Abstract
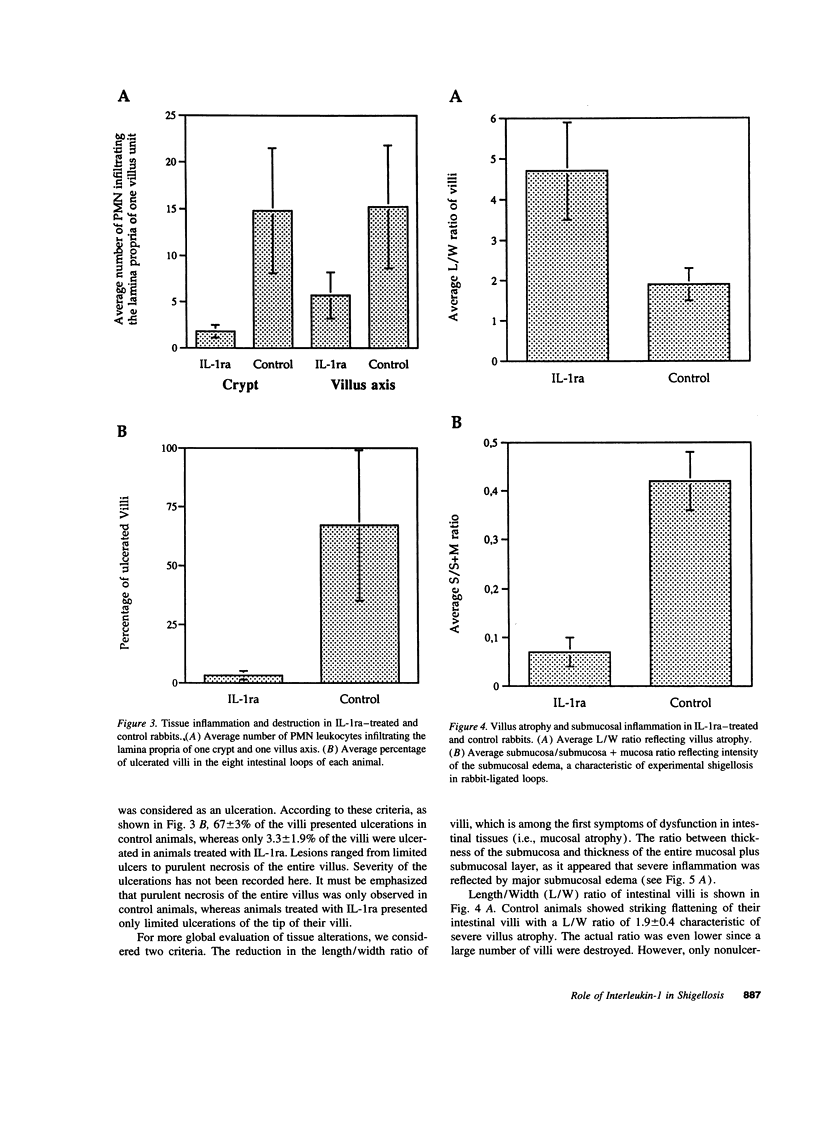
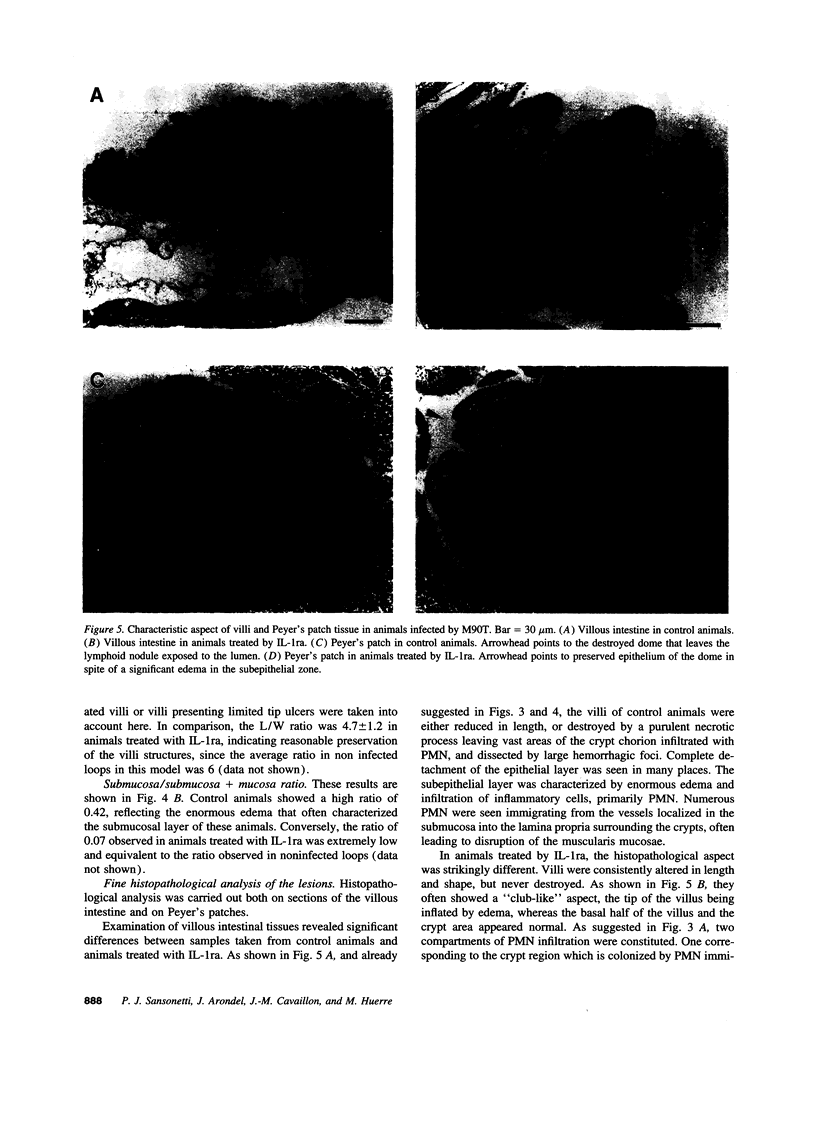
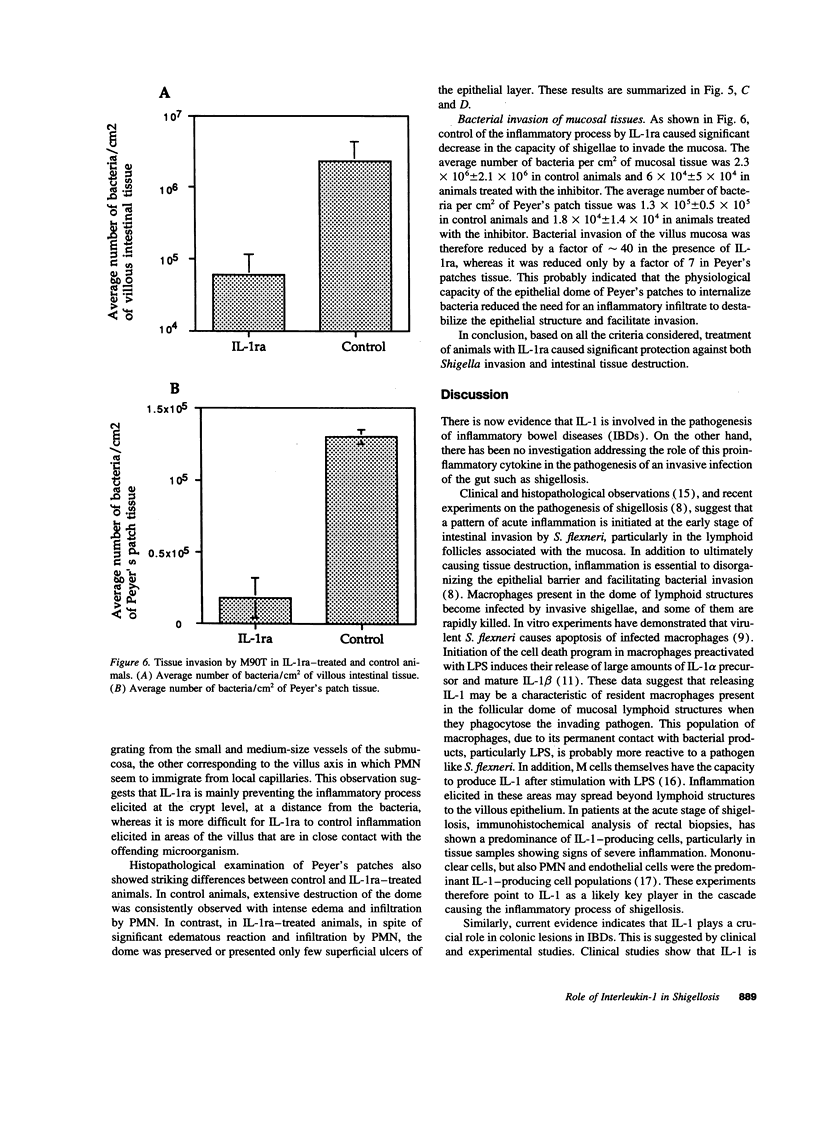
The effect of human recombinant interleukin-1 receptor antagonist on intestinal inflammation, tissue destruction, and bacterial invasion during experimental shigellosis caused by Shigella flexneri was studied in the rabbit-ligated loop infection model. Intravenous infusion of the inhibitor at a dose of 2 mg/kg per h, was initiated 30 min before intestinal loops were ligated and infected, and continued during the 8-h period of infection. The animals treated with IL-1 receptor antagonist showed a striking decrease in inflammation, destruction, and bacterial invasion of their tissues, both at the level of the villous intestine and Peyer's patches. This is conclusive evidence that interleukin-1 plays a critical role in the pathogenesis of shigellosis. This proinflammatory cytokine is here proposed as a major trigger of the inflammatory reaction which is characteristic of this invasive disease of the intestine, due to the particular interaction existing between S. flexneri and macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- Brynskov J., Tvede N., Andersen C. B., Vilien M. Increased concentrations of interleukin 1 beta, interleukin-2, and soluble interleukin-2 receptors in endoscopical mucosal biopsy specimens with active inflammatory bowel disease. Gut. 1992 Jan;33(1):55–58. doi: 10.1136/gut.33.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Haeffner-Cavaillon N., Kirsch S. J., Warren H. S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990 Jul;58(7):2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Clark B. D., Schindler R., Lierena R., Eysselein V. E., Thompson R. C., Dinarello C. A. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990 Sep;86(3):972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Duchini A., Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992 Jul;103(1):65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Kelly N., Bernton E., Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989 Jun 1;169(6):2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Recombinant murine interleukin-1 alpha enhancement of nonspecific antibacterial resistance. Infect Immun. 1987 Sep;55(9):2061–2065. doi: 10.1128/iai.55.9.2061-2065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect Immun. 1994 Feb;62(2):457–461. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A. The role of interleukin-1 in host responses to infectious diseases. Infect Agents Dis. 1992 Oct;1(5):227–236. [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M. F., Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993 Nov;61(11):4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fischer E., Van Zee K. J., Marano M. A., Rock C. S., Kenney J. S., Poutsiaka D. D., Dinarello C. A., Lowry S. F., Moldawer L. L. Interleukin-1 receptor antagonist circulates in experimental inflammation and in human disease. Blood. 1992 May 1;79(9):2196–2200. [PubMed] [Google Scholar]
- Goldberg M. B., Sansonetti P. J. Shigella subversion of the cellular cytoskeleton: a strategy for epithelial colonization. Infect Immun. 1993 Dec;61(12):4941–4946. doi: 10.1128/iai.61.12.4941-4946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F., Goto K., Miyata T., Ohkawara S., Takao T., Mori S., Furukawa S., Maeda T., Iwanaga S., Shimonishi Y. Interleukin-1 receptor antagonist in inflammatory exudate cells of rabbits. Production, purification and determination of primary structure. Immunology. 1992 Oct;77(2):235–244. [PMC free article] [PubMed] [Google Scholar]
- Granowitz E. V., Porat R., Mier J. W., Pribble J. P., Stiles D. M., Bloedow D. C., Catalano M. A., Wolff S. M., Dinarello C. A. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992 Sep;4(5):353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- Granowitz E. V., Santos A. A., Poutsiaka D. D., Cannon J. G., Wilmore D. W., Wolff S. M., Dinarello C. A. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet. 1991 Dec 7;338(8780):1423–1424. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Moldawer L. L., Helfgott D., Kilian P. L., Sehgal P. B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992 Mar 1;148(5):1486–1492. [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla J., García P., Dinarello C. A. The interleukin-1 receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniae sepsis in newborn rats. Infect Immun. 1993 Mar;61(3):926–932. doi: 10.1128/iai.61.3.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S314–S318. doi: 10.1093/clinids/13.supplement_4.s314. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am J Pathol. 1986 Apr;123(1):25–38. [PMC free article] [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K. Interleukin-1 administration to C3H/HeJ mice after but not prior to infection increases resistance to Salmonella typhimurium. Infect Immun. 1991 Dec;59(12):4729–4731. doi: 10.1128/iai.59.12.4729-4731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Vasselon T., Hellio R., Lesourd M., Sansonetti P. J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992 Jan;60(1):237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Hutton A. K., Garside P., Steel M. A role for interleukin-1 alpha in immunologically mediated intestinal pathology. Immunology. 1993 Sep;80(1):110–115. [PMC free article] [PubMed] [Google Scholar]
- Nicholls S., Stephens S., Braegger C. P., Walker-Smith J. A., MacDonald T. T. Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol. 1993 Aug;46(8):757–760. doi: 10.1136/jcp.46.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Minami A., Nakamura S. Enhanced resistance of mice to bacterial infection induced by recombinant human interleukin-1a. Infect Immun. 1987 Jun;55(6):1436–1440. doi: 10.1128/iai.55.6.1436-1440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo J., Mahlman R. T. Follicle epithelial M cells are a source of interleukin-1 in Peyer's patches. Immunology. 1993 Mar;78(3):505–507. [PMC free article] [PubMed] [Google Scholar]
- Pelkonen S., Pluschke G. Recombinant interleukin-1 stimulates clearance of Escherichia coli K1 bacteraemia. Microb Pathog. 1989 Jun;6(6):415–424. doi: 10.1016/0882-4010(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Perdomo J. J., Gounon P., Sansonetti P. J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest. 1994 Feb;93(2):633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo O. J., Cavaillon J. M., Huerre M., Ohayon H., Gounon P., Sansonetti P. J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994 Oct 1;180(4):1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Lindberg A. A., Wretlind B., Bardhan P. K., Andersson U., Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995 Jan;63(1):289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Arondel J., Fontaine A., d'Hauteville H., Bernardini M. L. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991 Jun;9(6):416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor R. B. Pathogenetic and clinical relevance of cytokines in inflammatory bowel disease. Immunol Res. 1991;10(3-4):465–471. doi: 10.1007/BF02919743. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Wolstencroft R. A., Cason J., Ainley C. C., Dumonde D. C., Thompson R. P. Interleukin 1 in Crohn's disease. Clin Exp Immunol. 1987 Mar;67(3):594–605. [PMC free article] [PubMed] [Google Scholar]
- Schuerer-Maly C. C., Eckmann L., Kagnoff M. F., Falco M. T., Maly F. E. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994 Jan;81(1):85–91. [PMC free article] [PubMed] [Google Scholar]
- Thomas T. K., Will P. C., Srivastava A., Wilson C. L., Harbison M., Little J., Chesonis R. S., Pignatello M., Schmolze D., Symington J. Evaluation of an interleukin-1 receptor antagonist in the rat acetic acid-induced colitis model. Agents Actions. 1991 Sep;34(1-2):187–190. doi: 10.1007/BF01993274. [DOI] [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman K. R., Simon P. L., West G. A., Cominelli F., Rachmilewitz D., Klein J. S., Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993 Mar;104(3):749–758. doi: 10.1016/0016-5085(93)91010-f. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Fitting C., Cavaillon J. M., Sansonetti P. J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Invest. 1994 Sep;94(3):1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A., Kenny B., Ménard R., Prévost M. C., Holland I. B., Sansonetti P. J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994 Feb;11(4):619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]