteoclast activity in the presence of increased homocysteine concentrations. Clin Chem 51:2348–2353 10.1373/clinchem.2005.053363 [DOI] [PubMed] [Google Scholar]
- 22.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ Jr, Kohl B, Rao V, Kisiel W, et al (2001) Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest 107:675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald DS, Law M, Morris JK (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325:1202–1208 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Splaver A, Lamas GA, Hennekens CH (2004) Homocysteine and cardiovascular disease: biological mechanisms, observational epidemiology, and the need for randomized trials. Amer Heart J 148:34–40 10.1016/j.ahj.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 25.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K (2006) Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 354:1578–1588 10.1056/NEJMoa055227 [DOI] [PubMed] [Google Scholar]
- 26.Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G (2002) NORCCAP (Norwegian Colorectal Cancer Prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut 50:604–607 10.1136/gut.50.5.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Windelberg A, Årseth O, Kvalheim G, Ueland PM (2005) Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin Chem 51:2103–2109 10.1373/clinchem.2005.053835 [DOI] [PubMed] [Google Scholar]
- 28.Midttun Ø, Hustad S, Solheim E, Schneede J, Ueland PM (2005) Multianalyte quantification of vitamin B6 and B2 species in the nanomolar range in human plasma by liquid chromatography-tandem mass spectrometry. Clin Chem 51:1206–1216 10.1373/clinchem.2005.051169 [DOI] [PubMed] [Google Scholar]
- 29.Molloy AM, Scott JM (1997) Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol 281:43–53 [DOI] [PubMed] [Google Scholar]
- 30.Kelleher BP, Broin SD (1991) Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol 44:592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulvik A, Ueland PM (2001) Single nucleotide polymorphism (SNP) genotyping in unprocessed whole blood and serum by real-time PCR: application to SNPs affecting homocysteine and folate metabolism. Clin Chem 47:2050–2053 [PubMed] [Google Scholar]
- 32.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH (1993) Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 270:2693–2698 10.1001/jama.270.22.2693 [DOI] [PubMed] [Google Scholar]
- 33.Zhang SM, Willett WC, Selhub J, Hunter DJ, Giovannucci EL, Holmes MD, Colditz GA, Hankinson SE (2003) Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst 95:373–380 [DOI] [PubMed] [Google Scholar]
- 34.Smith GD, Ebrahim S (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32:1–22 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 35.Hustad S, Nedrebø BG, Ueland PM, Schneede J, Vollset SE, Ulvik A, Lien EA (2004) Phenotypic expression of the methylenetetrahydrofolate reductase 677C→T polymorphism and flavin cofactor availability in thyroid dysfunction. Am J Clin Nutr 80:1050–1057 [DOI] [PubMed] [Google Scholar]
- 36.Halsted CH (2001) Lifestyle effects on homocysteine and an alcohol paradox. Am J Clin Nutr 73:501–502 [DOI] [PubMed] [Google Scholar]
- 37.Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE (2001) Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 22:195–201 10.1016/S0165-6147(00)01675-8 [DOI] [PubMed] [Google Scholar]
- 38.Hustad S, Ueland PM, Vollset SE, Zhang Y, Bjørke-Monsen AL, Schneede J (2000) Riboflavin as a determinant of plasma total homocysteine: effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clin Chem 46:1065–1071 [PubMed] [Google Scholar]
- 39.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J (2001) Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr 73:613–621 [DOI] [PubMed] [Google Scholar]
- 40.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R (1996) Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 93:7–9 [DOI] [PubMed] [Google Scholar]
- 41.Shimakawa T, Nieto FJ, Malinow MR, Chambless LE, Schreiner PJ, Szklo M (1997) Vitamin intake: a possible determinant of plasma homocyst(e)ine among middle-aged adults. Ann Epidemiol 7:285–293 10.1016/S1047-2797(97)00004-5 [DOI] [PubMed] [Google Scholar]
- 42.McCormick DB (1989) Two interconnected B vitamins: riboflavin and pyridoxine. Physiol Rev 69:1170–1198 [DOI] [PubMed] [Google Scholar]
- 43.Leclerc D, Odièvre MH, Wu Q, Wilson A, Huizenga JJ, Rozen R, Scherer SW, Gravel RA (1999) Molecular cloning, expression and physical mapping of the human methionine synthase reductase gene. Gene 240:75–88 10.1016/S0378-1119(99)00431-X [DOI] [PubMed] [Google Scholar]
- 44.Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, Ludwig ML (1999) The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nature Struct Biol 6:359–365 10.1038/7594 [DOI] [PubMed] [Google Scholar]
- 45.Yamada K, Chen ZT, Rozen R, Matthews RG (2001) Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Nat Acad Sci USA 98:14853–14858 10.1073/pnas.261469998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ames BN, Elson-Schwab I, Silver EA (2002) High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am J Clin Nutr 75:616–658 [DOI] [PubMed] [Google Scholar]
- 47.D’Angelo A, Coppola A, Madonna P, Fermo I, Pagano A, Mazzola G, Galli L, Cerbone AM (2000) The role of vitamin B-12 in fasting hyperhomocysteinemia and its interaction with the homozygous C677T mutation of the methylenetetrahydrofolate reductase (MTHFR) gene—a case-control study of patients with early-onset thrombotic events. Thromb Haemost 83:563–570 [PubMed] [Google Scholar]
- 48.Molloy AM (2004) Folate and homocysteine interrelationships including genetics of the relevant enzymes. Curr Opin Lipidol 15:49–57 10.1097/00041433-200402000-00010 [DOI] [PubMed] [Google Scholar]
- 49.Monsen ALB, Refsum H, Markestad T, Ueland PM (2003) Cobalamin status and its biochemical markers methylmalonic acid and homocysteine in different age groups from 4 days to 19 years. Clin Chem 49:2067–2075 10.1373/clinchem.2003.019869 [DOI] [PubMed] [Google Scholar]
- 50.McNulty H, Dowey LRC, Strain JJ, Dunne A, Ward M, Molloy AM, McAnena LB, Hughes JP, Hannon-Fletcher M, Scott JM (2006) Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C→T polymorphism. Circulation 113:74–80 10.1161/CIRCULATIONAHA.105.580332 [DOI] [PubMed] [Google Scholar]
- 51.Selhub J, Miller JW (1992) The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr 55:131–138 [DOI] [PubMed] [Google Scholar]
- 52.McKinley MC, McNulty H, McPartlin J, Strain JJ, Pentieva K, Ward M, Weir DG, Scott JM (2001) Low-dose vitamin B-6 effectively lowers fasting plasma homocysteine in healthy elderly persons who are folate and riboflavin replete. Am J Clin Nutr 73:759–764 [DOI] [PubMed] [Google Scholar]
- 53.Ubbink JB, van der Merwe A, Delport R, Allen RH, Stabler SP, Riezler R, Vermaak WJ (1996) The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest 98:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brattström L (1996) Vitamins as homocysteine-lowering agents. J Nutr 126:1276S–1280S [DOI] [PubMed] [Google Scholar]
- 55.Guttormsen AB, Ueland PM, Nesthus I, Nygård O, Schneede J, Vollset SE, Refsum H (1996) Determinants and vitamin responsiveness of intermediate hyperhomocysteinemia (⩾40 μmol/liter)—the Hordaland homocysteine study. J Clin Invest 98:2174–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carmel R (2001) Cobalamin deficiency. In: Carmel R, Jacobsen DW (eds) Homocysteine in health and disease. Cambridge University Press, Cambridge, United Kingdom, pp 289–305 [Google Scholar]
- 57.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, et al (2006) Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 354:1567–1577 10.1056/NEJMoa060900 [DOI] [PubMed] [Google Scholar]
- 58.Lichtenstein AH, Russell RM (2005) Essential nutrients: food or supplements? Where should the emphasis be? JAMA 294:351–358 10.1001/jama.294.3.351 [DOI] [PubMed] [Google Scholar]
- 59.Verhoef P, Rimm EB, Hunter DJ, Chen J, Willett WC, Kelsey K, Stampfer MJ (1998) A common mutation in the methylenetetrahydrofolate reductase gene and risk of coronary heart disease: results among U.S. men. J Am Coll Cardiol 32:353–359 10.1016/S0735-1097(98)00244-7 [DOI] [PubMed] [Google Scholar]
- 60.Backstrand JR (2002) The history and future of food fortification in the United States: a public health perspective. Nutr Rev 60:15–26 10.1301/002966402760240390 [DOI] [PubMed] [Google Scholar]
- 61.Le Marchand L, Donlon T, Hankin JH, Kolonel LN, Wilkens LR, Seifried A (2002) B-vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control 13:239–248 10.1023/A:1015057614870 [DOI] [PubMed] [Google Scholar]
- 62.Ulrich CM, Kampman E, Bigler J, Schwartz SM, Chen C, Bostick R, Fosdick L, Beresford SA, Yasui Y, Potter JD (1999) Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev 8:659–668 [PubMed] [Google Scholar]
- 63.Ulvik A, Evensen ET, Lien EA, Hoff G, Vollset SE, Majak BM, Ueland PM (2001) Smoking, folate and methylenetetrahydrofolate reductase status as interactive determinants of adenomatous and hyperplastic polyps of colorectum. Am J Med Genet 101:246–254 10.1002/ajmg.1370 [DOI] [PubMed] [Google Scholar]
- 64.Stern LL, Mason JB, Selhub J, Choi SW (2000) Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev 9:849–853 [PubMed] [Google Scholar]
- 65.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, Olivieri O, Jacques PF, Rosenberg IH, Corrocher R, et al (2002) A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Nat Acad Sci USA 99:5606–5611 10.1073/pnas.062066299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shelnutt KP, Kauwell GPA, Gregory JF, Maneval DR, Quinlivan EP, Theriaque DW, Henderson GN, Bailey LB (2004) Methylenetetrahydrofolate reductase 677C→T polymorphism affects DNA methylation in response to controlled folate intake in young women. J Nutr Biochem 15:554–560 10.1016/j.jnutbio.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 67.Quinlivan EP, Davis SR, Shelnutt KP, Henderson GN, Ghandour H, Shane B, Selhub J, Bailey LB, Stacpoole PW, Gregory JF (2005) Methylenetetrahydrofolate reductase 677C→T polymorphism and folate status affect one-carbon incorporation into human DNA deoxynucleosides. J Nutr 135:389–396 [DOI] [PubMed] [Google Scholar]