Abstract
Syntaxin and Munc18 are, in tandem, essential for exocytosis in all eukaryotes. Recently, it was shown that Munc18 inhibition of neuronal syntaxin 1 can be overcome by arachidonic acid, indicating that this common second messenger acts to disrupt the syntaxin–Munc18 interaction. Here, we show that arachidonic acid can stimulate syntaxin 1 alone, indicating that it is syntaxin 1 that undergoes a structural change in the syntaxin 1–Munc18 complex. Arachidonic acid is incapable of dissociating Munc18 from syntaxin 1 and, crucially, Munc18 remains associated with syntaxin 1 after arachidonic-acid-induced syntaxin 1 binding to synaptosomal-associated protein 25 kDa (SNAP25). We also show that the same principle operates in the case of the ubiquitous syntaxin 3 isoform, highlighting the conserved nature of the mechanism of arachidonic acid action. Neuronal soluble N-ethyl maleimide sensitive factor attachment protein receptors (SNAREs) can be isolated from brain membranes in a complex with endogenous Munc18, consistent with a proposed function of Munc18 in vesicle docking and fusion.
Keywords: Munc18, syntaxin, SNAP25, arachidonic acid, SNARE, vesicle fusion
Introduction
Membrane-associated helical proteins known as soluble N-ethyl maleimide sensitive factor attachment protein receptors (SNAREs) are crucial for vesicle fusion. In the brain, fusion of synaptic vesicles with the plasma membrane requires three SNARE proteins: syntaxin 1, SNAP25 (synaptosomal-associated protein 25 kDa) and synaptobrevin (Sollner et al, 1993; Montecucco & Schiavo, 1994). The three SNARE proteins form a four-helical bundle—the SNARE complex that mediates membrane fusion (Sutton et al, 1998).
Several proteins regulate SNARE function in membrane fusion. Of particular importance is the cytosolic protein Munc18, also known as neuronal (n) Sec1, which binds syntaxin and is conserved throughout the eukaryotic kingdom (Hata et al, 1993; Garcia et al, 1994). Initial in vitro experiments indicated that a syntaxin–Munc18 interaction is incompatible with SNARE assembly (Pevsner et al, 1994; Yang et al, 2000). However, the yeast Munc18 homologue sec1 associates with the SNARE complex (Carr et al, 1999), and recent in vivo studies indicate that Munc18 has a pivotal function in vesicle docking and fusion—that is, the stages in which SNARE assembly takes place (Fisher et al, 2001; Voets et al, 2001; Martinez-Arca et al, 2003; Toonen et al, 2006). Importantly, knockout or mutagenesis of Munc18 homologues in diverse organisms result in markedly decreased secretion, indicating a positive role for Munc18 in exocytosis (Novick et al, 1981; Verhage et al, 2000).
In addition to proteins, membrane transport is regulated by lipids (Wenk & De Camilli, 2004; Rigoni et al, 2005; Rohrbough & Broadie, 2005). Many studies have implicated arachidonic acid, an important polyunsaturated fatty acid, in stimulation of vesicle fusion (Frye & Holz, 1984; Williams et al, 1989; Brown et al, 2003; Latham et al, 2007). Arachidonic acid can be released from the lipid bilayer by phospholipases and is then metabolized into a variety of active compounds, such as prostaglandins. It was recently shown that arachidonic acid activates SNARE assembly by relieving Munc18 inhibition of syntaxin 1, indicating a disruption of the syntaxin–Munc18 complex (Rickman & Davletov, 2005).
Here, we have investigated whether arachidonic acid acts to dissociate Munc18 from syntaxin. We show that arachidonic acid does not release Munc18 from syntaxin 1 but causes a conformational change in syntaxin 1, allowing formation of a tripartite Munc18–syntaxin–SNAP25 complex. Similarly, Munc18-bound syntaxin 3, a ubiquitous isoform, can also be activated by arachidonic acid, showing that the association of Munc18 with syntaxins is not necessarily inhibitory. All plasma membrane syntaxins tested are sensitive to arachidonic acid to varying degrees. Our results reconcile data from different model organisms and provide a clearer picture of the mode of arachidonic acid action in membrane fusion.
Results And Discussion
We have recently shown that the syntaxin 3 isoform involved in neurite outgrowth is activated by arachidonic acid in the absence of Munc18 (Darios & Davletov, 2006). Thus, we analysed the action of this second messenger on other plasma membrane syntaxins. Figure 1A shows that syntaxin 1 and syntaxin 2 are able to assemble, in short 20 min reactions, into a SNARE complex but arachidonic acid still has a significant stimulatory effect, particularly in the case of syntaxin 1. Strikingly, syntaxin 3 and syntaxin 4 are completely inhibited except in the presence of arachidonic acid. Together, these results emphasize the importance of syntaxin properties in SNARE assembly.
Figure 1.
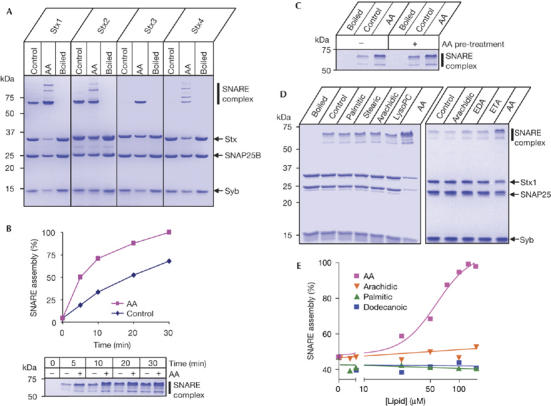
Action of arachidonic acid on plasma membrane syntaxins assessed by Coomassie-stained SDS–polyacrylamide gel electrophoresis. (A) Degree of SNARE assembly varies between syntaxins (Stx) 1 to 4 as a function of arachidonic acid (AA, 100 μM). SNAP25B and synaptobrevin 2 (Syb) were used. Boiling disrupts SNARE complexes, thus showing input protein quantities. (B) The graph shows the kinetics of SNARE assembly by syntaxin 1. A representative gel is shown with AA induced increases in the SNARE complex. AA, 100 μM. (C) Syntaxin 1 pre-treated with 100 μM AA (and then purified by gel filtration, not shown) still requires AA addition for efficient SNARE assembly. (D) Specificity of AA action on syntaxin 1. The left-hand gel shows SNARE reactions in the presence of 100 μM saturated palmitic (C16:0), stearic (C18:0), arachidic (C20:0) acids, brain lysophosphatidylcholine (LysoPC) and unsaturated AA (20:4). The right-hand gel shows SNARE assembly in the presence of C20 fatty acids with increasing unsaturation. EDA, eicosadienoic acid (C20:2); ETA, eicosatrienoic acid (C20:3). (E) Titration of AA, dodecanoic (C12:0), palmitic and arachidic acids in the SNARE assembly assay. EC50 for syntaxin 1 activation by AA is about 60 μM. SNAP25, synaptosomal-associated protein 25 kDa; SNARE, soluble NSF attachment protein receptors.
We further investigated the effect of arachidonic acid using syntaxin 1, the isoform pivotal for neuronal exocytosis. Kinetic analysis showed that stimulation of SNARE assembly occurs quickly, within 5 min (Fig 1B). To test whether arachidonic acid causes a lasting effect on syntaxin function, syntaxin 1 was incubated with arachidonic acid for 10 min and then isolated by size-exclusion chromatography to remove arachidonic acid. This pre-treated syntaxin still required arachidonic acid for full activation (Fig 1C), indicating that accelerated SNARE assembly seems to coincide with the presence of this lipid molecule.
The specificity of arachidonic acid action on syntaxin 1 was tested using a range of saturated and unsaturated fatty acids, and prostaglandins A1, A2, B1, B2, E1, F1 and I2. In addition, another product of phospholipid hydrolysis implicated in vesicle fusion—lysophosphatidylcholine—was also tested on SNARE assembly. Only arachidonic acid potently accelerated SNARE complex formation (Fig 1D; data not shown). Titration of arachidonic acid gave an EC50 of ∼60 μM (Fig 1E), a value within bulk estimates reported previously in the cytosol of activated cells (Wolf et al, 1991). Activation of syntaxin is apparently unrelated to the formation of lipid micelles, as dodecanoic acid, palmitic acid and arachidic acid are all ineffective, despite the presence or absence of micelles within the concentration range used (supplementary Fig 1A online). Furthermore, syntaxin 1 itself was sufficient to suppress micelle formation by arachidonic acid (supplementary Fig 1B online). Therefore, it is probably the specific properties of arachidonic acid (Brash, 2001) rather than micelle formation that underlie syntaxin activation.
Next, we tested whether arachidonic acid affects SNARE conformation by protein intrinsic fluorescence. Addition of arachidonic acid to syntaxin 1 led to an instant and marked change in intrinsic fluorescence, indicating rapid interaction (Fig 2A,B). Synaptobrevin and SNAP25 did not show a similar response (Fig 2A), indicating that arachidonic acid stimulates SNARE assembly primarily at the syntaxin level and supporting the view that syntaxin properties are important in SNARE interactions (Fig 1A). Consistent with the results of SNARE assembly (Fig 1D), the effect of arachidonic acid was specific (Fig 2C).
Figure 2.
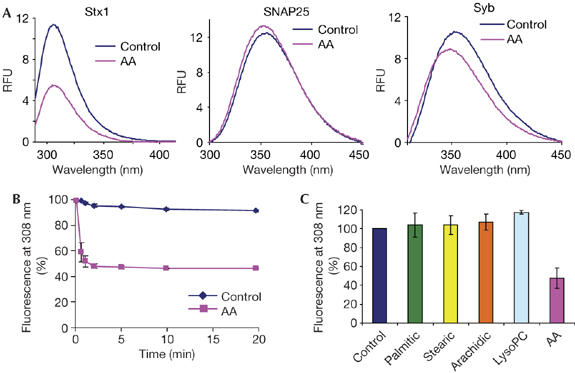
Arachidonic acid triggers a rapid change in syntaxin 1 intrinsic fluorescence. (A) Fluorescence spectra of syntaxin 1, SNAP25 and synaptobrevin±arachidonic acid (AA) with excitation at 280 nm. The peaks reflect either tyrosine (syntaxin 1; Stx1) or tryptophan fluorescence (SNAP25 and synaptobrevin; Syb). (B) Decrease in syntaxin 1 fluorescence at 308 nm occurs within 30 s of addition of arachidonic acid. (C) Neither saturated fatty acids nor lysophosphatidylcholine (LysoPC) affect syntaxin 1 structure in the same way as arachidonic acid. SNAP25, synaptosomal-associated protein 25 kDa.
Knowing that arachidonic acid can act directly on syntaxin 1, we turned our attention to the syntaxin 1–Munc18 molecular interaction. Syntaxin 1 has traditionally been considered to be in two mutually exclusive states: in complex with either Munc18 or its SNARE partners (Pevsner et al, 1994; Dulubova et al, 1999). We tested whether activation of syntaxin 1 leads to dissociation of Munc18. Native Munc18 was first attached to syntaxin 1 by incubating glutathione S-transferase (GST)–syntaxin 1 beads with bovine brain cytosol. Syntaxin 1–Munc18 beads were then treated for 30 min with arachidonic acid or 1 M NaCl, but these conditions were unable to disrupt the binary association (Fig 3A). Next, we released syntaxin 1–Munc18 from the beads by thrombin cleavage and isolated an equimolar complex by size-exclusion chromatography (supplementary Fig 2 online). Syntaxin 1, when associated with Munc18, was fully inhibited, but was able to form a SNARE complex on addition of arachidonic acid (Fig 3B), as reported previously (Rickman & Davletov, 2005). Addition of syntaxin 1–Munc18 to GST–SNAP25 beads, in the presence of arachidonic acid, resulted in attachment of syntaxin 1 without dissociation of Munc18 (Fig 3C).
Figure 3.
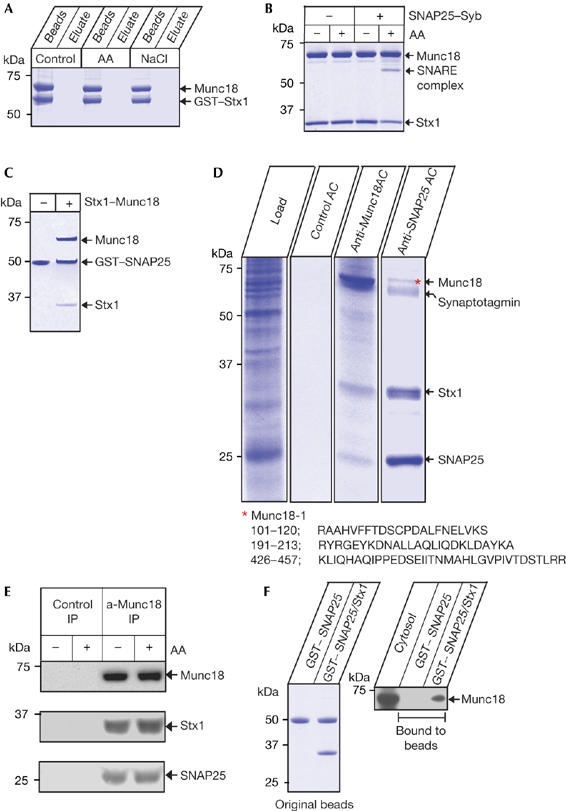
Interaction of native Munc18 with syntaxin 1 persists after arachidonic-acid-induced SNAP25 engagement. (A) GST–syntaxin 1 (GST–Stx1), on glutathione beads, binds readily to bovine brain Munc18 and this association is not disrupted by 100 μM arachidonic acid (AA) or 1 M NaCl; 30 min reactions. Coomassie-stained gel. (B) AA (100 μM) allows SNARE assembly with an equimolar syntaxin 1–Munc18 complex. Coomassie-stained gel. (C) Incubation of GST–SNAP25 on glutathione beads with Stx1–Munc18 binary complex, in the presence of 100 μM AA, leads to pull-down of both syntaxin 1 and Munc18. Coomassie-stained gel. (D) Munc18 co-purifies with syntaxin 1–SNAP25 from brain extract (load) during preparative affinity chromatographies (AC) using anti-Munc18 or anti-SNAP25 BrCN beads. The control was BrCN Sepharose beads alone. Note the presence of SNAP25 in Munc18 AC and of Munc18 (red asterisk) in SNAP25 AC. Coomassie-stained gels. Representative peptides matching the Munc18 mass spectrometry profile are shown. (E) AA (200 μM) does not disrupt the native link between Munc18 and syntaxin 1–SNAP25 in synaptic membranes as judged by anti-Munc18 immunoprecipitation (IP) and western immunoblotting. (F) Syntaxin 1 presence on GST–SNAP25 beads (left) (Coomassie-stained gel) allows Munc18 pull-down from bovine brain cytosol. Munc18 was identified by western immunoblotting (right). GST, glutathione S-transferase; SNAP25, synaptosomal-associated protein 25 kDa; SNARE, soluble NSF attachment protein receptors; Syb, synaptobrevin.
Previously, immunoprecipitation using the syntaxin antibody ‘hippocampus-1' (HPC-1) showed that native Munc18 does not co-purify with assembled SNAREs (Pevsner et al, 1994). However, this antibody cannot recognize Munc18-bound syntaxin 1, probably owing to occlusion of the binding epitope (Rickman & Davletov, 2005). Two recent studies indicated that exogenously added, recombinant GST–Munc18 binds to assembled SNARE complexes (Latham et al, 2007; Shen et al, 2007). As the existence of a native mammalian Munc18–SNARE association has not been shown, we investigated native Munc18 interactions using two alternative immunopreparative chromatographies. We covalently attached Munc18 or SNAP25 monoclonal antibodies to BrCN beads and incubated them with rat brain detergent extract. After extensive washing, bound protein was eluted from the antibodies using a low-pH solution. Analysis of eluates using SDS–polyacrylamide gel electrophoresis (SDS–PAGE; Fig 3D), followed by western immunoblotting (data not shown) and/or mass spectrometry, showed that SNAP25 co-purifies with Munc18-bound syntaxin 1 (lane 3) and, reciprocally, Munc18 can be found in SNAP25 isolates (lane 4), despite all syntaxin 1 being in an ‘open', SNAP25-bound conformation.
We next tested whether this link between native Munc18 and SNAREs can be disrupted by exogenously adding arachidonic acid. Intact synaptic membranes were pretreated with arachidonic acid for 30 min and membrane proteins were then solubilized. Munc18 was immunoprecipitated and syntaxin–SNAP25 detected by western immunoblotting (Fig 3E). As in the case of syntaxin alone (Fig 3A), arachidonic acid was not able to disrupt native Munc18–SNARE association. The above experiments showed that Munc18 can still associate with activated syntaxin, leading us to assess the ability of native Munc18 to bind to an exogenous syntaxin–SNAP25 heterodimer. We attached syntaxin 1 to GST–SNAP25 beads and incubated these beads, or GST–SNAP25 alone as a control, with bovine brain cytosol, which contains Munc18 but lacks membrane-associated SNAREs. Western immunoblotting of the bound material showed that Munc18 can attach to syntaxin 1–SNAP25 (Fig 3F), illustrating that syntaxin 1 interaction with SNAP25 does not necessarily prohibit cytosolic Munc18 association.
To test the wider applicability of our findings, we investigated the interaction of ubiquitous syntaxin 3 with native Munc18 in the presence of arachidonic acid. We first incubated GST–syntaxin 3 with bovine brain cytosol, and the bound Munc18 was determined by mass spectrometry to be Munc18-1 (Fig 4A; data not shown). Incubation of the GST–syntaxin 3–Munc18 beads with either arachidonic acid or 1 M NaCl led to dissociation of Munc18 only in the case of NaCl. The result showed that even in the case of this weaker syntaxin 3–Munc18 interaction, as compared with syntaxin 1 (Fig 3A), arachidonic acid still cannot disrupt it. After isolation of an equimolar syntaxin 3–Munc18 complex as for syntaxin 1 (supplementary Fig 2 online), we tested the role of Munc18 in syntaxin 3 activation. Bound Munc18 did not change the ability of arachidonic acid or ω-3 docosahexaenoic acid—another common polyunsaturated fatty acid—to stimulate SNARE assembly (Darios & Davletov, 2006), highlighting the fact that, in the presence of specific fatty acids, Munc18 is not inhibitory (Fig 4B). In addition, as in the case of syntaxin 1 (Fig 3C), syntaxin 3 and Munc18 bind simultaneously to GST–SNAP25 beads in the presence of docosahexaenoic acid (supplementary Fig 3 online).
Figure 4.
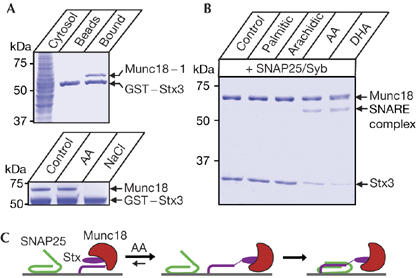
Arachidonic acid is able to activate syntaxin 3 in the presence of Munc18. (A) GST–syntaxin 3, on glutathione beads, binds to Munc18-1 from bovine brain cytosol (top). Syntaxin 3–Munc18 association is not disrupted by 100 μM arachidonic acid but is sensitive to 1 M NaCl, indicating an electrostatic interaction (bottom). Coomassie-stained gels. GST, glutathione S-transferase. (B) Syntaxin 3, in equimolar complex with Munc18, is activated for SNARE assembly by 100 μM arachidonic acid (AA) and docosahexaenoic acid (DHA, C22:6). Coomassie-stained gel. (C) Schematic diagram showing the effect of AA on the syntaxin–Munc18 binary complex. AA allows SNAP25 engagement by syntaxin without Munc18 dissociation. SNAP25, synaptosomal-associated protein 25 kDa; Stx, syntaxin.
Our current results provide a coherent picture of the initial steps of SNARE assembly underlying vesicle fusion. Syntaxins require Munc18 for conformational stabilization during transport and they initially will be in a tight union (Martinez-Arca et al, 2003; Toonen et al, 2005). When at the membrane, a transient increase in arachidonic acid concentration might trigger a rapid conformational change in syntaxin, without Munc18 elution, allowing tripartite assembly of Munc18–syntaxin–SNAP25 (Fig 4C). This scheme is confirmed here for both neuronal and ubiquitous syntaxins, and it is in line with the coexistence of Munc18 with syntaxin and SNAP25 on the plasma membrane, as reported in a recent imaging study (Zilly et al, 2006). It is important to note that Zilly et al (2006) based their conclusions on correlative immunostaining in PC12 cells, whereas our results show a direct physical link between brain Munc18 and assembled syntaxin–SNAP25 and also describe the potential role of arachidonic acid in vesicle fusion. After arachidonic acid action, continued association of Munc18 with assembled syntaxin–SNAP25 is consistent with the crucial role of Munc18 in downstream vesicle docking and fusion. It was found that addition of recombinant Munc18 to partly assembled SNAREs upregulates liposomal fusion (Shen et al, 2007), but it is known that syntaxins partner with Munc18 even during transport to membrane destinations—that is, ab initio. Whereas Shen et al (2007) coexpressed syntaxin and SNAP-25 in the absence of Munc18 in bacteria, obtaining a preformed SNARE intermediate, our data indicate that a dynamic transition can be made from ‘closed' syntaxin–Munc18 into an active SNAP25-bound configuration without loss of Munc18. This transition requires arachidonic acid, consistent with the activating role of this lipid in vesicle fusion.
It is likely that, in the tripartite complex, syntaxin–Munc18 exists in a structurally different mode compared with crystallized binary syntaxin–Munc18 (Misura et al, 2000; Shen et al, 2007). It has been suggested that arachidonic acid might either reveal another hypothetical site on Munc18 for binding of other SNARE complexes or cause a conformational change in syntaxin 1 (Latham et al, 2007). Our results resolve this issue in favour of the second hypothesis. We did not observe an arachidonic-acid-induced increase in the interaction between Munc18 and SNAREs (Fig 3E), as suggested previously (Latham et al, 2007), probably because we investigated the native association. In the brain extract, Munc18 binding to activated syntaxin seems to be substoichiometric (Fig 3D, lane 4), indicating that in vivo additional endogenous factors contribute to weakening of the syntaxin–Munc18 association.
The ability of arachidonic acid to activate efficiently syntaxin 1, syntaxin 3 and syntaxin 4, which are involved in vital cellular processes, identifies these plasma membrane isoforms as valid pharmaceutical targets for drug screening. The observed specificity of arachidonic acid is remarkable (Fig 1D), indicating that the products of phospholipase A2 action—arachidonic acid and lysophospholipid—activate vesicle fusion through complementary pathways. Although lysophospholipids tend to remain in the bilayer, increasing the membrane fusion propensity owing to their cone-shaped morphology (Rossetto et al, 2006), arachidonic acid can be released, facilitating local activation of membrane-embedded syntaxins and other intracellular targets.
Methods
Recombinant protein production. Recombinant proteins and their isolation have been described previously (Bajohrs et al, 2005). Briefly, SNAREs were produced in BL21 Escherichia coli and purified on glutathione Sepharose beads (GE Healthcare, Amersham, UK). Proteins were washed with buffer A (20 mM HEPES (pH 7.3), 100 mM NaCl) and eluted after thrombin cleavage. Proteins were further purified by gel filtration on a Superdex 200 column (GE Healthcare) equilibrated in buffer A.
Isolation of native Munc18. All procedures were carried out at 4°C. Brain cortex (10 g) was homogenized in 50 ml of 10 mM HEPES (pH 7.3) and 2 mM EDTA in the presence of complete protease inhibitors cocktail (Roche, Basel, Switzerland), in a glass–teflon homogenizer. Ionic strength was adjusted to 200 mM NaCl and the homogenate was centrifuged at 100,000 g for 1 h. Supernatant cytosol was rotated with GST–syntaxin beads for 1.5 h. Syntaxin–Munc18 was released by thrombin action and purified by gel filtration on a Superdex 200 column.
Immunoaffinity chromatographies from brain extract. Monoclonal antibodies against SNAP25 (clone SMI81) and Munc18–nSec1 (clone 31) were from Sternberger Monoclonals and BD Biosciences (San Jose, CA, USA), respectively. Antibodies were covalently coupled to BrCN Sepharose beads (GE Healthcare) according to the manufacturer's instructions. Triton X-100 extracts of rat brain or synaptic plasma membranes were prepared as described by Rickman & Davletov (2005) and transferred to columns containing antibody-coupled beads. After 2 h of rotations at 4°C, the beads were washed in buffer A in the presence of 0.1% Triton X-100. Bound protein was eluted by glycine-HCl (pH 2.5), 0.15 M NaCl and 0.1% Triton X-100 and, after neutralization, analysed by SDS–PAGE and mass spectrometry of individual protein bands, as described by Bajohrs et al (2005).
Protein reactions and fluorescence assay. All binding reactions were carried out in buffer A at 22°C with 1–2 μg of each protein. Fatty acids, prostaglandins and brain lysophosphatidylcholine were from Sigma (Poole, UK) and Biomol (Plymouth Meeting, PA, USA). Final protein reactions contained 2% dimethyl sulphoxide, used for preparation of control and all lipid solutions. Formation of SDS-resistant SNARE complex was quantified by monomeric SNAP25 depletion, as the SNARE complex has a complex migration pattern. Band intensities were quantified by using a 12-bit CCD camera in the ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA), and Quantity One software. Prism4 (GraphPad, San Diego, CA, USA) was used for statistical analysis. Binding of syntaxin (or syntaxin–Munc18) to GST–SNAP25 was described previously (Darios & Davletov, 2006). SDS–PAGE was carried out in 12% Ready gels (Bio-Rad). For intrinsic fluorescence measurements, SNARE proteins (0.15 mg/ml) in buffer A were added to a Hellma quartz cuvette and autofluorescence spectra were collected on Jobin-Yvon SPEX Fluoromax II fluorometer with excitation at 280 nm. Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures
References
- Bajohrs M, Darios F, Peak-Chew SY, Davletov B (2005) Promiscuous interaction of SNAP-25 with all plasma membrane syntaxins in a neuroendocrine cell. Biochem J 392: 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash AR (2001) Arachidonic acid as a bioactive molecule. J Clin Invest 107: 1339–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WJ, Chambers K, Doody A (2003) Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic 4: 214–221 [DOI] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ (1999) Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol 146: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Davletov B (2006) ω-3 and ω-6 fatty acids stimulate cell membrane expansion by acting on syntaxin3. Nature 440: 813–817 [DOI] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J (1999) A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J 18: 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Pevsner J, Burgoyne RD (2001) Control of fusion pore dynamics during exocytosis by Munc18. Science 291: 875–878 [DOI] [PubMed] [Google Scholar]
- Frye RA, Holz RW (1984) The relationship between arachidonic acid release and catecholamine secretion from cultured bovine adrenal chromaffin cells. J Neurochem 43: 146–150 [DOI] [PubMed] [Google Scholar]
- Garcia EP, Gatti E, Butler M, Burton J, De Camilli P (1994) A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc Natl Acad Sci USA 91: 2003–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Slaughter CA, Sudhof TC (1993) Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366: 347–351 [DOI] [PubMed] [Google Scholar]
- Latham CF, Osborne SL, Cryle MJ, Meunier FA (2007) Arachidonic acid potentiates exocytosis and allows neuronal SNARE complex to interact with Munc18a. J Neurochem 100: 1543–1554 [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S, Proux-Gillardeaux V, Alberts P, Louvard D, Galli T (2003) Ectopic expression of syntaxin1 in the ER redirects TI-VAMP- and cellubrevin-containing vesicles. J Cell Sci 116: 2805–2816 [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI (2000) Three-dimensional structure of the neuronal-Sec1–syntaxin1a complex. Nature 404: 355–362 [DOI] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G (1994) Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol 13: 1–8 [DOI] [PubMed] [Google Scholar]
- Novick P, Ferro S, Schekman R (1981) Order of events in the yeast secretory pathway. Cell 25: 461–469 [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, Scheller RH (1994) Specificity and regulation of a synaptic vesicle docking complex. Neuron 13: 353–361 [DOI] [PubMed] [Google Scholar]
- Rickman C, Davletov B (2005) Arachidonic acid allows SNARE complex formation in the presence of Munc18. Chem Biol 12: 545–553 [DOI] [PubMed] [Google Scholar]
- Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C (2005) Equivalent effects of snake PLA2 neurotoxins and lysophospholipid–fatty acid mixtures. Science 310: 1678–1680 [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Broadie K (2005) Lipid regulation of the synaptic vesicle cycle. Nat Rev Neurosci 6: 139–150 [DOI] [PubMed] [Google Scholar]
- Rossetto O, Morbiato L, Caccin P, Rigoni M, Montecucco C (2006) Presynaptic enzymatic neurotoxins. J Neurochem 97: 1534–1545 [DOI] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128: 183–195 [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418 [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395: 347–353 [DOI] [PubMed] [Google Scholar]
- Toonen RF, de Vries KJ, Zalm R, Sudhof TC, Verhage M (2005) Munc18-1 stabilizes syntaxin1, but is not essential for syntaxin1 targeting and SNARE complex formation. J Neurochem 93: 1393–1400 [DOI] [PubMed] [Google Scholar]
- Toonen RF, Kochubey O, de Wit H, Gulyas-Kovacs A, Konijnenburg B, Sorensen JB, Klingauf J, Verhage M (2006) Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J 25: 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287: 864–869 [DOI] [PubMed] [Google Scholar]
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Sudhof TC, Neher E, Verhage M (2001) Munc18-1 promotes large dense-core vesicle docking. Neuron 31: 581–591 [DOI] [PubMed] [Google Scholar]
- Wenk MR, De Camilli P (2004) Protein–lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA 101: 8262–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Errington ML, Lynch MA, Bliss TV (1989) Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature 341: 739–742 [DOI] [PubMed] [Google Scholar]
- Wolf BA, Pasquale SM, Turk J (1991) Free fatty acid accumulation in secretagogue-stimulated pancreatic islets and effects of arachidonate on depolarization-induced insulin secretion. Biochemistry 30: 6372–6379 [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC Jr, Scheller RH (2000) nSec1 binds a closed conformation of syntaxin1A. J Cell Biol 148: 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilly FE, Sorensen JB, Jahn R, Lang T (2006) Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS Biol 4: e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


