Abstract
In mammals, immediate-early transcription of the Period 1 (Per1) gene is crucial for resetting the mammalian circadian clock. Here, we show that CLOCK is a real signalling molecule that mediates the serum-evoked rapid induction of Per1 in fibroblasts through the Ca2+-dependent protein kinase C (PKC) pathway. Stimulation with serum rapidly induced nuclear translocation, heterodimerization and Ser/Thr phosphorylation of CLOCK just before the surge of Per1 transcription. Serum-induced CLOCK phosphorylation was abolished by treatment with PKC inhibitors but not by other kinase inhibitors. Consistently, the interaction between CLOCK and PKC was markedly increased shortly after serum shock, and the Ca2+-dependent PKC isoforms PKCα and PKCγ phosphorylated CLOCK in vitro. Furthermore, phorbol myristic acetate treatment triggered immediate-early transcription of Per1 and also CLOCK phosphorylation, which were blocked by a Ca2+-dependent PKC inhibitor. These findings indicate that CLOCK activation through the Ca2+-dependent PKC pathway might have a substantial role in phase resetting of the circadian clock.
Keywords: circadian rhythm, CLOCK, phase shift, phosphorylation, PKC
Introduction
The circadian clock is an intrinsic timing device that typically runs with a period of about 24 h. Its main function is to optimize metabolic and physiologic behaviour in anticipation of cyclic changes in environmental conditions. The phase of the day and night cycle changes depending on the position of the Earth in its orbit around the sun; therefore, periodic resetting of the biological clock is required not only to maintain accurate timekeeping, but also to synchronize circadian rhythms with the solar day (Reppert & Weaver, 2002; Schibler & Sassone-Corsi, 2002). Although the phase resetting of the master clock in the suprachiasmatic nucleus (SCN) and the peripheral clocks throughout the body is regulated by different input signals, their downstream pathways are known to converge on the same, or similar, intracellular signalling cascades (Balsalobre et al, 2000; Yagita et al, 2001). The most prominent molecular event that leads to clock resetting is a rapid surge of Period 1 (Per1) transcription in both the SCN and peripheral tissues. Furthermore, in cultured cell lines, diverse extracellular stimuli, including serum shock, can trigger immediate-early transcription of Per1, and this in turn seems to elicit a robust circadian oscillation of clock gene expression (Akashi & Nishida, 2000; Balsalobre et al, 2000; Yagita et al, 2001).
Intensive genetic studies on Clock mutants have provided an insight into the role of CLOCK in phase resetting. The homozygous Clock mutation significantly reduces the amplitude of the photic induction of Per genes in the SCN and abolishes the light-induced phase shift of behavioural rhythm (Vitaterna et al, 1994; Shearman & Weaver, 1999; Low-Zeddies & Takahashi, 2001). Although heterozygous Clock mutants maintained circadian rhythmicity under constant darkness, they were defective in phase shifting in response to both photic and non-photic stimuli (Challet et al, 2000; Low-Zeddies & Takahashi, 2001). In addition, we have recently shown that overexpression of CLOCKΔ19, a dominant-negative form of CLOCK, reduces the serum-evoked surge of Per1 transcription without any significant effect on other immediate-early genes (Jung et al, 2003). Thus, CLOCK seems to be important in resetting the circadian clock and also in maintaining circadian gene oscillation. In this report, we provide the first evidence for the direct involvement of the core clock component CLOCK in the signalling pathways responsible for resetting the circadian rhythm.
Results And Discussion
Serum shock induces nuclear translocation of CLOCK
Most transcription factors that belong to the basic helix–loop–helix (bHLH)/PER-AHR/ARNT-SIM (PAS) family are crucial components of the signal-transduction pathways that allow organisms to adapt to environmental changes (Kewley et al, 2004). The CLOCK/brain and muscle Arnt-like protein-1 (BMAL1) heterodimer is a bHLH/PAS family transcription factor complex that regulates clock gene expression in response to the light and dark cycle, but its role in the signal-transduction pathway has not been described. Phylogenetic and structural analyses indicate that CLOCK is a Class I sensing factor and its partner BMAL1 is a Class II factor (Gu et al, 2000). The heterodimer that they form specifically recognizes the E-box located in the upstream regulatory regions of target genes, such as Per1, leading to transcriptional initiation. It is, therefore, reasonable to postulate that CLOCK is involved in the detection of, and adaptation to, changes in environmental conditions. To test this hypothesis, we examined the effect of serum shock (50% horse serum) on the subcellular distributions of CLOCK and BMAL1, and their interaction in NIH3T3 cells. Subcellular fractionation and Western blot analysis showed that CLOCK molecules rapidly translocated to the nucleus without significant alteration of the distribution of BMAL1 (Fig 1A; supplementary Fig 1 online). These observations were confirmed by immunocytochemistry (Fig 1B). The diffuse signal of CLOCK throughout the cells was concentrated in the nucleus shortly after serum shock (30 min), whereas BMAL1 showed a predominantly nuclear distribution regardless of serum treatment. We recently showed that BMAL1 has a functional nuclear localization signal (NLS) at its amino terminus, whereas CLOCK has no such signal (Kwon et al, 2006). Thus, heterodimerization with BMAL1 seems to be required for the rapid nuclear accumulation of CLOCK in response to serum. In favour of this idea, serum treatment markedly increased the amount of BMAL1 co-precipitated with CLOCK antibody and vice versa (Fig 1C; supplementary Fig 1 online). These results indicate that serum shock induces rapid nuclear translocation of CLOCK through heterodimerization with BMAL1.
Figure 1.
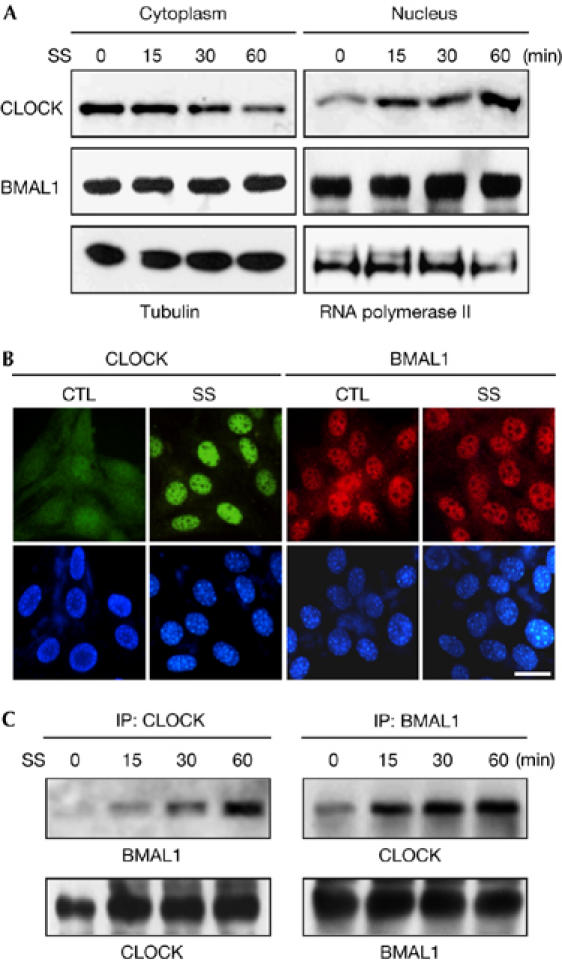
Serum shock induces nuclear translocation and heterodimerization of CLOCK. (A) NIH3T3 cells were divided into cytoplasmic and nuclear fractions at the indicated time points after serum shock (SS) and subjected to immunoblotting by using specific antibodies for the indicated proteins. (B) Representative fluorescence images showing the nuclear accumulation of CLOCK. Upper panels show cellular distribution of CLOCK (green) and BMAL1 (red) in the cells before and after serum shock (30 min). DAPI staining (lower panels) is shown to define the nuclei. Scale bar, 25 μm. CTL, control. (C) The protein–protein interaction between CLOCK and BMAL1 is increased after serum shock in the cells. Duplicate whole-cell lysates were immunoprecipitated (IP) with anti-CLOCK and anti-BMAL1, respectively and immunoblotted with both antibodies. BMAL1, brain and muscle Arnt-like protein 1; DAPI, 4,6-diamidino-2-phenylindole.
PKC mediates the serum-evoked CLOCK phosphorylation
If CLOCK is a real signalling molecule modulating the circadian clock, it ought to be activated by post-translational modification in response to the resetting stimuli. Therefore, we conducted prolonged gel electrophoresis to examine the post-translational modification of CLOCK, and found transient but marked changes in its electrophoretic mobility (Fig 2A). The slowly migrating bands peaked at 30–60 min after serum shock and then gradually decreased. When the CLOCK immunoprecipitates were treated with λ protein phosphatase, the high-molecular-weight forms of CLOCK were reduced by more than 90%, and the addition of vanadate completely blocked this effect (Fig 2B, upper panel). In the same blot, the phospho-Ser antibody showed strong signals overlapping with the slow-migrating bands (Fig 2B, lower panel); the phospho-Thr antibody gave similar results (data not shown). These observations show that serum shock rapidly induces Ser/Thr phosphorylation of CLOCK in cultured fibroblasts.
Figure 2.
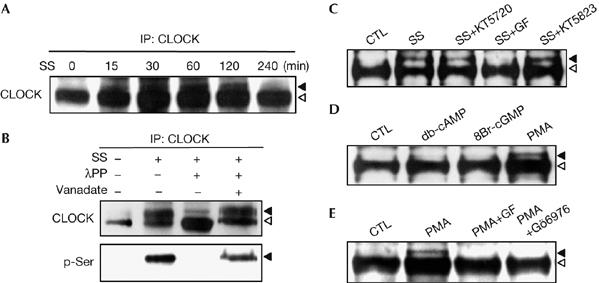
Protein kinase C-dependent phosphorylation of CLOCK. (A) Time-course changes of the electrophoretic mobility of CLOCK. CLOCK immunoprecipitates (IP) were prepared at the indicated time points after serum shock (SS), separated by long SDS–PAGE on 6% gels and visualized by immunoblotting with anti-CLOCK. Arrowheads indicate the slow-migrating band (black) and the fast-migrating band (white). (B) CLOCK immunoprecipitates from the cells before and after serum shock (30 min) were incubated with λ protein phosphatase (λPP) in the absence or presence of vanadate (Na3VO4) and were assessed by immunoblotting with the indicated antibodies. (C) The effect of various kinase inhibitors on CLOCK phosphorylation induced by serum shock. Cells were treated with 0.5 μM KT5720, 5 μM GF109203X (GF) or 1 μM KT5823 1 h before serum shock. Arrowheads indicate the phosphorylated form (black) and the naked form (white). (D) The effect of diverse kinase activators on phosphorylation of CLOCK. Cells were treated with 1 mM db-cAMP, 1 mM 8Br-cGMP or 1 μM PMA for 30 min and whole-cell lysates were subjected to immunoblotting with anti-CLOCK. (E) Characterization of PMA-induced CLOCK phosphorylation. Cells were treated with 5 μM GF109203X (GF) or 5 μM Gö6976 1 h before incubation with PMA for 30 min and analysed as described in (D). CTL, control; PMA, phorbol myristic acetate; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis.
To identify the Ser/Thr kinase(s) responsible for the CLOCK phosphorylation, we used various kinase inhibitors and activators. Pretreatment of cells with GF109203X, a PKC inhibitor, completely prevented serum-induced CLOCK phosphorylation, whereas other kinase inhibitors including the PKA inhibitor KT5720 and the PKG inhibitor KT5823 failed to do so (Fig 2C). Furthermore, treatment with the PKC activator phorbol myristic acetate (PMA) caused an electrophoretic mobility shift similar to serum shock, whereas neither the PKA activator db-cAMP nor the PKG activator 8-Br-cGMP had this effect (Fig 2D). Therefore, the PKC pathway seems to mediate the rapid phosphorylation of CLOCK induced by serum.
PKC comprises a family of isoenzymes that differ in Ca2+ requirement, substrate specificity and cell–tissue-specific expression. To identify the PKC pathway that regulates CLOCK phosphorylation, we tested the effects of GF109203X, a broad-specificity PKC inhibitor, and Gö6976, a specific inhibitor of the classic PKC that requires Ca2+ as co-activator. As shown in Fig 2E, both drugs eliminated PMA-induced CLOCK phosphorylation, indicating that CLOCK phosphorylation is mediated by the Ca2+-dependent PKC pathway. Consistently, immunoprecipitation analysis using an antibody specific for the classic PKC together with CLOCK antibody showed a marked increase in the interaction between CLOCK and PKC after serum shock (Fig 3A). To determine whether CLOCK acts as a substrate for PKC and, if so, which PKC isoform is responsible, we carried out in vitro PKC assays. We first generated histidine-tagged CLOCK by using a cell-free protein synthesis system and incubated it with purified classic PKC in the presence and absence of Gö6976. The full-length CLOCK was readily phosphorylated by the PKC, which was completely blocked by the competitive PKC inhibitor (Fig 3B). Next, we carried out kinase assays for each isoform of the classic PKC family. Of the four isoforms both PKCα and PKCγ labelled CLOCK with [γ-32P]ATP, but PKCγ was more effective than PKCα (Fig 3C). Importantly, these PKC isoforms were highly expressed in the SCN (Van der Zee & Bult, 1995), and their messenger RNA levels peaked during early and/or late subjective night—that is, the permissive time period for phase resetting (Cagampang et al, 1998). Furthermore, inhibition of the PKC pathway abrogated the phase shift of SCN activity induced by melatonin and neuropeptide Y, whereas activation of the PKC pathway advanced circadian neuronal activity in the SCN (Biello et al, 1997; McArthur et al, 1997). Our results therefore provide an insight into the possible role of PKC in phase resetting of the mammalian central clock.
Figure 3.

Phosphorylation of CLOCK by Ca2+-dependent protein kinase C isoforms in vitro. (A) Serum-induced interaction between CLOCK and PKC. Cells were exposed to serum-rich medium for 30 min and then subjected to immunoprecipitation (IP) with CLOCK and PKC antibodies, followed by immunoblotting with both antibodies. (B) Histidine-tagged recombinant CLOCK was purified and subjected to in vitro PKC kinase assay in the absence or presence of 5 μM Gö6976. The reaction mixture was resolved by SDS–PAGE and transferred to a polyvinylidene fluoride membrane. The membrane was exposed to X-ray film for 24 h (upper panel). The same blot was then probed with CLOCK antibodies (lower panel). (C) Isoform specific kinase assays were carried out as described in (B). CTL, control; cPKC, classic PKC; PKC, protein kinase C; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis; SS, serum shock.
PKC mediates Per1 activation and phase resetting
To investigate whether PKC-mediated CLOCK phosphorylation is important in the immediate-early response of Per1, we assessed the effects of PKC activation on the DNA-binding activity of CLOCK using chromatin immunoprecipitation (ChIP) assays (Fig 4A; supplementary Fig 2 online). PMA treatment enhanced the binding of both CLOCK and BMAL1 to E-box elements of the Per1 promoter in NIH3T3 fibroblasts and these effects were blocked by pretreatment with Gö6976 and GF109203X. Furthermore, Re-ChIP assays with CLOCK and phospho-Ser antibodies showed that the PMA-induced DNA-binding activity of CLOCK was mediated by the PKC pathway. Next, we evaluated the effects of PMA on Per1 gene expression in the cells (Fig 4B). Per1 mRNA levels increased rapidly and reached a maximum as early as 1 h after the drug treatment; pretreatment with Gö6976 completely abrogated it. These data indicate that CLOCK is an effector in the PKC signalling cascade that leads to rapid induction of Per1 mRNA.
Figure 4.
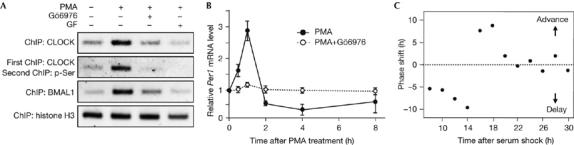
Involvement of protein kinase C-mediated CLOCK phosphorylation in the rapid activation of Per1 and phase resetting of the molecular clock. (A) Chromatin was extracted from NIH3T3 cells that were treated with 1 μM PMA for 1 h in the presence and absence of Gö6976 or GF109203X (GF). ChIP assays were carried out with specific antibodies for the indicated proteins. Re-ChIP experiments were carried out with a two-step immunoprecipitation with CLOCK and phospho-Ser antibodies (pSer). The association of each protein with the Per1 promoter was analysed by PCR using a primer set flanking the proximal E-box. ChIP data of histone H3 are shown as a control. (B) Cells were treated with 1 μM PMA for 2 h, and the medium was replaced with serum-free medium. A set of cells was incubated with Gö6976 for 1 h before treatment with PMA. Per1 messenger RNA levels were determined by reverse transcription–PCR and normalized with those of G3PDH. Data are shown as means±s.e.m. of three independent experiments. (C) Phase responses to a 15 min PMA pulse. NIH3T3 cells stably transfected with a plasmid containing the Per1-dsLuc were synchronized by serum shock and then treated with PMA for 15 min at various time points. The Per1-dsLuc activities were monitored continuously using a real-time measuring system and the resulting shift in the second peak time of the bioluminescence was expressed as phase shift of the molecular clock. BMAL1, brain and muscle Arnt-like protein 1; ChIP, chromatin immunoprecipitation; PMA, phorbol myristic acetate.
To verify the role of PKC signalling in phase resetting, we first generated genetically modified NIH3T3 cells expressing Per1 promoter-controlled destabilized luciferase reporter (Per1-dsLuc) and showed that serum shock induced robust oscillation of the bioluminescence with an approximate 24 h periodicity in these cells (supplementary Fig 3A online). Then, at various time points after serum shock, the effects of a 15 min PMA pulse on the phase shift and immediate-early response of Per1 gene expression were determined in the cells (Fig 4C; supplementary Fig 3B online). Depending on the circadian time point at which PMA was applied, both phase shift and Per1 mRNA induction varied. This indicates that activation of the PKC pathway can indeed act as a phase-resetting signal of the molecular clock and also as an inducer of Per1 gene expression in cultured fibroblasts. Recently, CLOCK-deficient mice have been established and characterized (Debruyne et al, 2006). Surprisingly, but consistent with our present hypothesis, these animals showed abnormal responses to light stimuli but continued to show robust circadian rhythms of locomotor activity similar to wild-type animals. Together, these results further emphasize the role of CLOCK in the resetting of the circadian clock rather than in generating circadian rhythmicity.
CLOCK activation is independent of the CREB pathway
It is widely accepted that cAMP response element-binding protein (CREB) seems to mediate the rapid induction of Per gene expression, as it is rapidly phosphorylated by the light stimulus in the SCN (Ginty et al, 1993), and both Per1 and Per2 carry a canonical CRE in their promoter (Travnickova-Bendova et al, 2002). This prompted us to test the possibility that activation of the PKC pathway also affects CREB activation. Indeed, treatment with PMA stimulated phosphorylation of CREB and also CLOCK (Fig 5A). However, the Ca2+-dependent PKC inhibitor Gö6976 did not prevent CREB phosphorylation, whereas PD98059, a MAPK kinase (MEK) inhibitor, completely eliminated it without significant effect on CLOCK phosphorylation and Per1 expression (Fig 5A,B). Furthermore, PMA augmented CLOCK/BMAL1-induced Per1 transcription in a PKC-dependent manner. As shown in Fig 5C, treatment with PMA significantly elevated CLOCK/BMAL1-induced Per1 promoter activity, which was blocked by Gö6976 but not by PD98059. PMA alone also increased Per1 promoter activity, presumably reflecting phosphorylation of endogenous CLOCK. Thus, the Ca2+-dependent PKC pathway seems to be crucial in both CLOCK phosphorylation and Per1 transcription.
Figure 5.
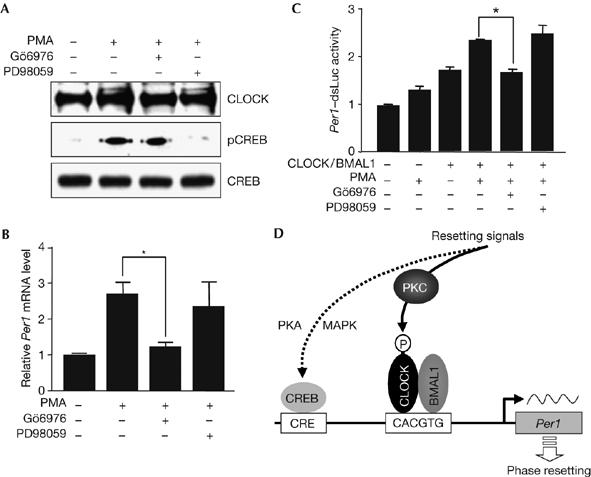
Characterization of the signal-transduction pathways leading to the rapid induction of Per1. (A) Cells were stimulated with 1 μM PMA for 30 min in the absence or presence of Gö6976 and PD98059, and subjected to immunoblotting with the indicated antibodies. (B) Under the same conditions, messenger RNA levels of Per1 were determined as described in Fig 4B (*P<0.05). (C) Cells were transiently transfected with Per1-dsLuc reporter construct with or without plasmids expressing CLOCK and BMAL1. One day after serum depletion, they were treated with PMA for 4 h. Inhibitors were added 1 h before the addition of PMA. The resulting luciferase activities were expressed as mean fold±s.e.m. of the control group (*P<0.01). (D) Schematic model for the rapid induction of Per1 gene expression by resetting signals. The immediate-early response of the Per1 gene is mediated by independent signalling pathways. The Ca2+-dependent PKC pathway leads to CLOCK activation, whereas the PKA and MAPK pathways converge on CREB. BMAL1, brain and muscle Arnt-like protein 1; CRE, cAMP response element; CREB, cAMP response element-binding protein; MAPK, MAP kinase; PKC, protein kinase C; P, phosphate; PMA, phorbol myristic acetate.
Intuitively, rapid activation of CREB alone cannot account for the induction of the immediate-early response of the Per genes as CREB activation causes rapid activation of only a few genes, including c-fos, Per1 and Per2, despite the existence of various genes containing functional CREs in their upstream regulatory regions. Furthermore, there are some kinetic differences in the expression profiles of c-fos and the Per genes. It is therefore likely that the induction of Per gene expression does not depend on CREB activation, alone. Indeed, the promoters of Per1 and Per2 contain the configuration of CRE and E-boxes appropriate for cooperative action as shown in other systems (Benjanirut et al, 2006), and combined treatment with forskolin and PMA synergistically enhanced Per1 promoter activity (supplementary Fig 4 online). This synergism further explains the previous study showing that serum shock evokes selective transcription of Per1 and Per2 among CLOCK/BMAL1-controlled genes (Balsalobre et al, 1998). On the basis of these observations, we propose a schematic model for the rapid induction of Per1 gene expression triggered by the resetting signals (Fig 5D).
In conclusion, we have provided the first evidence that CLOCK is phosphorylated by Ca2+-dependent PKC isoforms immediately after the application of extracellular stimuli that lead to synchronization of individual cellular clocks. This phosphorylation-dependent CLOCK activation is essential for the rapid surge of Per1 transcription and also for activation of circadian gene expression in cultured cells. Furthermore, our results strongly indicate that CLOCK is a real signalling molecule mediating the resetting of the circadian clock in a process distinct from its role in the positive limb of the core clock feedback loop. In the SCN, this type of clock-specific signalling mechanism would be applicable to the selective induction of Per gene expression among the diverse CREB target genes that can be triggered by Ca2+ influx.
Methods
Experimental procedures are provided in detail in the supplementary information online.
Cell culture and transient transfection. NIH3T3 and mouse embryonic fibroblast cells were cultivated in DMEM supplemented with 10% fetal bovine serum (FBS). For drug application, confluent cells were serum-starved (1% FBS) for 1 day before treatment. DNA transfections were carried out using Lipofectamine plus reagents (Invitrogen, Carlsbad, CA, USA) and reporter gene activity was measured using the dual luciferase assay reagents (Promega, Madison, WI, USA).
Synthesis of CLOCK protein and protein kinase C kinase assay. Histidine-tagged CLOCK protein was prepared in a cell-free protein synthesis system and purified with a commercial histidine-Bind kit (Novagen, Darmstadt, Germany). In vitro PKC kinase assays were carried out at 30°C for 15 min with [γ-32P]-ATP (10 μCi) and purified rat brain PKC (Promega)/recombinant PKC isozymes (Sigma, St Louis, MO, USA) in the presence or absence of a PKC inhibitor (Gö6976, 5 μM).
Chromatin immunoprecipitation assay. ChIP assays were carried out using a commercial ChIP assay kit (Upstate Biotech, Chicago, IL, USA) according to the manufacturer's instructions. The primer sets used for ChIP assays are provided as supplementary information online.
Real-time measurement of bioluminescence. Cells were transfected with 0.5 μg of the full-length Per1 promoter (5.7 kb) fused with a destabilized firefly luciferase (dsLuc) and preincubated in serum-free DMEM for 24 h. The medium was then replaced with 3 ml of DMEM supplemented with 10% FBS, 10 mM HEPES (pH 7.4) and 0.1 mM luciferin (Ueda et al, 2002). GF109203X (5 μM) was added 1 h before the serum treatment. Bioluminescence was measured with photomultiplier tube detector assemblies (AB-2500 Kronos, ATTO, Tokyo, Japan). Photon counts were integrated at 1 min intervals. Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by the Brain Research Center of the 21st Century Frontier Program in Neuroscience and the Biotool R&D Project for Cell Research from the Korean Ministry of Science and Technology. K.H.L. was supported by the BK 21 program from the Korea Ministry of Education.
References
- Akashi M, Nishida E (2000) Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev 14: 645–649 [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Marcacci L, Schibler U (2000) Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 10: 1291–1294 [DOI] [PubMed] [Google Scholar]
- Benjanirut C, Paris M, Wang WH, Hong SJ, Kim KS, Hullinger RL, Andrisani OM (2006) The cAMP pathway in combination with BMP2 regulates Phox2a transcription via cAMP response element binding sites. J Biol Chem 281: 2969–2981 [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Schak KM, Harrington ME (1997) Circadian phase shifts to neuropeptide Y in vitro: cellular communication and signal transduction. J Neurosci 17: 8468–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagampang FR, Rattray M, Campbell IC, Powell JF, Coen CW (1998) Variation in the expression of the mRNA for protein kinase C isoforms in the rat suprachiasmatic nuclei, caudate putamen and cerebral cortex. Mol Brain Res 53: 277–284 [DOI] [PubMed] [Google Scholar]
- Challet E, Takahashi JS, Turek FW (2000) Nonphotic phase-shifting in clock mutant mice. Brain Res 859: 398–403 [DOI] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM (2006) A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 50: 465–477 [DOI] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME (1993) Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 260: 238–241 [DOI] [PubMed] [Google Scholar]
- Gu YZ, Hogenesch JB, Bradfield CA (2000) The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 40: 519–561 [DOI] [PubMed] [Google Scholar]
- Jung H, Choe Y, Kim H, Park N, Son GH, Khang I, Kim K (2003) Involvement of CLOCK:BMAL1 heterodimer in serum-responsive mPer1 induction. Neuroreport 14: 15–19 [DOI] [PubMed] [Google Scholar]
- Kewley RJ, Whitelaw ML, Chapman-Smith A (2004) The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol 36: 189–204 [DOI] [PubMed] [Google Scholar]
- Kwon I, Lee J, Chang SH, Jung NC, Lee BJ, Son GH, Kim K, Lee KH (2006) BMAL1 shuttling controls transactivation and degradation of the CLOCK/BMAL1 heterodimer. Mol Cell Biol 26: 7318–7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low-Zeddies SS, Takahashi JS (2001) Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell 105: 25–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AJ, Hunt AE, Gillette MU (1997) Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology 138: 627–634 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418: 935–941 [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P (2002) A web of circadian pacemakers. Cell 111: 919–922 [DOI] [PubMed] [Google Scholar]
- Shearman LP, Weaver DR (1999) Photic induction of Period gene expression is reduced in Clock mutant mice. Neuroreport 10: 613–618 [DOI] [PubMed] [Google Scholar]
- Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P (2002) Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA 99: 7728–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S (2002) A transcription factor response element for gene expression during circadian night. Nature 418: 534–539 [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Bult A (1995) Distribution of AVP and Ca2+-dependent PKC-isozymes in the suprachiasmatic nucleus of the mouse and rabbit. Brain Res 701: 99–107 [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, van Der Horst GT, Okamura H (2001) Molecular mechanisms of the biological clock in cultured fibroblasts. Science 292: 278–281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


