Abstract
Pitt-Hopkins syndrome (PHS) is a rare syndromic encephalopathy characterized by daily bouts of hyperventilation and a facial gestalt. We report a 1.8-Mb de novo microdeletion on chromosome 18q21.1, identified by array–comparative genomic hybridization in one patient with PHS. We subsequently identified two de novo heterozygous missense mutations of a conserved amino acid in the basic region of the TCF4 gene in three additional subjects with PHS. These findings demonstrate that TCF4 anomalies are responsible for PHS and provide the first evidence of a human disorder related to class I basic helix-loop-helix transcription-factor defects (also known as “E proteins”). Moreover, our data may shed new light on the normal processes underlying autonomic nervous system development and maintenance of an appropriate ventilatory neuronal circuitry.
Pitt-Hopkins syndrome (PHS) was originally described in 1978 in two unrelated patients with mental retardation, recurrent episodes of hyperventilation, and a wide mouth.1 Six additional cases have been reported subsequently, allowing further clinical delineation of the syndrome.2–5 PHS is defined by severe psychomotor delay, epilepsy, and daily bouts of diurnal hyperventilation starting in infancy; mild postnatal growth retardation; postnatal microcephaly; and distinctive facial features. Since most hitherto reported cases have been sporadic, with males and females equally affected, PHS is regarded as an autosomal dominant condition with de novo mutations in index cases. We ascertained four cases of PHS and performed a systematic 1-Mb–resolution genomewide BAC-array screening for microdeletions or duplications in all subjects. Using this approach, we identified a 1.8-Mb de novo microdeletion on chromosome 18q21.1 in one of the four subjects. Further sequence analyses of the genes located within this interval revealed causative de novo mutations in the TCF4 gene (MIM 602272; GenBank accession number NM_003199) in three further subjects with PHS. TCF4, a member of the ubiquitous E-protein family, is therefore responsible for a severe CNS and autonomous nervous system dysfunction when mutated in humans.
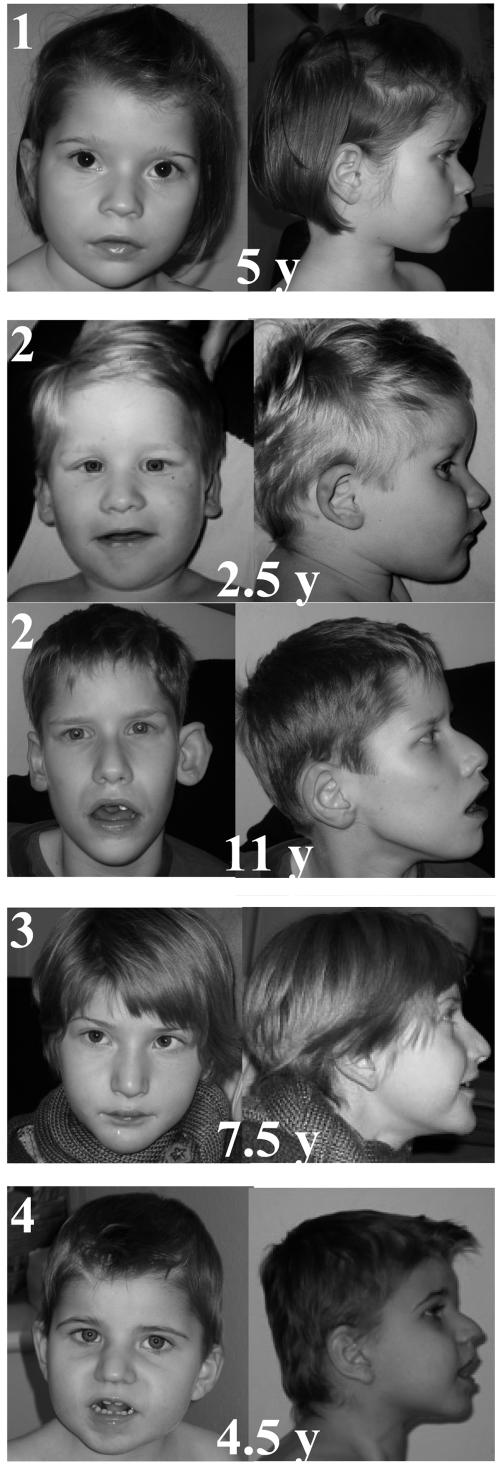
A total of four patients were included in the study. Inclusion criteria were (i) severe psychomotor delay, (ii) moderate postnatal growth retardation and microcephaly, and (iii) a PHS facial gestalt (fig. 1). Indeed, facial features consistently included enophthalmia, strabismus, and thin eyebrows in their midline portion; a large nose with high bridge and flared nostril; a protruding philtrum; M-shaped Cupid’s bow; fleshy lips and wide mouth with shallow and broad palate; and widely spaced teeth. The helices of the ears were dysplastic and thick. The traits coarsened with age, and the lower face got more protruding. All patients were born at term to unrelated parents after uneventful pregnancies (but subject 4 had nuchal edema), with normal birth parameters and unremarkable medical family history (table 1). Psychomotor delay was severe: no patients sat unaided until age 13–18 mo, none walked unassisted, and language was absent or limited to a few single words. All but the youngest one developed seizures and/or stereotypic movements of hands (clapping and washing, but with persistent voluntary use of hands) and head (rolling movements of the head) and daily episodes of hyperventilation, which is the hallmark of the syndrome. These episodes started abruptly, lasted several minutes, and were followed by apnea, cyanosis, and occasional loss of consciousness. These events happened during wakefulness only and were not associated with epileptic changes but were increased by emotions and fatigue. Hypoxia was severe and frequent enough to generate clubbing of fingers in the oldest of our patients. Other features included small penis and cryptorchidism, severe constipation, gastroesophageal reflux, small hands with slender fingers and single palmar crease (SPC), and small feet with pes planus. No congenital malformation was reported except for a unilateral supernumerary nipple in two subjects. All were usually placid and happy, although one patient experienced daily episodes of agitation and shouting, and another had a sleep disorder with difficulty falling asleep. He is currently receiving treatment of melatonin. One patient had mild thoracic scoliosis; none were spastic. Finally, dilated pupils with sluggish response to light were noted in three patients.
Figure 1. .
Frontal and profile views of patients 1–4 with PHS, with ages given in years (y). The consistent facial features associate enophthalmia, strabismus, and thin eyebrows in their midline portion; a large nose with high bridge and flared nostril; a protruding philtrum; M-shaped Cupid’s bow; fleshy lips, wide mouth with shallow and broad palate, and widely spaced teeth. The helices of the ears are dysplastic and thick. The traits coarsen with age, and the lower face gets more protruding.
Table 1. .
Clinical and Molecular Findings in Patients with PHS[Note]
| Patient |
||||
| Phenotype | 1 | 2 | 3 | 4 |
| Sex | F | F | M | M |
| Age at diagnosis (years) | 4.5 | 6.5 | 10 | 4.5 |
| Birth parameters (percentile): | ||||
| Weight and length | 50th | 50th | 50th | 50th |
| Occipitofrontal circumference | 25th | 10th | 50th | 50th |
| Growth parameters (percentile): | ||||
| Height | 25th | 25th | 10th | 50th |
| Weight | 25th | 25th | 10th | 25th |
| Neurologic findings: | ||||
| Postnatal microcephaly (percentile) | 3rd | <2nd | 3rd | 3rd |
| Epilepsy (age at onset, in years) | − | + (3.5) | + (8) | + (4.5) |
| Hyperventilation (age at onset, in years) | − | + (3.5) | + (5) | + (4.5) |
| Stereotypic movements (hands/head) | (+/−) | (+/+) | (+/+) | (+/−) |
| Genitalia | Normal | Normal | Abnormala | Abnormala |
| Scoliosis | Mild | − | − | − |
| Hands (small size, SPC, clubbing of fingers) | (+, +, −) | (+, +, −) | (+, +, +) | (+, +, −) |
| Other | 3 Nipples | Sleeping disorder | 3 Nipples | − |
| EEG abnormalities (testing age, in years) | + (2) | ++ (3) | ++ (6) | + (3) |
| Brain MRI abnormalities (testing age, in years) | + (4.5) | + (6.5) | + (6.5) | Not performed |
| Results of TCF4 gene screening | Del RP11-397A16 | R576Q | R576W | R576W |
Note.— All patients have severe mental retardation, strabismus, the PHS facial gestalt, and severe chronic constipation and gastroesophageal reflux. A plus sign (+) indicates presence of a train; a minus sign (−) indicates absence of a trait; a double plus sign (++) indicates that the trait is severe.
Unilateral cryptorchidism and small penis.
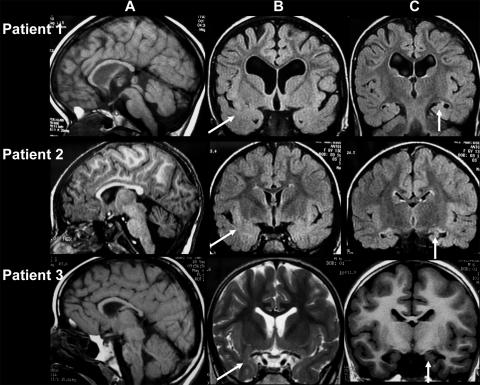
Electroencephalogram (EEG) was recorded for all subjects. No specific pattern was found in the two younger patients (subjects 1 and 4), but occipital or central delta waves unusual for that age were seen. In the older patients (subjects 2 and 3), pseudoperiodic complexes were present during wakefulness, on central or occipital regions, mixed with spikes and slow spikes and waves. This pattern was observed before the onset of epilepsy in subject 3. Magnetic resonance imaging (MRI) of the brain was available for three patients and consistently showed a thin corpus callosum, marked white-matter hyperintensity in the temporal poles, small hippocampi, and moderate hypoplasia of the frontal lobes (fig. 2).
Figure 2. .
Brain MRI findings for patients with PHS. A, T1-weighted sagittal images. B and C, Coronal flair or T2 sequences. Anomalies consistently noticed include a thin corpus callosum and moderate hypoplasia of the frontal lobes (A), marked white-matter hyperintensity in the temporal poles (B), and small hippocampi (C). The brain stem is normal (A). Patient 1, Mild ventricular dilatation and moderate hyperintensity of the dentate nuclei at age 4.5 years. Patient 2, No ventricular dilatation but moderate hyperintensity of the dentale nuclei, observed at age 6.5 years. Patient 3, Mild ventricular dilatation and no abnormalities of the posterior fossa at age 6.5 years.
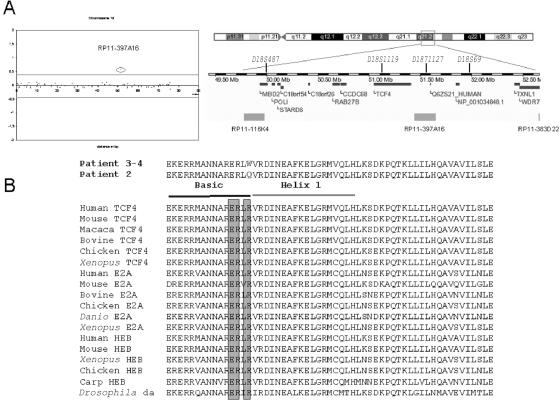
We analyzed the four patients by comparative genomic hybridization (CGH) on a microarray developed by the Wellcome Trust Sanger Institute and composed of 3,523 large insert clones ranging in size from 80 to 240 kb and spread over the entire genome, with a mean resolution of 1 clone per megabase.6 An abnormal pattern involving one single clone was suggestive of an 18q21.1 monosomy in patient 1 (1.8 Mb) (fig. 3A). With use of clone RP11-397A16 as a probe, FISH analyses on metaphase nuclei from blood lymphocytes of the proband and her parents confirmed our array-CGH findings and showed that the deletion had occurred de novo (not shown). Further genotyping analysis with use of microsatellite DNA markers showed irregular inheritance of markers D18S1119 and D18S1127, with a single maternal contribution. FISH analyses with use of probe RP11-397A16 failed to detect anomalies of chromosome 18 in the other three patients with PHS.
Figure 3. .
Genetic analyses of patients with PHS. A, Chromosome 18 array-CGH ratio profile obtained for patient 1 that identifies deletion of a single clone at chromosome 18q21.1. The X-axis represents the distance, in base pairs, along the chromosome from the p telomere. The Y-axis represents the hybridization ratio (reference DNA vs. test DNA) plotted on a log2 scale. Gray lines indicate thresholds for clone deletion or duplication (array ratio mean±4 SD). The figure also shows the Ensembl display of this region. B, TCF4 gene mutations and phylogenetic analysis of TCF4 proteins. The two de novo missense mutations (R276Q and R276W) identified in patients 2–4 are shown. ClustalW (National Center for Biotechnology Information) alignment of class I bHLH proteins orthologs are shown. Shadowed amino acids correspond to conserved glutamic and arginine residues constituting the E box–recognition motif.
Among the 11 annotated/predicted genes located within the chromosome 18q21.1–deleted interval, 2 genes were regarded as strong candidates for PHS—namely, methyl-CpG–binding domain protein 2 (MBD2 [MIM 603547])7 and transcription factor 4 (TCF4).8,9 The coding sequences and flanking introns of TCF4 were PCR amplified and sequenced by the fluorometric method on both strands (Big DyeTerminator Cycle Sequencing Kit [Applied Biosystems]) (primers and PCR conditions available on request). Remarkably, we identified a heterozygous point mutation at the same arginine 576 codon in all three of the analyzed subjects. The base change was a C→T transition at position 1726 leading to the substitution of an arginine by a tryptophane (R576W) in patients 3 and 4, whereas a G→A transition at position 1727 led to the substitution of an arginine by a glutamine (R576Q) in patient 2. In addition, the three base changes occurred de novo, since they were found neither in the parent DNA nor in a panel of 338 control chromosomes.
TCF4—also referred to as “ITF-2” (for immunoglobulin transcription factor 2), “E2-2,” or “SEF2” (for SL3-3 enhancer factor 2)—belongs to the class I basic helix-loop-helix (bHLH) protein family, also known as the “E-protein family.” Ubiquitously expressed class I bHLHs consist of TCF4, HEB,10 and the differentially spliced products of the E2A gene: E12 and E47.11 E proteins contain a common bHLH structural motif that mediates homo- and heterodimerization between bHLH proteins via their HLH domain, whereas the adjacent basic region mediates the binding of the dimers to a common DNA sequence (CANNTG) known as an “E box.”12 All four members of the mammalian class I bHLH transcription factors (TFs) are evolutionarily conserved and play critical roles in cell-fate determination and differentiation.13 TCF4 is a downstream target of the WNT/β-catenin/TCF pathway and, like cMYC and cyclinD1, has been shown to function as an oncogene when deregulated.14 Remarkably, the three TCF4 missense mutations identified in subjects with PHS altered the same arginine 576 residue. This amino acid lies within the basic region of the bHLH domain and is extremely conserved among bHLH proteins (fig. 3B). Moreover, this residue is known to play an essential role for DNA recognition, since it stabilizes the glutamic residue that directly interacts with DNA.15 Interestingly, an engineered missense mutation of the homologous arginine residue impaired DNA-binding properties but not dimerization in other bHLH proteins, thus resulting in a dominant negative effect (by trapping partners in the homo- or heterodimeric complex) by inhibition of E box–mediated transcription (E2A and BMAL1).16,17 It is worth noting that, although PHS is difficult to assess because of the severity of the handicap and the small number of patients, patient 1 (harboring the large-scale deletion) is not more severely affected than the other three patients who harbor a TCF4 missense mutation. Hence, a contiguous gene syndrome is unlikely, and a loss-of-function of at least 50% is the most likely pathophysiological mechanism underlying PHS. This hypothesis is further supported by the observation that, in mice, embryonic development has been shown to be sensitive to E-protein copy number, since the absence of three copies causes embryonic lethality.18 Tcf4-null mice were born with a frequency <25% and subsequently died within the 1st wk of life.18 No malformation has been reported, and the cause of death remains unclear. Conditional Tcf4-deficient mice generated with the Cre/loxP system exhibit impaired B and T cell development.19
Despite expression of TCF4 in human embryonic buds, no congenital malformation has been reported elsewhere for PHS except for the supernumerary nipple in two subjects (this study) and Hirschsprung disease (HSCR) in one subject.5 By contrast, TCF4 is also expressed in adult brain, skeletal muscle, lung, and heart, which is consistent with the postnatal growth retardation and microcephaly observed in the course of the disease. Similarly, epileptic encephalopathy and bouts of hyperventilation could be ascribed to the dysregulation of neurogenic bHLHs that form heterodimers with TCF4, especially the human Achaete-Scute homolog 1 (ASCL1 [MIM 100790]).20 Indeed, TCF4 forms a functional complex with the ASCL1.21 Previous work on cellular and animal models has demonstrated that mutations affecting the ASCL1–PHOX developmental pathway impaired the development of neurons of the noradrenergic lineage, resulting in abnormal respiratory network in the brain stem and periphery.22,23 Finally, several clinical features observed in patients with PHS could also result from deregulation of TCF4 downstream targets. Along these lines, it is worth remembering that the respiratory neuron homeobox gene (RNX, also known as “TLX3” and “HOX11L2” [MIM 604640])24 includes several E boxes in its 5′ regulating region and that Rnx-deficient mice display an abnormal respiratory pattern, with alternating tachypnea and apnea.25
An abnormal ventilatory pattern characterized by daily bouts of diurnal hyperventilation is the hallmark of PHS but is not reported for patients younger than 3 years. Epilepsy generally occurs later in the course of the disease, and 24-h EEG recordings of patient 2 (the present report) and of case 1 reported by Peippo5 confirmed that the two features are unrelated. Episodes of hyperventilation occur only during wakefulness, but, because of the mental handicap of the patients, voluntary triggering remains a difficult option to assess. Conversely, these hyperpneic episodes could be included in the spectrum of dysautonomic features observed in patients with PHS. Indeed, dilated pupils with sluggish response to light have been noticed. Finally, one patient had short-segment HSCR, a congenital malformation of the enteric nervous system (case 2 reported by Peippo5), and other patients have severe gastrointestinal dysfunction. The identification of TCF4 as the disease-causing gene in PHS may bring new insight into normal autonomic nervous system development and maintenance of appropriate ventilation.
The association of HSCR (or severe constipation) with severe mental retardation also points to Mowat-Wilson (MIM 235730) and Goldberg-Shprintzen (MIM 609460) syndromes as differential diagnoses. Similarly, the occurrence of hyperventilation frequently also raises the issue of Rett syndrome (MIM 312750) in female patients with PHS. Yet, in addition to distinctive facial features, all patients with PHS reported to date had abnormal psychomotor development from the neonatal period. Although progressive, the disease does not have a regressive course after a period of near-normal development. Conversely, neither loss of purposeful hand movements nor severe progressive scoliosis is reported in PHS. Angelman syndrome (MIM 105830) is also invariably discussed and tested, although the facial gestalt and EEG findings clearly differ between the two conditions.
In conclusion, on the basis of the facial gestalt, PHS should be regarded as a clinically recognizable entity, even before the occurrence of hyperventilation and epilepsy. Electrophysiologic findings and brain MRI should be of valuable help at that time. We believe that the syndrome will now be recognized with a greater frequency than was initially expected, and we suggest that TCF4-mutation screening be recommended for patients for whom overlapping syndromes were unsuccessfully tested—namely, Angelman, Rett, and Mowat-Wilson syndromes.
Acknowledgments
We are thankful to the patients and their families for their cooperation. We thank Heike Fiegler and the Sanger Microarray Facility for providing the 1-Mb arrays. This study was supported by the Centre National de la Recherche Scientifique, the Agence National pour la Recherche, and the Wellcome Trust. R.R. was supported by a Sanger Institute Postdoctoral Fellowship.
Web Resources
The accession number and URLs for data presented herein are as follows:
- Ensembl Genome Browser, http://www.ensembl.org/
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for TCF4 [accession number NM_003199])
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for TCF4, MBD2, ASCL1, HOX11L2, Mowat-Wilson syndrome, Goldberg-Shprintzen syndrome, Rett syndrome, and Angelman syndrome)
Reference
- 1.Pitt D, Hopkins I (1978) A syndrome of mental retardation, wide mouth and intermittent overbreathing. Aust Paediatr J 14:182–184 [DOI] [PubMed] [Google Scholar]
- 2.Singh HA (1993) Mental retardation, macrostomia and hyperpnoea syndrome. J Paediatr Child Health 29:156–157 [DOI] [PubMed] [Google Scholar]
- 3.Van Balkom ID, Quartel S, Hennekam RC (1998) Mental retardation, “coarse” face, and hyperbreathing: confirmation of the Pitt-Hopkins syndrome. Am J Med Genet 75:273–276 [DOI] [PubMed] [Google Scholar]
- 4.Orrico A, Galli L, Zappella M, Lam CW, Bonifacio S, Torricelli F, Hayek G (2001) Possible case of Pitt-Hopkins syndrome in sibs. Am J Med Genet 103:157–159 10.1002/ajmg.1523 [DOI] [PubMed] [Google Scholar]
- 5.Peippo MM, Simola KO, Valanne LK, Larsen AT, Kahkonen M, Auranen MP, Ignatius J (2006) Pitt-Hopkins syndrome in two patients and further definition of the phenotype. Clin Dysmorphol 15:47–54 10.1097/01.mcd.0000184973.14775.32 [DOI] [PubMed] [Google Scholar]
- 6.Shaw-Smith C, Redon R, Rickman L, Rio M, Willatt L, Fiegler H, Firth H, Sanlaville S, Winter R, Colleaux L, et al (2004) Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. J Med Genet 41:241–248 10.1136/jmg.2003.017731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrich B, Bird A (1998) Identification and characterization of a family of mammalian methyl-CpG binding proteins. Molec Cell Biol 18:6538–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henthron P, Kiledjian M, Kadesch T (1990) Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science 247:467–470 10.1126/science.2105528 [DOI] [PubMed] [Google Scholar]
- 9.Corneliussen B, Thornell A, Hallberg B, Grundström T (1991) Helix-loop-helix transcriptional activators bind to a sequence in glucocorticoid response elements of retrovirus enhancers. J Virol 65:6084–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB, et al (1989) Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58:537–544 10.1016/0092-8674(89)90434-0 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Babin J, Feldhaus AL, Singh H, Sharp PA, Bina M (1991) HTF4: a new human helix-loop-helix protein. Nucleic Acids Res 19:4555–4558 10.1093/nar/19.16.4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murre C, McCaw PS, Baltimore D (1989) A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56:777–783 10.1016/0092-8674(89)90682-X [DOI] [PubMed] [Google Scholar]
- 13.Pagliuca A, Gallo P, De Luca P, Lania L (2000) Class A helix-loop-helix proteins are positive regulators of several cyclin-dependent kinase inhibitors’ promoter activity and negatively affect cell growth. Cancer Res 60:1376–1382 [PubMed] [Google Scholar]
- 14.Kolligs FT, Nieman MT, Winer I, Hu G, Van Mater D, Feng Y, Smith IM, Wu R, Zhai Y, Cho KR, et al (2002) ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with β-catenin defects and promotes neoplastic transformation. Cancer Cell 1:145–155 10.1016/S1535-6108(02)00035-1 [DOI] [PubMed] [Google Scholar]
- 15.Ma PC, Rould MA, Weintraub H, Pabo CO (1994) Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell 77:451–459 10.1016/0092-8674(94)90159-7 [DOI] [PubMed] [Google Scholar]
- 16.Zhuang Y, Barndt RJ, Pan L, Kelley R, Dai M (1998) Functional replacement of the mouse E2A gene with a human HEB cDNA. Mol Cell Biol 18:3340–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosoda H, Motohashi J, Kato H, Masushige S, Kida S (2004) A BMAL1 mutant with arginine 91 substituted with alanine acts as a dominant negative inhibitor. Gene 338:235–241 10.1016/j.gene.2004.05.022 [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Y, Cheng P, Weintraub H (1996) B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol Cell Biol 16:2898–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergqvist I, Eriksson M, Saarikettu J, Eriksson B, Corneliussen B, Gundström T, Holmberg D (2000) The basic helix-loop-helix transcription factor E2-2 is involved in T lymphocyte development. Eur J Immunol 30:2857–2863 [DOI] [PubMed] [Google Scholar]
- 20.Ball DW, Azzoli CG, Baylin SB, Chi D, Dou S, Donis-Keller H, Cumaraswamy A, Borges M, Nelkin BD (1993) Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Nat Acad Sci 90:5648–5652 10.1073/pnas.90.12.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persson P, Jogi A, Grynfeld A, Pahlman S, Axelson H (2000) HASH-1 and E2-2 are expressed in human neuroblastoma cells and form a functional complex. Biochem Biophys Res Commun 274:22–31 10.1006/bbrc.2000.3090 [DOI] [PubMed] [Google Scholar]
- 22.Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463–476 10.1016/0092-8674(93)90381-Y [DOI] [PubMed] [Google Scholar]
- 23.de Pontual L, Nepote V, Attie-Bitach T, Al Halabiah H, Trang H, Elghouzzi V, Levacher B, Benihoud K, Auge J, Faure C, et al (2003) Noradrenergic neuronal development is impaired by mutation of the proneural HASH-1 gene in congenital central hypoventilation syndrome (Ondine’s curse). Hum Molec Genet 12:3173–3180 10.1093/hmg/ddg339 [DOI] [PubMed] [Google Scholar]
- 24.Dear TN, Sanchez-Garcia I, Rabbitts TH (1993) The HOX11 gene encodes a DNA-binding nuclear transcription factor belonging to a distinct family of homeobox genes. Proc Nat Acad Sci 90:4431–4435 10.1073/pnas.90.10.4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirasawa S, Arata, A, Onimaru, H, Roth, KA, Brown GA, Horning S, Arata, S, Okumura, K, Sasazuki T, Korsmeyer SJ (2000) Rnx deficiency results in congenital central hypoventilation. Nat Genet 24:287–290 10.1038/73516 [DOI] [PubMed] [Google Scholar]