Abstract
The endocycle is a developmentally programmed variant cell cycle in which cells undergo repeated rounds of DNA replication with no intervening mitosis. In Drosophila, the endocycle is driven by the oscillations of Cyclin E/Cdk2 activity. How the periodicity of Cyclin E/Cdk2 activity is achieved during endocycles is poorly understood. Here, we demonstrate that the p21cip1/p27kip1/p57kip2-like cyclin-dependent kinase inhibitor (CKI), Dacapo (Dap), promotes replication licensing during Drosophila endocycles by reinforcing low Cdk activity during the endocycle Gap-phase. In dap mutants, cells in the endocycle have reduced levels of the licensing factor Double Parked/Cdt1 (Dup/Cdt1), as well as decreased levels of chromatin-bound minichromosome maintenance (MCM2–7) complex. Moreover, mutations in dup/cdt1 dominantly enhance the dap phenotype in several polyploid cell types. Consistent with a reduced ability to complete genomic replication, dap mutants accumulate increased levels of DNA damage during the endocycle S-phase. Finally, genetic interaction studies suggest that dap functions to promote replication licensing in a subset of Drosophila mitotic cycles.
Keywords: Cdt1, dacapo , Drosophila , endoreplication, p27
Introduction
The endocycle, also known as the endoreplicative cycle, is a variant cycle used by both plants and animals to increase cell size and ploidy (Edgar and Orr-Weaver, 2001; Lilly and Duronio, 2005). During endocycle, cells undergo repeated rounds of DNA replication without intervening mitosis. Recently, considerable progress has been made in understanding how the core cell cycle machinery is modified to convert a mitotic cycle to an endocycle (Sigrist and Lehner, 1997; Shcherbata et al, 2004). Entry into the endocycle is contingent on the downregulation of the mitotic kinase Cdk1. Indeed, in Drosophila, removing the mitotic cyclins or the mitotic kinase Cdk1 shunts cells programmed to be in the mitotic cycle into a self-sustaining endocycle (Sauer et al, 1995; Hayashi, 1996; Weigmann et al, 1997). However, relative to the mitotic cycle, our basic understanding of the biochemical oscillator that drives the endocycle remains limited.
Current data suggest that the Drosophila endocycle is driven by the oscillations of Cyclin E/Cdk2 activity (Edgar and Orr-Weaver, 2001; Lilly and Duronio, 2005). In Drosophila, Cyclin E/Cdk2 activity is required for DNA replication during endocycle and the mitotic cycle (Lane et al, 1996). Paradoxically, continuous overexpression of Cyclin E inhibits endocycle progression (Follette et al, 1998; Weiss et al, 1998). These data suggest that cells in the endocycle require a Gap-phase when overall Cyclin E/Cdk2 activity is low, in order to relicense DNA replication origins for successive rounds of DNA replication. An important factor controlling the periodicity of Cyclin E/Cdk2 activity is the regulated accumulation and destruction of the Cyclin E protein (Moberg et al, 2001). However, the exact nature of the regulator inputs that drive the oscillations of Cyclin E/Cdk2 activity during the endocycle is poorly defined.
Perfect duplication of the genome depends on two sequential steps of DNA replication having opposite requirements for Cdk activity (Bell and Dutta, 2002; Diffley and Labib, 2002). Whereas the formation of pre-replication complexes (pre-RCs) or licensing of the DNA, requires low Cdk activity, the initiation of DNA replication is triggered by high Cdk activity. Pre-RCs are formed in late mitosis and G1 when the origin recognition complex recruits Cdt1 and Cdc6, which load the minichromosome maintenance (MCM2–7) complex onto the origin (reviewed in Bell and Dutta, 2002). In Drosophila, Cdt1 is referred to as Double Parked (Dup) (Whittaker et al, 2000). It has been shown in multiple organisms that increasing Cdk activity during the Gap-phase, by misexpressing G1 cyclins, inhibits pre-RC formation, resulting in genomic instability (Spruck et al, 1999; Lengronne and Schwob, 2002; Tanaka and Diffley, 2002; Ekholm-Reed et al, 2004b). Thus, precise regulation of Cdk activity is critical to the faithful duplication of the genome.
The dacapo (dap) gene encodes a p21CIP/p27KIP1/p57KIP2-like cyclin-dependent kinase inhibitor that inhibits the activity of Cyclin E/Cdk2 complexes in Drosophila (de Nooij et al, 1996, 2000; Lane et al, 1996). In dap-null alleles, which are embryonic lethal, cells of the epidermis progress through an extra embryonic division cycle (de Nooij et al, 1996; Lane et al, 1996). Thus, as observed with other cyclin-dependent kinase inhibitors (CKIs), dap functions to coordinate cell cycle exit with terminal differentiation. However, a positive role has been proposed for dap in the regulation of the endocycle (de Nooij et al, 2000; Edgar and Orr-Weaver, 2001). Central to this proposal are the following two observations. First, Dap oscillations closely follow those of Cyclin E during the endocycle in the polyploid nurse cells of the ovary (de Nooij et al, 2000). Second, in many tissues of Drosophila, Cyclin E positively influences the accumulation of Dap (de Nooij et al, 2000). These two findings suggest a possible feedback loop in which increased Cyclin E promotes DNA replication, as well as accumulation of Dap. Ultimately, Dap levels may go high enough to inhibit Cyclin E/Cdk2 activity, which either directly and/or indirectly brings S-phase to a halt, shunting cells into the Gap-phase. A similar role has been proposed for the mammalian Cdk inhibitor p57KIP2 during the endocycle of mammalian trophoblasts (Hattori et al, 2000). In the giant trophoblasts of the placenta, the levels of p57KIP2 oscillate with cell cycle kinetics similar to that of Dap in the polyploid nurse cells. Thus, the oscillation of one or more CKIs may be a common feature of endocycles in multiple species.
In the Drosophila ovary, both germ-line derived nurse cells and somatic follicle cells enter the endocycle and become polyploid. Drosophila oogenesis takes place within a 16-cell interconnected cyst. However, only one of the 16 cells proceeds through meiosis and becomes a viable gamete, whereas the other 15 cells in the cyst enter the endocycle and develop as highly polyploid nurse cells. Individual egg chambers are produced when somatically derived follicle cells encapsulate the ovarian cysts. The follicle cells continue to divide mitotically until mid-oogenesis (stage 6), when they synchronously exit the mitotic cycle and enter the endocycle in a process requiring the Notch signaling pathway (Deng et al, 2001; Lopez-Schier and St Johnston, 2001). Because the complete process of polyploidization can be followed in two independent cell types, Drosophila oogenesis provides an excellent model system to examine mechanisms of endocycle regulation.
Here, we present evidence that the CKI Dap promotes the accumulation of Dup/Cdt1 and licensing of DNA replication origins during Drosophila endocycles. In the absence of Dap, cells in the endocycle have low levels of Dup/Cdt1, as well as dramatically reduced levels of chromatin-bound MCM2–7 complex. Moreover, upon entering the endocycle, dap−/− cells accumulate DNA damage, consistent with the number of pre-RCs assembled during the endocycle Gap-phase being below the threshold required to complete genomic replication. Finally, reducing the dosage of dup/cdt1 in a dap−/− background reveals a possible role for Dap in replication licensing during the mitotic cycle. These studies represent one of the first reports of a CKI acting to promote replication licensing in a metazoan.
Results
Dap influences endoreplication in the polyploid nurse cells
To explore the role of Dap in endoreplication in Drosophila, we used FLP/FRT-mediated mitotic recombination (Xu and Rubin, 1993) to generate dap−/− mutant clones using the dap4 allele. The dap4 allele is an amorph, which contains a deletion of the conserved Cdk binding domain (Lane et al, 1996). In developing wild-type egg chambers, the 15 polyploid nurse cells either have equal amounts of DNA, as measured by DAPI staining, or, in some stages of oogenesis, the posterior nurse cells are one or two endocycles ahead of the anterior nurse cells (Figure 1A; reviewed in Spradling, 1993). In Figure 1B, we used the FLP/FRT system to create an egg chamber in which all 16 cells within the germ-cyst are dap−/−. Note that the egg chamber containing the dap−/− germ-line clone has a posterior nurse cell, indicated by an arrowhead, with a dramatically smaller nuclear size than an anterior nurse cell, indicated by the arrow. Additionally, dap−/− nurse cells have a condensed chromatin structure (Figure 1C), similar to that observed in mutants that affect the oscillation of the Cyclin E protein (Lilly and Spradling, 1996; Doronkin et al, 2003). The smaller nuclear size often observed in dap−/− polyploid nurse cells suggests that in the absence of Dap, the nurse cell endocycle is compromised (Lilly and Spradling, 1996).
Figure 1.
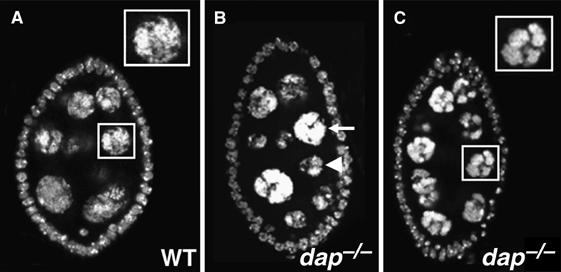
dap−/− egg chambers have abnormal nurse cell nuclei. (A) Wild-type and (B, C) egg chamber containing a dap−/− germ-line clone stained with the DNA dye DAPI. Boxes in (A) and (C) are blow-ups of the indicated nurse cells. Note that dap−/− nurse cells have a condensed chromatin structure. Arrowhead in (B) denotes an example of a dap−/− nurse cell with an apparent DNA content lower than the anterior nurse cell indicated by an arrow.
dap−/− mutants extend the endocycle S-phase
To examine directly if the mitotic and/or endocycle S-phase is altered in dap−/− cells, we labeled ovaries containing dap−/− somatic and germ-line clones with the nucleotide analog, BrdU. When cells are in the mitotic cycle, the pattern of BrdU labeling in dap−/− germ-line and follicle cell clones is indistinguishable from wild type (data not shown). However, the pattern of BrdU incorporation diverges from wild type when the cells in dap−/− clones enter the endocycle. After a 1-h incubation, approximately 32±7% (n=81) of endocycling nurse cells from heterozygous (dap+/−) egg chambers were BrdU positive, indicating that approximately 1/3 of the nurse cells were in S-phase during the 1-h period of BrdU incorporation (Figure 2A and B). In contrast, in dap−/− homozygous germ-line clones almost twice as many cells, 62±7% (n=73), incorporate BrdU (Figure 2C and D). Similar to what is observed in dap−/− nurse cells, upon entry into the endocycle in stage 6 of oogenesis, an increased proportion of dap−/− follicle cells incorporate BrdU relative to adjacent heterozygous (dap+/−) cells (Figure 2E–G). Thus, in the absence of the CKI Dap, the endocycle is modified, such that cells spend a greater proportion of their cell cycle in S-phase.
Figure 2.
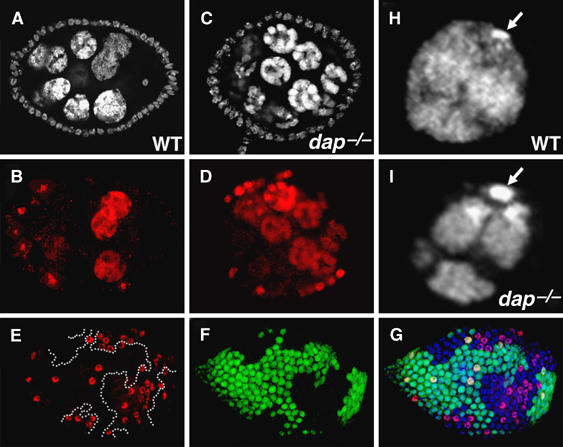
The endocycle S-phase is lengthened in dap−/− mutants. The FLP/FRT technique was used to generate dap−/− clones in a wild-type (dap+/−) background. (A–D) Egg chambers containing (A, B) wild-type and (C, D) dap−/− germ-line clones labeled with (A, C) DAPI and (B, D) the nucleotide analog BrdU. (E–G) dap−/− follicle cell clone from a stage 7 egg chamber labeled with (E) αBrdU and (F) αGFP. In (F) and (G), dap−/− clones are identified by the absence of αGFP staining. (G) An overlay of αBrdU (red), αGFP (green) and DAPI staining (blue). (H) Wild-type and (I) dap−/− nurse cell nuclei stained with DAPI. Arrows indicate the primarily heterochromatic fourth chromosome.
During the Drosophila endocycle, S-phase is often truncated before the entire genome is replicated. This truncation, or premature entry into the Gap-phase, leads to underrepresentation of late-replicating heterochromatic sequences in many polyploid cell types (Gall et al, 1971; Spradling and Orr-Weaver, 1987). Several recently proposed models suggest that Dap oscillations may help bring down Cyclin E/Cdk2 activity at the end of each endocycle S-phase (de Nooij et al, 2000; Edgar and Orr-Weaver, 2001). One prediction from this model is that in dap−/− mutants, Cyclin E/Cdk2 kinase activity may stay above the threshold that will support DNA replication for an extended period of time. As an assay for the lengthening of the endocycle S-phase, we examined if nuclei from dap−/− polyploid nurse cells contain increased amounts of heterochromatin relative to wild-type nurse cells. One of the largest blocks of heterochromatin in the Drosophila genome corresponds to a stretch of 1.672 satellite DNA on the primarily heterochromatic fourth chromosome (Lohe and Brutlag, 1987). This block of heterochromatin can be identified as a bright blob of DAPI staining near the nuclear envelope (Figure 2H, arrow) (Dej and Spradling, 1999). Consistent with a global increase in the copy number of late-replicating heterochromatic sequences in the polyploid genome, in dap−/− nurse cells, the DAPI-stained fourth chromosome appears considerably larger and brighter than in similarly staged wild-type nurse cells (Figure 2I, arrow). This phenotype is nearly identical to that observed in the cycE01672 female sterile mutant, which has an increase in the proportion of nurse cells that are Cyclin E positive and is known to have a 2–3-fold increase in the copy number of several late-replicating sequences (Lilly and Spradling, 1996).
To confirm that dap−/− mutants extend the endocycle S-phase, we compared the copy number of late-replicating sequences with the copy number of early replicating sequences in endocycling wild-type versus dap−/− nurse cells. We used the R1 (Endow and Glover, 1979) and the Bari-1 (Marsano et al, 2003) transposable elements as markers for late-replicating heterochromatic DNA and euchromatic sequences from the mio and CCR4 genes as markers for early replicating DNA (Iida and Lilly, 2004; Morris et al, 2005). Diploid nuclei, as well as all nuclei from 64C to 128C, were collected by FACS analysis from wild-type and dap−/− ovaries (Lilly and Spradling, 1996). The dap−/− ovaries were obtained from the small percentage of homozygous dap−/− females that develop to adulthood. The proportion of nuclei contributed by the 64C peak versus 128C peak was approximately equal in both mutant and wild-type samples. DNA was extracted from the samples and real-time PCR was performed to determine the copy number of the heterochromatic and euchromatic regions. DNA from the 2C peak, representing DNA from diploid cells, was assumed to be 100% represented for all heterochromatic sequences and was used for normalization. Real-time PCR using two probes from the R1 element (R1 and R1′) confirm that whereas the R1 element is underrepresented approximately four-fold in wild-type nurse cells from the 64–128C samples, it is nearly fully represented in nuclei from the dap−/− 64–128C sample (Table I). Similar to what is observed with the R1 element, the Bari-1 element is nearly fully represented in dap−/− nurse cells but is underrepresented nearly two-fold in 64–128C nuclei from wild-type nurse cells (Table I). Taken together, these data support the model that the endocycle S-phase is lengthened in dap−/− mutants to include late replication.
Table 1.
dap−/− nurse cells have an increased copy number of two heterochromatic sequences
| Bari-1 | R1 | R1′ | mio | CCR4 | |
|---|---|---|---|---|---|
| Wild type | 0.49±0.12 | 0.16±0.20 | 0.36±0.16 | 0.93±0.38 | 1.00±0.22 |
| dap−/− | 0.96±0.13 | 1.05±0.06 | 0.87±0.17 | 1.14±0.17 | 1.00±0.24 |
| Data are presented as the ratio of the >64C versus 2C for mutant versus wild-type cell lines, normalized for CCR4, +s.e.m. mio is a euchromatic sequence. | |||||
To further explore if the levels of Cyclin E/Cdk2 activity are increased in dap−/− nurse cells, as is suggested by the lengthening of S-phase, we stained ovaries that contained dap−/− clones with the αMPM2 antibody (Davis et al, 1983). Although traditionally used to follow mitotic phosphoepitopes, the αMPM2 antibody is a useful marker for monitoring Cyclin E/Cdk2 activity in Drosophila, with the presence of one or more αMPM2-positive subnuclear spheres correlating with high levels of Cyclin E/Cdk2 activity (Calvi et al, 1998; Royzman et al, 1999). In endocycling nurse cells, Cyclin E levels oscillate (Lilly and Spradling, 1996). αMPM2 staining levels also oscillate, with approximately 30% of wild-type nurse cells being αMPM2 positive at any given time (Supplementary Figure 1A). In dap−/− clones, the proportion of nurse cells that are αMPM2 positive is dramatically increased, with greater than 95% of the mutant nurse cells containing αMPM2 subnuclear spheres (Supplementary Figure 1B). These data suggest that in dap−/− nurse cells, the baseline level of Cyclin E/Cdk2 activity is increased. Similarly, dap−/− follicle cell clones contain a higher proportion of cells that are αMPM2 positive (Supplementary Figure 1C–E). Thus, Dap may influence the dynamics of Cyclin E/Cdk2 kinase activity in multiple cell types. These data support the model that it is increased Cyclin E/Cdk2 activity that drives the lengthening of the endocycle S-phase in dap−/− mutants.
Dap promotes the accumulation of Dup/Cdt1 during the endocycle
In human cells, Cyclin E overexpression inhibits pre-RC assembly (Ekholm-Reed et al, 2004a). Experiments described above suggest that Cyclin E/Cdk2 activity is increased in dap−/− mutants. To explore the possibility that high Cyclin E/Cdk2 kinase activity in dap−/− mutant cells inhibits the licensing of DNA replication origins, we compared the behavior of the licensing factor Dup/Cdt1 in wild-type versus dap−/− clones. The Dup/Cdt1 protein accumulates during the Gap-phase but rapidly disappears once cells enter the S-phase (Whittaker et al, 2000; Thomer et al, 2004). In Drosophila, Cyclin E/Cdk2 activity promotes the destruction of Dup/Cdt1 (Thomer et al, 2004). In wild-type nurse cells, the levels of Dup/Cdt1 oscillate during the endocycle, with nurse cells with low, medium and high levels of Dup/Cdt1 found within a single egg chamber (Figure 3C, asterisk). In contrast, nurse cells in dap−/− germ-line clones, marked by the absence of anti-β-gal staining, invariably have low levels of Dup/Cdt1 (Figure 3B and C, arrowhead). The decrease in Dup/Cdt1 levels observed in dap−/− germ-line clones in region 3 of the germarium is temporally coincident with entry into the endocycle. Earlier in oogenesis, dap−/− germ-line clones undergoing the mitotic cysts divisions in region 1 of the germarium have wild-type levels of Dup/Cdt1 (Figure 3D–F, arrowhead). Dap is also differentially required for the accumulation of Dup/Cdt1 during the endocycles in the somatic follicle cells. Before stage 6 of oogenesis, when the cells are in the mitotic cycle, dap−/− follicle cell clones have wild-type levels of Dup/Cdt1 (data not shown). However, upon entry into the endocycle in stage 6, dap−/− follicle cells exhibit decreased levels of Dup/Cdt1 relative to adjacent wild-type cells (Figure 3G–I). Additionally, consistent with a role for Dap in promoting the accumulation of Dup/Cdt1, the overexpression of Dap in endocycling follicle cells results in the increased accumulation of the Dup/Cdt1 protein (Figure 3J–L).
Figure 3.
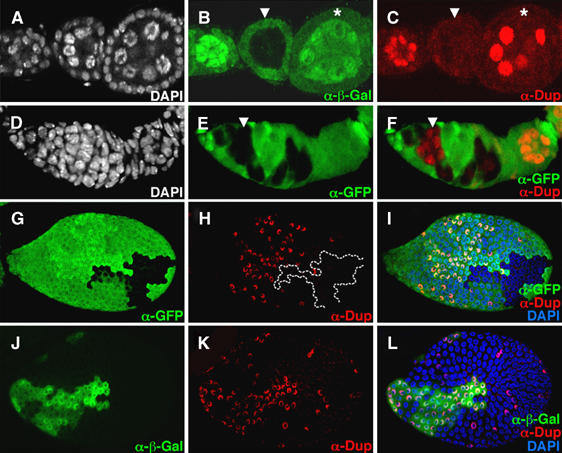
Dap differentially affects the accumulation of Dup/Cdt1 in endocycling versus mitotic cells. The FLP/FRT technique was used to generate dap−/− clones in a wild-type (dap+/−) background. (A–I) Ovaries containing dap−/− clones were stained with (A, D and I) DAPI, (B) α-β-gal, (E, F, G, I) αGFP and (C, F, H, I) αDup/Cdt1 antibodies. In (B), dap−/− clones are identified by the absence of α-β-gal staining, whereas in (E, F, G, and I), dap−/− clones are identified by the absence of αGFP staining. (A–C) An egg chamber containing a dap−/− germ-line clone, arrowhead, flanked by two wild-type egg chambers. Note the low levels of Dup/Cdt1 in the polyploid nurse cells of the dap−/− cyst. (D–F) In contrast, Dup/Cdt1 is present in dap−/− germ-line clones undergoing the mitotic cyst division in region 1 of the germarium (F, arrowhead). (G–I) dap−/− follicle cell clones, marked by the absence of (G) αGFP, have reduced levels of (H) αDup/Cdt1 staining compared with adjacent wild-type cells. (J–L) The FLP/Gal4 system was used to clonally express Dap with β-gal. Follicle cells overexpressing Dap, marked by (J) α-β-gal staining, have higher levels of (K) α-Dup/Cdt1 staining than neighboring wild-type cells.
dap−/− nurse cells have reduced levels of the chromatin-bound MCM2–7 complex
The loading of the MCM2–7 complex onto chromatin requires Dup/Cdt1 (Bell and Dutta, 2002). Therefore, one might predict that in dap−/− nurse cells, where Dup levels are low, the loading of the MCM2–7 complex onto chromatin may be compromised. To explore this possibility, we stained dap−/− ovaries with an antibody that recognizes a conserved epitope present in all six Drosophila MCM2–7 subunits (Claycomb et al, 2002). Immunostainings were performed under two conditions: first, using a low-salt wash, we examined the total nucleoplasmic pool of MCM2–7 proteins in wild-type versus dap−/− nurse cell nuclei (Figure 4A–D). From this experiment, we determined that dap−/− nurse cells have overall levels of MCM2–7 proteins similar to that observed in wild-type egg chambers. However, when we used high-salt wash to remove non-chromatin-bound MCM proteins from the nuclei in order to reveal what fraction of MCM proteins are loaded onto chromatin, we saw a striking difference between dap−/− and wild-type nurse cells (Figure 4E–H). Wild-type egg chambers contain a fraction of nurse cells with chromatin-bound MCM2–7, as well as a fraction of nurse cells with no obvious chromatin-bound MCM2–7 (Figure 4E). This pattern reflects the asynchrony of nurse cell endocycles within a single egg chamber, with some cells in the S-phase and others in the Gap-phase. In contrast, dap−/− nurse cells have uniformly low levels of chromatin-bound MCM2–7 (Figure 4G). Thus, consistent with the low levels of Dup/Cdt1, endocycling dap−/− nurse cells have decreased levels of the chromatin-bound MCM2–7 complex.
Figure 4.
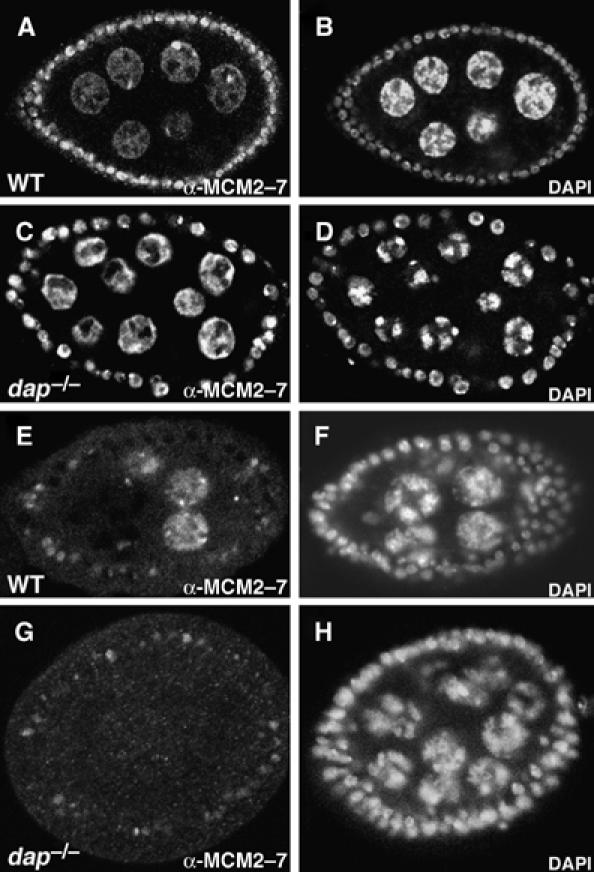
dap−/− nurse cells have reduced levels of the chromatin-bound MCM2–7 complex. (A, B) Wild-type and (C, D) dap−/− egg chambers stained with (A, C) αMCM2–7 antibody and (B, D) DAPI, and washed with low salt to reveal the nucleoplasmic pool of MCM2–7. (E, F) Wild-type and (G–H) dap−/− egg chambers stained with (E, G) αMCM2–7 antibody and (F, H) DAPI and washed with high salt to reveal chromatin-bound MCM2–7.
dap−/− mutants accumulate increased γ-H2Av staining upon endocycle entry
In budding yeast and mammals, overexpression of G1 cyclins results in genomic instability owing to inhibition of pre-RC formation (Tanaka and Diffley, 2002). The genomic instability is thought to arise, at least in part, because a lower density of licensed origins results in the production of stalled replication forks during the S-phase. Stalled replication forks often collapse and break, resulting in DNA damage (Zhou and Elledge, 2000). Thus, we wanted to determine if the apparent decrease in pre-RC formation observed in endocycling cells in dap−/− mutants results in a corresponding increase in DNA damage.
In order to address this question, we used an antibody against the phosphorylated form of the variant histone H2Av (Madigan et al, 2002). In Drosophila, the variant histone H2Av is phosphorylated on its C-terminal tail in response to the presence of a double-stranded break (Madigan et al, 2002). H2Av is the only H2A histone variant present in Drosophila and is thought to be the functional homolog of both H2Az and H2Ax (Madigan et al, 2002). Phosphorylated H2Av is referred to as γ-H2Av. In wild-type ovaries, coincident with entry into the first endocycle, γ-H2Av staining abruptly appears in stage 6 follicle cells (Figure 5, compare panel D with B). The staining is not uniform throughout the nucleus, but is found as a series of small intranuclear dots or clumps, often near the nuclear envelope. During the very first endocycle S-phase, follicle cells fail to replicate approximately 25% of their genome and thus are predicted to contain numerous stalled replication forks that in subsequent rounds of S-phase would produce truncated DNAs (Lilly and Spradling, 1996; Leach et al, 2000). The truncated DNAs are predicted to form between the junctions of the fully replicated euchromatin and the underreplicated heterochromatin (Leach et al, 2000). Consistent with this model, γ-H2Av staining in wild-type endocycling follicle cell nuclei is often present near the DAPI bright chromocenter, which contains the centric heterochromatin as well as the fourth chromosome (Figure 5C and D, arrows). In mammals, stalled DNA replication forks often collapse, resulting in DNA breaks (Kurose et al, 2006). Thus, the intranuclear foci of γ-H2Av staining likely mark the sites of accumulated stalled replication forks and/or truncated DNAs that accumulate during the incomplete endocycle S-phase.
Figure 5.
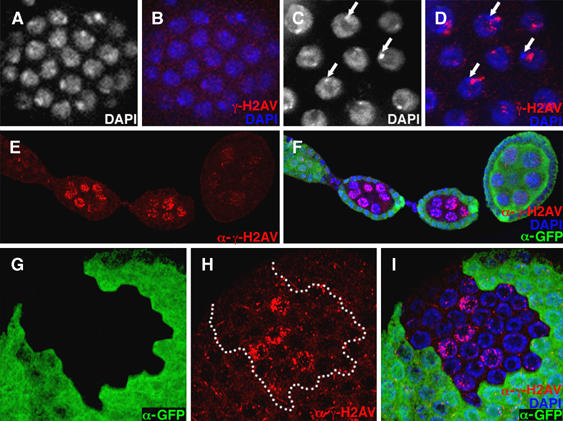
dap−/− mutants accumulate increased α-γ-H2Av staining upon entry into the endocycle. Wild-type (A, B) mitotic and (C, D) endocycling follicle cells stained with (A, C, white; B, D, blue) DAPI and (B, D, red) α-γ-H2Av antibody. (A, B) Wild-type mitotic follicle cells have little α-γ-H2Av staining, whereas (C, D) α-γ-H2Av staining accumulates near the chromocenter upon entry into the endocycle (C, D, arrows). (E, F) Two dap−/− germ-line cysts marked by the absence of (F) α-GFP staining have increased levels of (E, F) γ-H2Av staining compared with a neighboring older wild-type cyst. (G–I) A stage 9 egg chamber containing a dap−/− clone of follicle cells that have entered the endocycle. In the dap−/− cells (G), marked by the absence of α-GFP staining (H, I), γ-H2Av staining is often increased and not restricted to the region near the chromocenter.
The nurse cells approach full replication of their genomes during the first four endocycles, but then significantly truncate S-phase during the fifth and all subsequent endocycles (Dej and Spradling, 1999). We observe a notable increase in γ-H2Av staining in wild-type nurse cells, coincident with entry into the first endocycle in stage 1 egg chambers (data not shown). These data indicate that even though a greater proportion of the genome is replicated during each of the early nurse cell endocycles, DNA replication is unlikely to be fully completed, as is found during a mitotic S-phase. Also, note that because of the unique organization of the nurse cell nuclei, where each chromosomal arm remains in defined nuclear domains (Dej and Spradling, 1999), γ-H2Av staining appears somewhat more diffuse than in the somatic follicle cells that contain a chromocenter (Figure 5E).
Nurse cell nuclei in dap−/− clones have dramatically increased levels of γ-H2Av staining relative to wild-type nurse cells in the same ovariole (Figure 5E and F). In dap−/− nurse cells the levels of γ-H2Av staining increase with each endocycle S-phase (data not shown). However, it is not clear how much of the increased DNA damage observed in dap−/− nurse cells can be attributed to the presence of a reduced number of licensed origins available for the endocycle S-phase. We find that dap−/− germ-line clones have increased γ-H2Av staining, relative to wild-type cells, before the nurse cells enter the endocycle in region 3 of the germarium (data not shown). This observation indicates that DNA damage may be occurring during pre-meiotic S-phase or alternatively that dap−/− germ-line cysts do not adequately repair the double-stranded breaks that initiate meiotic recombination. Thus, although dap−/− nurse cells have increased γ-H2Av staining, consistent with the inhibition of pre-RC formation, currently we cannot distinguish how much of the damage is due to inadequate pre-RC formation during the endocycle or alternatively to DNA damaged that occurred during the previous meiotic cycle. However, as γ-H2Av staining in dap−/− nurse cells increases with each ensuing endocycle, at least some of the increased staining seen is due to alterations accrued after the foregoing meiotic cycle.
To avoid complication caused by DNA damage induced during meiosis, we examined γ-H2Av staining in dap−/− endocycling follicle cells. Whereas there is little to no difference observed between wild-type and dap−/− clones when the cells are in the mitotic cycle (data not shown), coincident with entry into the endocycle in stage 6, dap−/− follicle cells accumulate increased γ-H2Av staining relative to adjacent wild-type cells (Figure 5G–I). Specifically, we find that in approximately 20% (n=93) of dap−/− polyploid follicle cells, γ-H2Av staining is no longer confined to the region near the chromocenter, but is present throughout the nucleus (Figure 5H). Follicle cells from the hypomorphic allele dupPA77 exhibit a very similar pattern of γ-H2Av in endocycling follicle cells (Supplementary Figure 2B′). Thus, directly lowering the levels of Dup/Cdt1 results in a DNA damage phenotype similar to that observed in dap−/− mutants. These data support the model that the reduced ability of dap−/− follicle cells to accumulate Dup/Cdt1 leads to a low density of licensed origins along the chromosome, ultimately resulting in the incomplete DNA replication throughout the polyploid genome.
dap and dup/cdt1 genetically interact in endocycling and mitotic tissues
Reducing the levels of Dup/Cdt1 in a dap−/− background enhances the ovarian phenotype. Mutant egg chambers from dap−, dupa1/dap−, +ovaries have nurse cells DNA contents that are reduced relative to dap−/− egg chambers, as indicated by the size and intensity of the nucleus as assessed by DAPI staining (Figure 6A–C). dap−, dupa1/dap−, + egg chambers also exhibit a more condensed nurse cell nuclear structure relative to that observed in dap−/− homozygotes (Figure 6D–F). Additionally, the examination of dap−, dupa1/dap−, + and dap−, dupa3/dap−, + adults revealed a role for dap in endoreplication outside of the ovary. dap−, dupa1/dap−, + flies have a strong reduction in the size and thickness of the macrochaetae, large prominent bristles on the notum of the adult (Figure 6G–J, arrowheads). The cells that produce the socket and the shaft of the macrochaetae, referred to as the tormogen and trichogen cell respectively, become polyploid during pupal development. Bristle size correlates with the ploidy of the underlying trichogen cell, which secretes cuticle to produce the bristle shaft (Weng et al, 2003). Upon closer inspection, dap−/− homozygous adults also exhibit subtle defects in macrochaetae growth (Figure 6I, arrowhead). However, the penetrance and expressivity of the macrochaetae phenotype is greatly enhanced in dap−, dupa1/dap−, + flies (Figure 6J, arrowhead). A similar enhancement of the dap−/− macrochaetae phenotype is observed using the dup/cdt1 allele, dupa3 (data not shown). These data demonstrate that in a dap−/− background Dup/Cdt1 levels are limiting in multiple polyploid cell types.
Figure 6.
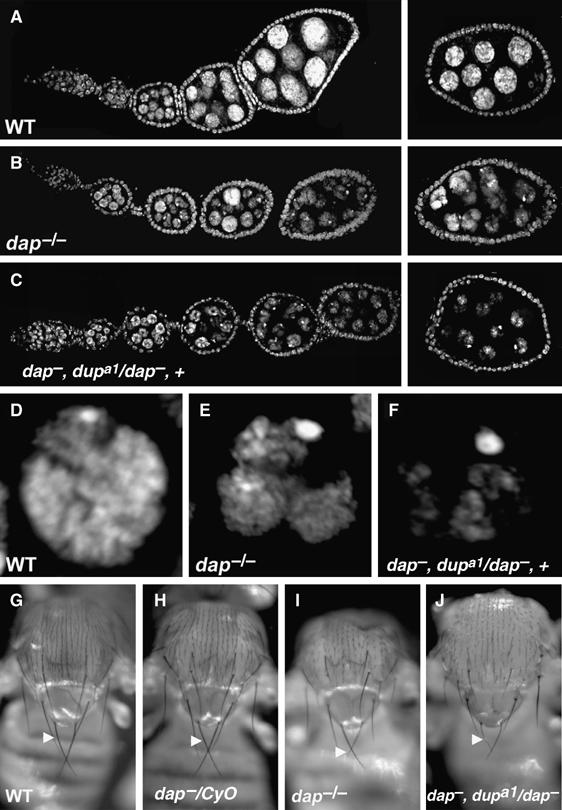
Reducing the dosage of dup/cdt1 enhances the dap−/− phenotype in several polyploid cell types. (A–C) DAPI staining of (A) wild-type, (B) dap−/− and (C) dap−, dupa1/dap−, + ovarioles. (D–F) Magnification of (D) wild-type, (E) dap−/− and (F) dap−, dupa1/dap−, + nurse cell nuclei. (G–J) Thorax of (G) wild-type, (H) dap−/CyO, (I) dap−/− and (J) dap−, dupa1/dap−, + flies. dap−/− mutant macrochaetae (I, arrowhead) are often thinner than the control (G, H, arrowheads). A single copy of dupa1 mutation dominantly enhances the dap−/− reduced macrochaetae phenotype (J, arrowhead).
Can the over-expression of Dup/Cdt1 promote replication relicensing in dap−/− nurse cells? In order to answer this question, we used two transgenes that contained either full-length Dup/Cdt1, FL-Dup, or a stabilized form of Dup/Cdt1, Dup10(A), in which all putative Cdk phosphorylation sites had been mutated (Thomer et al, 2004). Both transgenes were under the control of the inducible heat-shock promoter. In metazoans, overexpression of Cdt1 results in the unregulated relicensing of DNA replication origins. Recent evidence indicates that the DNA fragmentation and checkpoint activation associated with Cdt1-dependent relicensing, occurs as a direct result of DNA replication (Davidson et al, 2006). We find that the overexpression of Dup10(A) in a wild-type background, results in increased levels of DNA damage in both the mitotic and endocycling cells of the ovary (Supplementary Figure 3E and F). Importantly, four hours after a 1-h induction of Dup10(A) expression, only a fraction of nurse cells exhibit increased γ-H2Av staining (Supplementary Figure 3F). These data are consistent with the proposal that the DNA damage induced by Dup/Cdt1 overexpression is contingent on the relicensing of replication origins and subsequent DNA replication, and thus only occurs in cells in the S-phase (Davidson et al, 2006). Similar results were observed after the overexpression of FL-Dup. The overexpression of Dup10(A) and FL-Dup in a dap−/− background also results in a dramatic increase in DNA damage in all cell types of the ovary (Supplementary Figure 3K and L, and data not shown). Specifically, four hours after the induction of Dup10(A) expression, dap−/−, Dup10(A)/+ nurse cells have a greater than three-fold increase in γ-H2Av staining relative to similarly treated dap−/− nurse cells (Supplementary Table 1). These data strongly suggest that, in dap−/− mutants, the addition of Dup/Cdt1 can promote relicensing of DNA replication origins during nurse cell and follicle cell endocycles. Moreover, they indicate that the overexpression of Dup/Cdt1 is epistatic to other potential deficits in replication licensing observed in dap−/− mutants and strongly suggest that the licensing defects seen in dap−/− nurse cells are not due to a general block to cell cycle progression.
Intriguingly, we find that dup/cdt1 acts as a dominant enhancer of dap in the diploid cells of the Drosophila eye. The eyes of dap−/− homozygotes appear mostly wild type with only an occasional duplicated and/or missing bristle observed upon close inspection (Figure 7B). In contrast dap−, dupa1/dap−, + and dap−, dupa3/dap−, + adults have rough eyes containing misaligned rows of ommatidia, irregularly shaped ommatidia, as well as a large number of ommatidia with missing, misplaced, or duplicated bristles (Figure 7C). Sectioning and staining adult eyes with toluidine blue reveals that eyes from dap−, dupa1/dap−, + animals frequently have irregularly shaped and misoriented ommatidia that are missing cells (Figure 7F, arrows). dap−, dupa3/dap−, + eyes have similar, but milder, phenotypes, reflecting the weaker allelic strength of the dapa3 allele (data not shown). Thus, the CKI dap genetically interacts with the licensing factor dup/cdt1 in at least some mitotic cycles.
Figure 7.
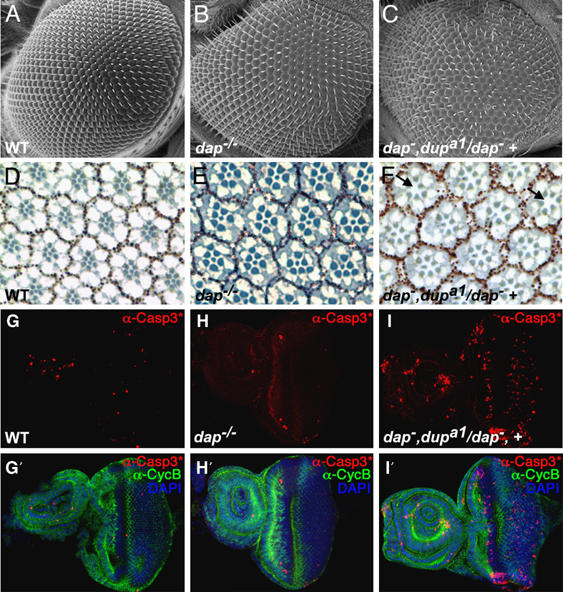
dap−, dup−/dap−, + flies have rough eyes. (A–C) Scanning electron micrographs of (A) wild-type, (B) dap−/− and (C) dap−, dupa1/dap−, + eyes. (C) Note that the dap−, dupa1/dap−, + compound eye has an irregular pattern of ommatidia relative to both wild-type and dap−/− eyes. Tangential sections (D–F) of (D) wild-type, (E) dap−/−, and (F) dap−,dupa1/dap−, + eyes, stained with toluidine blue. Note the missing photoreceptor cells and irregularly shaped and oriented ommatidia in dap−,dupa1/dap−, + (F, arrows). (G–I) Eye antennal imaginal discs from (G, G′) wild-type, (H, H′) dap−/− and (I, I′) dap−, dupa1/dap−, + stained with (G–I′, red) α-cleaved caspase 3*, (G′–I′, green) α-Cyclin B antibodies and (G′–I′, blue) DAPI. Anterior is on the left. (I, I′) Discs from dap−, dupa1/dap−, + have increased numbers of cleaved caspase 3*-positive cells relative to both (G, G′) wild-type and (H, H′) dap−/−, indicating increased levels of cell death.
During mitotic cycle, apoptosis is often triggered by DNA damage and/or replication stress (Zhou and Elledge, 2000). The adult compound eye is derived from the eye antennal imaginal disc, which proliferates extensively during larval development. We stained wild-type, dap−/− and dap−, dupa1/dap−, + eye antennal discs, which are undergoing mitotic cycles, with an antibody against cleaved caspase 3*, a marker for apoptosis (Yu et al, 2002). Consistent with the rough eye phenotype and the observation that many ommatidal clusters are missing cells, dap−, dupa1/dap−, + eye-antennal imaginal discs have increased cleaved capase 3* levels relative to both dap−/− and wild-type controls (Figure 7G–I). Importantly, the increase in apoptosis found in dap−, dupa1/dap−, + eye-antennal discs is correlated with increased levels of DNA damage as measured by γ-H2Av staining (Supplementary Figure 4). However, these effects are cell type-specific in that, compared with dap−/− and dupa1/+ wild-type controls, the levels of cleaved caspase 3* and γ-H2Av are not notably increased in the wing discs of dap−, dupa1/dap−, + mutants (data not shown). Thus, our data suggest, that as we observe during the endocycle, during some mitotic cycles Dap acts to prevent DNA damage owing to inadequate replication licensing.
Discussion
In metazoans, CKIs have traditionally been thought of as cell cycle brakes, with the expression of specific CKIs correlating with exit from the cell cycle and differentiation. Here, we define a positive role for the CKI Dap in Drosophila endocycles. Specifically, our data support a model in which Dap oscillations promote endoreplication by reinforcing low Cyclin E/Cdk2 activity during the endocycle Gap-phase. The low Cdk activity afforded by the presence of Dap facilitates both a timely entrance into the endocycle Gap-phase as well as the licensing of the DNA for the next round of DNA replication. Taken together, our data strongly suggest that Dap is an intrinsic component of the cell cycle machinery that drives endoreplication in Drosophila. Additionally, genetic interaction studies suggest that Dap may play a similar role in replication licensing during some mitotic cycles.
Dap limits Cyclin E/Cdk2 activity during the endocycle
The licensing of DNA replication origins requires a period of low Cdk activity or Gap-phase (Bell and Dutta, 2002). In mitotic cells, this period of low Cdk activity occurs in late mitosis after the destruction of the mitotic cyclins by the APC/C and before the accumulation of the G1 cyclins. How a distinct Gap-phase is achieved during the endocycle is less clear.
Our data suggest a model in which Dap oscillations promote entry into the endocycle Gap-phase through the cyclic inhibition of Cyclin E/Cdk2 activity. Two lines of evidence suggest that Cyclin E/Cdk2 activity remains inappropriately high in dap−/− mutants. First, in dap−/− ovaries the fraction of cells containing nuclear αMPM2 staining is dramatically increased in several endocycling cell types. Previous work has shown a strong correlation between the presence of one or more αMPM2 subnuclear spheres and Cyclin E/Cdk2 activity (Calvi et al, 1998). Indeed, a similar increase in nuclear αMPM2 staining is observed when Cyclin E is overexpressed from a transgene (Calvi et al, 1998). Thus, increased nuclear αMPM2 staining is consistent with dap−/− having higher baseline level of Cyclin E/Cdk2 activity.
The lengthening of the endocycle S-phase to include late replication in dap−/− nurse cells provides additional support to the idea that Dap oscillations function to restrict Cyclin E/Cdk2 activity during endocycles. Previous work has shown that during the endocycle S-phase, Cyclin E/Cdk2 activity often falls below the level that will support DNA replication before complete replication of the genome (Lilly and Spradling, 1996). This early entry into the Gap-phase results in the underrepresentation of late-replicating heterochromatic sequences. Here, we demonstrate that, relative to wild-type nurse cells of a similar ploidy value, dap−/− nurse cells have an increased copy number of two late-replicating heterochromatic sequences, the R1 and Bari-1 transposable elements (Endow and Glover, 1979; Schubeler et al, 2002). The increased copy number of late-replicating sequences within the polyploid genome is consistent with dap−/− nurse cells, supporting a prolonged endocycle S-phase owing to increased baseline levels of Cyclin E/Cdk2 activity. Moreover, we find that dap−/− nurse cells exhibit many of the same phenotypes associated with the broadening of Cyclin E oscillations and/or increased levels of the Cyclin E protein, including an increase in the size of the primarily heterochromatic fourth chromosome and a condensed chromatin structure (Lilly et al, 2000; Doronkin et al, 2003). Taken together, our data strongly suggest that the CKI Dap restricts Cyclin E/Cdk2 activity during Drosophila endocycles.
Dap promotes replication licensing
Consistent with Dap functioning to establish low Cdk activity during the endocycle Gap-phase, we find that loss-of-function mutations in dap compromise replication licensing during the endocycle. Intriguingly, loss of Dap differentially effects the accumulation of the licensing factor Dup/Cdt1 in mitotic versus endocycling cells of the ovary. While in the mitotic cycle, cells in dap−/− mutant clones exhibit no apparent reduction in the levels of Dup/Cdt1 relative to wild type. However, once the dap−/− mutant cells enter the endocycle, Dup/Cdt1 levels fall below the level of detection. Consistent with the lower levels of Dup/Cdt1, dap−/− nurse cells have reduced levels of the chromatin-bound MCM2–7 complex, even though the total nucleoplasmic pool of MCM2–7 proteins remains unchanged relative to wild type. Additionally, upon entering the endocycle, dap−/− nurse cells and follicle cells accumulate high levels of γH2Av staining, consistent with an increase in the number of stalled replication forks or truncated DNAs. Stalled forks and/or truncated DNAs are predicted to form if the number of licensed origins is reduced below the threshold required to complete genomic replication and/or DNA helicase activity is inadequate to support fork progression. Previous work has shown that Cyclin E/Cdk2 activity promotes the destruction of Dup/Cdt1 (Thomer et al, 2004). Thus, the simplest model to explain our data is that Dap promotes replication licensing during endocycles, at least in part, by restricting Cyclin E/Cdk2-dependent destruction of Dup/Cdt1. This model is consistent with the observation in human cells that the overexpression of either p21 or p27 reduces both Cdt1 phosphorylation and proteolysis (Liu et al, 2004). The proposal that Dup/Cdt1 is limiting in a dap−/− background is supported by our observation that mutations in dup/cdt1 dominantly enhance the dap−/− phenotype in several polyploid cell types. However, it is important to note that while our data clearly implicate Dup/Cdt1 as an important downstream target, Dap may influences additional aspects of pre-RC formation or replication licensing during endocycles.
Intriguingly, removing one copy of dup/cdt1 dominantly enhances the dap−/− phenotype in the diploid cells of the adult eye. Specifically, relative to both dap−/− and wild-type controls, dap−, dapa1/dap−, + eye discs exhibit both increased DNA damage and cell death. These data suggest that dap may influence replication licensing in at least some mitotic cycles. Indeed, the precise importance of Dap during both the mitotic cycle and the endocycle may be cell type specific. Finally, it is important to note that because dap−/− mutants have no apparent problems in replicating their DNA during most mitotic cycles, any role for dap in reinforcing low Cyclin E/Cdk2 activity during G1 must be redundant with other mechanisms that restrict Cyclin E/Cdk2 activity, such as the regulated proteolysis of the Cyclin E protein.
A general role for CKIs in reinforcing low Cdk activity during the Gap-phase?
During the development of the placenta in mice, trophoblasts enter the endocycle and become highly polyploid, ultimately achieving ploidy values of greater than 1000C. Intriguingly, entry into the endocycle is accompanied by expression of the Cdk inhibitor p57kip2 (Hattori et al, 2000). Like Dap, p57kip2 is a member of the Cip/Kip family of Cdk inhibitors (Matsuoka et al, 1995). Similar to what is observed with Dap in the nurse and follicle cells of the Drosophila ovary, in mice, p57Kip2 protein levels oscillate during the endocycle in giant trophoblasts, with p57Kip2 accumulating in late S-phase, remaining high during most of the Gap-phase, and then rapidly disappearing before entry into the subsequent S-phase. Stabilization of p57 through removal of a C-terminal Cdk phosphorylation site blocks S-phase entry (Hattori et al, 2000). These data suggest Cdk activity and p57 stability may be components of a feedback loop that drive the licensing and firing of DNA replication origins during the trophoblast endocycle (Edgar and Orr-Weaver, 2001).
The proposed role of Dap and p57 during the endocycle is very similar to that of the CKI Sic1 during the mitotic cycle of Saccharomyces cerevisiae. Sic1 promotes origin licensing in late G1 by preventing precocious activation of Cdks (Lengronne and Schwob, 2002). Similar to what we find in dap−/− mutant nurse cells, deletions of Sic1 result in an extended S-phase, a reduction in the number of licensed origins and accumulation of double-stranded breaks (Lengronne and Schwob, 2002). Additionally, Sic1 and Cdt1 genetically interact with weak mutations in Cdt1 enhancing the S-phase defects of Sic1 deletions (Jacobson et al, 2001). This apparent conservation of function, along with our observations that Dap may influence replication licensing in mitotic as well as endocycling cells, suggests that Dap may play a redundant role during many Drosophila cell cycles in reinforcing low Cdk activity during the Gap-phase.
Materials and methods
Fly strains
The FRT42B, dap4 (Lane et al, 1996) stock was a gift from Iswar Hariharan. dap4 is refered as dap−/− in the text. hs-FLP1, w1118; Adv1/CyO, w1118; FRT42B ubi-GFP, w1118; FRT42B arm-lacZ, dupPA77 and Act>CD2>Gal4, UAS-lacZ/CyO were obtained from the Bloomington Stock Center. dupa1 and dupa3 (Whittaker et al, 2000) were a gift from Terry Orr-Weaver. The hsp70:Myc:FL-Dup (FL-Dup) line is an myc-tagged Dup/Cdt1 construct, whereas the hsp70:Myc:Dup10(A) (Dup10(A)) was created by mutating a putative phosphorylation site (Thomer et al, 2004), and were gifts from Brian Calvi. The UAS-Dap line was a gift from Christian Lehner. The dap4, dupa1 and dap4, dupa3 chromosomes were generated by meiotic recombination. Homozygous dap4 mutant germ-line and follicle cell clones were generated by FLP/FRT-mediated site-specific recombination (Xu and Rubin, 1993) and Dap somatic overexpression was performed by generating Flip-out/GAL4 clones (Pignoni and Zipursky, 1997) (see Supplementary data).
Immunocytochemistry and BrdU labeling
Immunocytochemistry of adult ovary staining was performed as described in McKearin and Ohlstein (1995). Labeling with αMCM2–7 was performed by using a high-salt wash as described in Claycomb et al (2002). For BrdU labeling, ovaries were dissected and stained according to Calvi and Lilly (2004). The following antibodies were used in this study: mouse monoclonal αGFP (1:200, Roche), rabbit polyclonal αGFP (1:500, Molecular Probes), mouse monoclonal α-β-galactosidase (1:500; Promega), rabbit polyclonal α-β-galactosidase (1:1500, Molecular Probes), rabbit polyclonal α-cleaved caspase 3* (1:50, Cell Signaling), mouse monoclonal αMPM2 (1:50, Dako), mouse monoclonal α-BrdU (1:20, Becton-Dickinson) and mouse monoclonal α-Cyclin B (DSHB). αDup/Cdt1 guinea-pig antibody (1:1000) was kindly provided by Terry Orr-Weaver, αMCM2–7 mouse monoclonal (1:200) antibody by Steve Bell, and αγH2AV rabbit polyclonal antibody (1:3000) by Bob Glaser. Fluorescence-conjugated secondary antibodies were purchased from Molecular Probes and were used at a 1:1000 dilution. All samples were mounted in cytifluor (Kent) or vectashield (Vector).
For the Dup/Cdt1 overexpression experiments, the expression of Dup10(A) and FL-Dup was induced by heat shocking flies for 1 h at 37°C. The flies were subsequently allowed to recover for four hours before the ovaries were dissected and processed for immunocytochemistry as indicated above. Fluorescent intensity (FI) of γ-H2Av staining was measured in individual nurse cell nuclei from stage 4–6 egg chambers using an Olympus Fluoview FV1000.
Eye sectioning and scanning electron microscopy
Eye sectioning and scanning electron microscopy were performed as described in Raabe et al (1996), except that toluidine blue at 0.5% was used to stain 1.3-μm-thick eye sections.
FACS sorting and real-time PCR
Nuclei were isolated from adult ovaries as indicated in Lilly and Spradling (1996). 2C and 64–128C nuclear fractions from wild-type and dap4 (dap−/−) ovaries were collected with a FACS Vantage (BD Bioscience, San Jose, CA). dap−/− ovaries were collected from the small percentage of dap−/− females that survive to adulthood. After DNA extraction, the abundance of specific sequences was measured by real-time PCR using an ABI 7900 thermocycler (Applied Biosystems International). Standard curves for each primer consisted of serial dilutions of genomic DNA from Schneider cells. The abundance ratio for each nuclear fraction was calculated as the abundance of a specific probe in the 64–128C fraction divided by the abundance of the same probe in the 2C fraction. The ratios were then normalized to that obtained for the CCR4 probe (see table), which was arbitrarily set to 1.0. Primers are listed in Supplementary data.
Supplementary Material
Supplementary Information and Figure Legends
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Table 1
Acknowledgments
We thank Steve Bell, Brian Calvi, Robert Glaser, Christian Lehner, Terry Orr-Weaver, the Developmental Hybridoma Bank and the Bloomington Stock Center for Drosophila stocks and antibodies. We thank Melvin Depamphilis and Deborah Hursh for comments on the manuscript. We thank CINVESTAV for use of SEM facility. This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development at the National Institutes of Health, and a CONACYT grant #U46133-Q to JRE.
References
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 [DOI] [PubMed] [Google Scholar]
- Calvi BR, Lilly MA (2004) Fluorescent BrdU labeling and nuclear flow sorting of the Drosophila ovary. Methods Mol Biol 247: 203–213 [DOI] [PubMed] [Google Scholar]
- Calvi BR, Lilly MA, Spradling AC (1998) Cell cycle control of chorion gene amplification. Genes Dev 12: 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb JM, MacAlpine DM, Evans JG, Bell SP, Orr-Weaver TL (2002) Visualization of replication initiation and elongation in Drosophila. J Cell Biol 159: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson IF, Li A, Blow JJ (2006) Deregulated replication licensing causes DNA fragmentation consistent with head-to-tail fork collision. Mol Cell 24: 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN (1983) Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA 80: 2926–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nooij JC, Graber KH, Hariharan IK (2000) Expression of the cyclin-dependent kinase inhibitor Dacapo is regulated by cyclin E. Mech Dev 97: 73–83 [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Letendre MA, Hariharan IK (1996) A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell 87: 1237–1247 [DOI] [PubMed] [Google Scholar]
- Dej KJ, Spradling AC (1999) The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development 126: 293–303 [DOI] [PubMed] [Google Scholar]
- Deng WM, Althauser C, Ruohola-Baker H (2001) Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128: 4737–4746 [DOI] [PubMed] [Google Scholar]
- Diffley JF, Labib K (2002) The chromosome replication cycle. J Cell Sci 115: 869–872 [DOI] [PubMed] [Google Scholar]
- Doronkin S, Djagaeva I, Beckendorf SK (2003) The COP9 signalosome promotes degradation of Cyclin E during early Drosophila oogenesis. Dev Cell 4: 699–710 [DOI] [PubMed] [Google Scholar]
- Edgar BA, Orr-Weaver TL (2001) Endoreplication cell cycles: more for less. Cell 105: 297–306 [DOI] [PubMed] [Google Scholar]
- Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI (2004a) Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol 165: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm-Reed S, Spruck CH, Sangfelt O, van Drogen F, Mueller-Holzner E, Widschwendter M, Zetterberg A, Reed SI (2004b) Mutation of hCDC4 leads to cell cycle deregulation of cyclin E in cancer. Cancer Res 64: 795–800 [DOI] [PubMed] [Google Scholar]
- Endow SA, Glover DM (1979) Differential replication of ribosomal gene repeats in polytene nuclei of Drosophila. Cell 17: 597–605 [DOI] [PubMed] [Google Scholar]
- Follette PJ, Duronio RJ, O'Farrell PH (1998) Fluctuations in cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Curr Biol 8: 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG, Cohen EH, Polan ML (1971) Repetitive DNA sequences in Drosophila. Chromosoma 33: 319–344 [DOI] [PubMed] [Google Scholar]
- Hattori N, Davies TC, Anson-Cartwright L, Cross JC (2000) Periodic expression of the cyclin-dependent kinase inhibitor p57(Kip2) in trophoblast giant cells defines a G2-like gap phase of the endocycle. Mol Biol Cell 11: 1037–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S (1996) A Cdc2 dependent checkpoint maintains diploidy in Drosophila. Development 122: 1051–1058 [DOI] [PubMed] [Google Scholar]
- Iida T, Lilly MA (2004) Missing oocyte encodes a highly conserved nuclear protein required for the maintenance of the meiotic cycle and oocyte identity in Drosophila. Development 131: 1029–1039 [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Munoz CX, Knox KS, Williams BE, Lu LL, Cross FR, Vallen EA (2001) Mutations in SID2, a novel gene in Saccharomyces cerevisiae, cause synthetic lethality with sic1 deletion and may cause a defect during S phase. Genetics 159: 17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose A, Tanaka T, Huang X, Traganos F, Darzynkiewicz Z (2006) Synchronization in the cell cycle by inhibitors of DNA replication induces histone H2AX phosphorylation: an indication of DNA damage. Cell Prolif 39: 231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H (1996) Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87: 1225–1235 [DOI] [PubMed] [Google Scholar]
- Leach TJ, Chotkowski HL, Wotring MG, Dilwith RL, Glaser RL (2000) Replication of heterochromatin and structure of polytene chromosomes. Mol Cell Biol 20: 6308–6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengronne A, Schwob E (2002) The yeast CDK inhibitor Sic1 prevents genomic instability by promoting replication origin licensing in late G(1). Mol Cell 9: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Lilly MA, de Cuevas M, Spradling AC (2000) Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev Biol 218: 53–63 [DOI] [PubMed] [Google Scholar]
- Lilly MA, Duronio RJ (2005) New insights into cell cycle control from the Drosophila endocycle. Oncogene 24: 2765–2775 [DOI] [PubMed] [Google Scholar]
- Lilly MA, Spradling AC (1996) The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev 10: 2514–2526 [DOI] [PubMed] [Google Scholar]
- Liu E, Li X, Yan F, Zhao Q, Wu X (2004) Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J Biol Chem 279: 17283–17288 [DOI] [PubMed] [Google Scholar]
- Lohe AR, Brutlag DL (1987) Identical satellite DNA sequences in sibling species of Drosophila. J Mol Biol 194: 161–170 [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D (2001) Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev 15: 1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan JP, Chotkowski HL, Glaser RL (2002) DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res 30: 3698–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano RM, Milano R, Minervini C, Moschetti R, Caggese C, Barsanti P, Caizzi R (2003) Organization and possible origin of the Bari-1 cluster in the heterochromatic h39 region of Drosophila melanogaster. Genetica 117: 281–289 [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A, Harper JW, Elledge SJ (1995) p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev 9: 650–662 [DOI] [PubMed] [Google Scholar]
- McKearin D, Ohlstein B (1995) A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121: 2937–2947 [DOI] [PubMed] [Google Scholar]
- Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK (2001) Archipelago regulates Cyclin E levels in Drosohila and is mutated in human cancer cell lines. Nature 413: 1183–1193 [DOI] [PubMed] [Google Scholar]
- Morris JZ, Hong A, Lilly MA, Lehmann R (2005) twin, a CCR4 homolog, regulates cyclin poly(A) tail length to permit Drosophila oogenesis. Development 132: 1165–1174 [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL (1997) Induction of Drosophila eye development by decapentaplegic. Development 124: 271–278 [DOI] [PubMed] [Google Scholar]
- Raabe T, Riesgo-Escovar J, Liu X, Bausenwein BS, Deak P, Maroy P, Hafen E (1996) DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell 85: 911–920 [DOI] [PubMed] [Google Scholar]
- Royzman I, Austin RJ, Bosco G, Bell SP, Orr-Weaver TL (1999) ORC localization in Drosophila follicle cells and the effects of mutations in dE2F and dDP. Genes Dev 13: 827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer K, Knoblich JA, Richardson H, Lehner CF (1995) Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev 9: 1327–1339 [DOI] [PubMed] [Google Scholar]
- Schubeler D, Scalzo D, Kooperberg C, van Steensel B, Delrow J, Groudine M (2002) Genome-wide DNA replication profile for Drosophila melanogaster: a link between transcription and replication timing. Nat Genet 32: 438–442 [DOI] [PubMed] [Google Scholar]
- Shcherbata HR, Althauser C, Findley SD, Ruohola-Baker H (2004) The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development 131: 3169–3181 [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90: 671–681 [DOI] [PubMed] [Google Scholar]
- Spradling A, Orr-Weaver T (1987) Regulation of DNA replication during Drosophila development. Annu Rev Genet 21: 373–403 [DOI] [PubMed] [Google Scholar]
- Spradling AC (1993) Developmental genetics of oogenesis. In Bate M, Martinez-Arias A (eds) The Development of Drosophila melanogaster, Vol. 1, pp 1–70. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Spruck CH, Won KA, Reed SI (1999) Deregulated cyclin E induces chromosome instability. Nature 401: 297–300 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Diffley JF (2002) Deregulated G1-cyclin expression induces genomic instability by preventing efficient pre-RC formation. Genes Dev 16: 2639–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer M, May NR, Aggarwal BD, Kwok G, Calvi BR (2004) Drosophila double-parked is sufficient to induce re-replication during development and is regulated by cyclin E/CDK2. Development 131: 4807–4818 [DOI] [PubMed] [Google Scholar]
- Weigmann K, Cohen SM, Lehner CF (1997) Cell cycle progression, growth and patterning in imaginal discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development 124: 3555–3563 [DOI] [PubMed] [Google Scholar]
- Weiss A, Herzig A, Jacobs H, Lehner CF (1998) Continuous cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Curr Biol 8: 239–242 [DOI] [PubMed] [Google Scholar]
- Weng L, Zhu C, Xu J, Du W (2003) Critical role of active repression by E2F and Rb proteins in endoreplication during Drosophila development. EMBO J 22: 3865–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker AJ, Royzman I, Orr-Weaver TL (2000) Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev 14: 1765–1776 [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, Baker NE (2002) A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development 129: 3269–3278 [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information and Figure Legends
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Table 1


