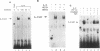Abstract
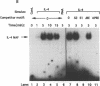
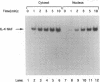
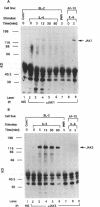
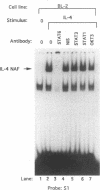
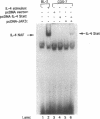
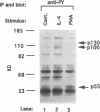
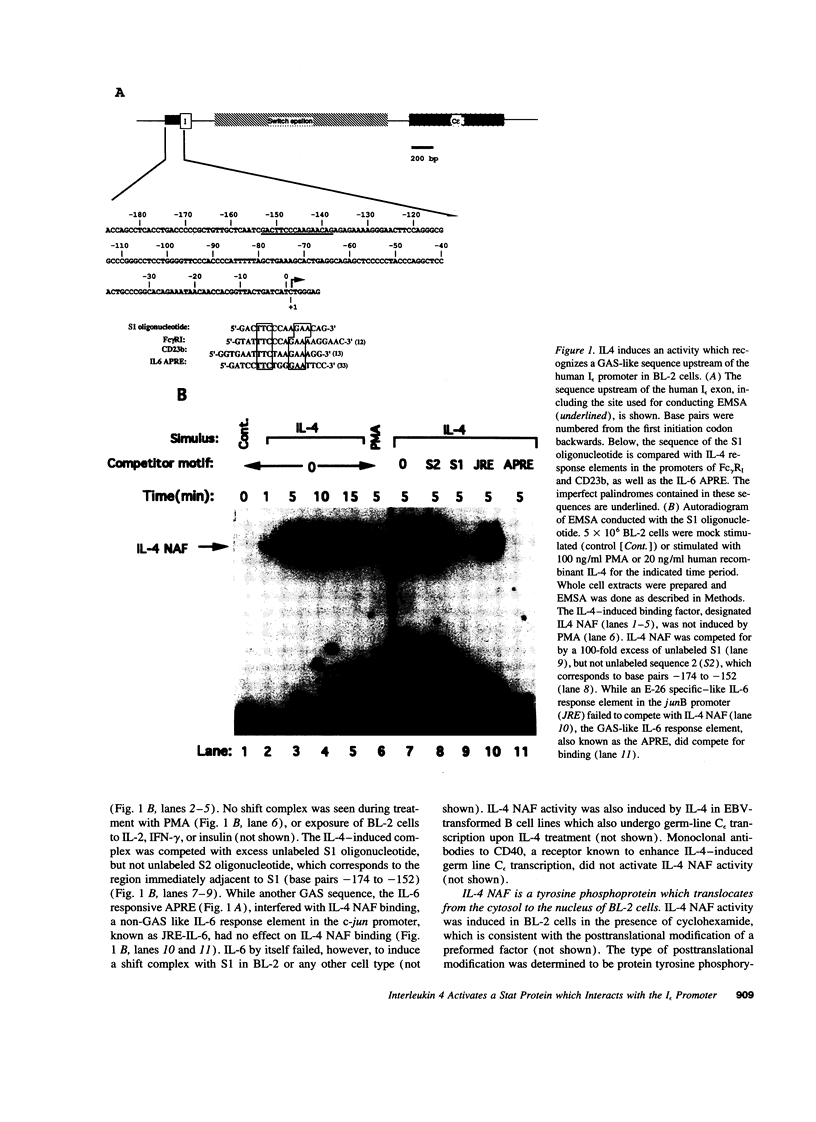
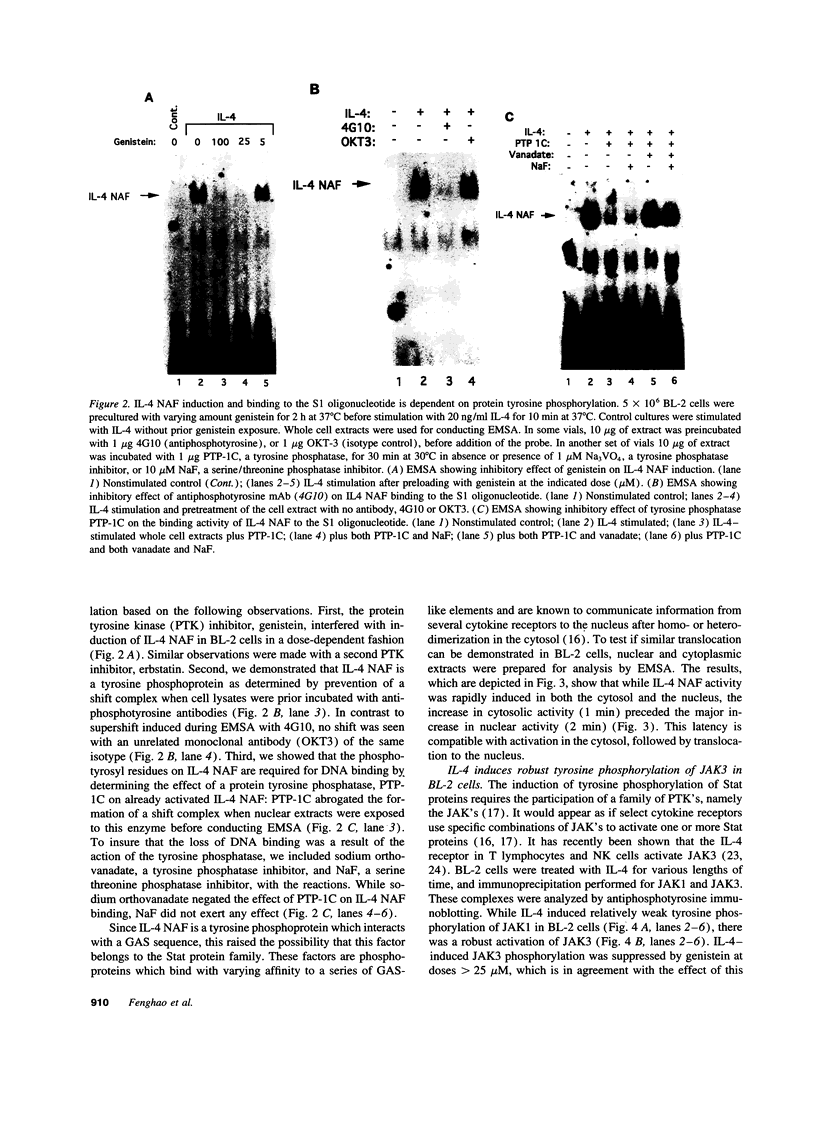
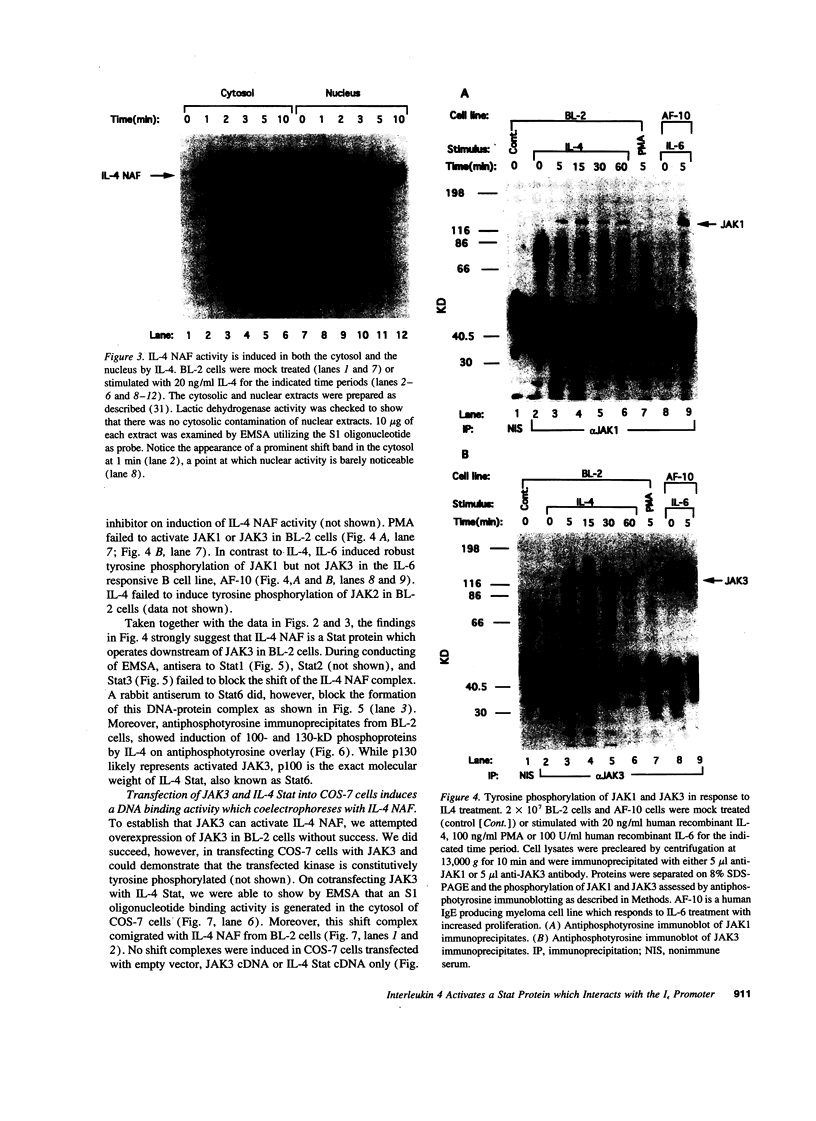
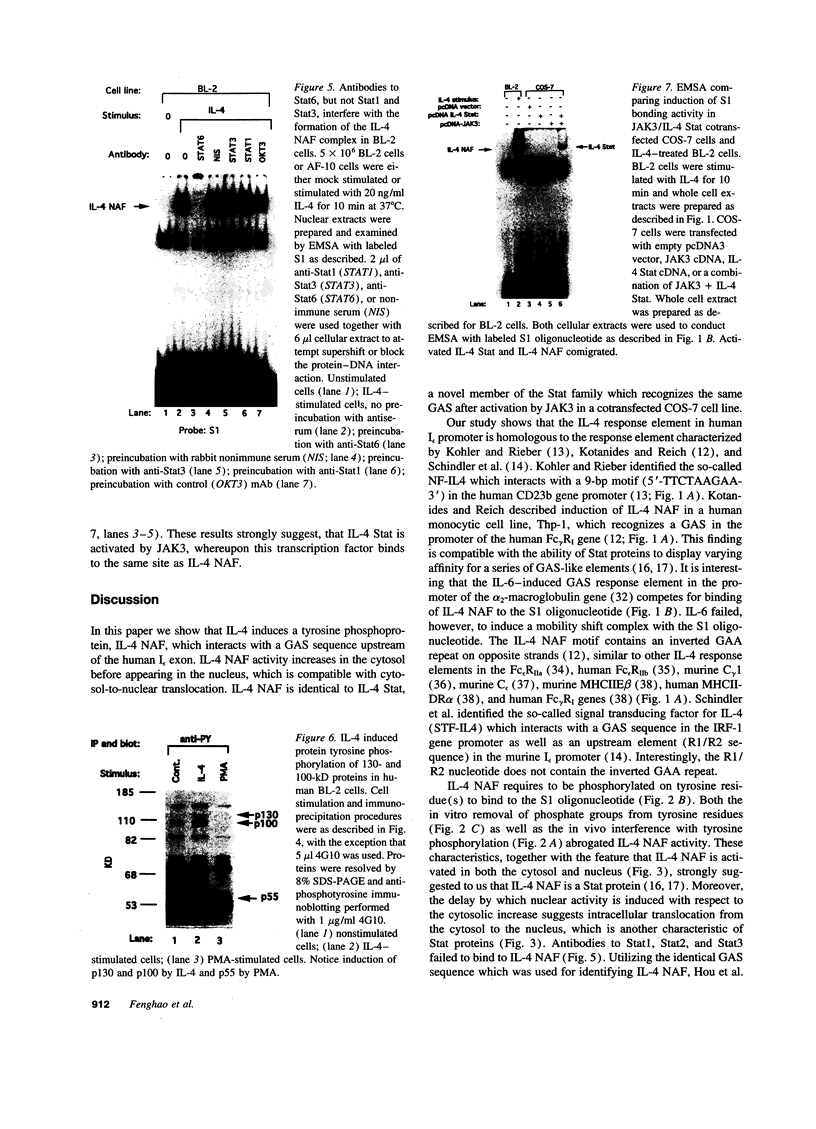
Germ line C transcripts can be induced by IL-4 in the human B cell line, BL-2. Utilizing a IFN-gamma activation site-like DNA sequence element located upstream of the I epsilon exon, we demonstrated by gel mobility shift assays that IL-4 induced a binding activity in the cytosol and nucleus of BL-2 cells. This factor was designated IL-4 NAF (IL-4-induced nuclear-activating factors) and was identified as a tyrosine phosphoprotein, which translocates from the cytosol to the nucleus upon IL-4 treatment. Because these are the characteristics of a signal transducer and activator of transcription (Stat) protein, we determined whether antibodies to Stat proteins will interfere with gel mobility shift and found that antibodies to IL-4 Stat, also known as Stat6, but not antibodies to other Stat proteins, interfere with the formation of the IL-4 NAF complex. Congruous with the involvement of a Stat protein, IL-4 induced robust Janus kinase 3 (JAK3) activity in BL-2 cells. Cotransfection of JAK3 with IL-4 Stat into COS-7 cells produced an intracellular activity which bound the same IFN-gamma activation site-like sequence and comigrated with IL-4 NAF in electrophoretic mobility shift assay. These results show that IL-4 NAF is IL-4 Stat, which is activated by JAK3 in response to IL-4 receptor engagement.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Albrecht B., Peiritsch S., Woisetschläger M. A bifunctional control element in the human IgE germline promoter involved in repression and IL-4 activation. Int Immunol. 1994 Aug;6(8):1143–1151. doi: 10.1093/intimm/6.8.1143. [DOI] [PubMed] [Google Scholar]
- Conrad D. H., Waldschmidt T. J., Lee W. T., Rao M., Keegan A. D., Noelle R. J., Lynch R. G., Kehry M. R. Effect of B cell stimulatory factor-1 (interleukin 4) on Fc epsilon and Fc gamma receptor expression on murine B lymphocytes and B cell lines. J Immunol. 1987 Oct 1;139(7):2290–2296. [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchat J. F., Gascan H., de Waal Malefyt R., de Vries J. E. Regulation of germ-line epsilon transcription and induction of epsilon switching in cloned EBV-transformed and malignant human B cell lines by cytokines and CD4+ T cells. J Immunol. 1992 Apr 1;148(7):2291–2299. [PubMed] [Google Scholar]
- Gauchat J. F., Lebman D. A., Coffman R. L., Gascan H., de Vries J. E. Structure and expression of germline epsilon transcripts in human B cells induced by interleukin 4 to switch to IgE production. J Exp Med. 1990 Aug 1;172(2):463–473. doi: 10.1084/jem.172.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher L. H., Kara C. J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Izuhara K., Harada N. Interleukin-4 (IL-4) induces protein tyrosine phosphorylation of the IL-4 receptor and association of phosphatidylinositol 3-kinase to the IL-4 receptor in a mouse T cell line, HT2. J Biol Chem. 1993 Jun 25;268(18):13097–13102. [PubMed] [Google Scholar]
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990 Dec 1;172(6):1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Jumper M. D., Splawski J. B., Lipsky P. E., Meek K. Ligation of CD40 induces sterile transcripts of multiple Ig H chain isotypes in human B cells. J Immunol. 1994 Jan 15;152(2):438–445. [PubMed] [Google Scholar]
- Keegan A. D., Nelms K., Wang L. M., Pierce J. H., Paul W. E. Interleukin 4 receptor: signaling mechanisms. Immunol Today. 1994 Sep;15(9):423–432. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Keegan A. D., Nelms K., White M., Wang L. M., Pierce J. H., Paul W. E. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994 Mar 11;76(5):811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Kotanides H., Reich N. C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993 Nov 19;262(5137):1265–1267. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- Köhler I., Rieber E. P. Allergy-associated I epsilon and Ec epsilon receptor II (CD23b) genes activated via binding of an interleukin-4-induced transcription factor to a novel responsive element. Eur J Immunol. 1993 Dec;23(12):3066–3071. doi: 10.1002/eji.1830231204. [DOI] [PubMed] [Google Scholar]
- Lebman D. A., Coffman R. L. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988 Sep 1;168(3):853–862. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills F. C., Brooker J. S., Camerini-Otero R. D. Sequences of human immunoglobulin switch regions: implications for recombination and transcription. Nucleic Acids Res. 1990 Dec 25;18(24):7305–7316. doi: 10.1093/nar/18.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Kawahara A., Fujii H., Nakagawa Y., Minami Y., Liu Z. J., Oishi I., Silvennoinen O., Witthuhn B. A., Ihle J. N. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994 Nov 11;266(5187):1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Kusafuka T., Takeda T., Fujitani Y., Nakae K., Hirano T. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol Cell Biol. 1993 May;13(5):3027–3041. doi: 10.1128/mcb.13.5.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991 May 1;77(9):1859–1870. [PubMed] [Google Scholar]
- Rothman P., Li S. C., Gorham B., Glimcher L., Alt F., Boothby M. Identification of a conserved lipopolysaccharide-plus-interleukin-4-responsive element located at the promoter of germ line epsilon transcripts. Mol Cell Biol. 1991 Nov;11(11):5551–5561. doi: 10.1128/mcb.11.11.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Johnston J. A., Noguchi M., Kawamura M., Bacon C. M., Friedmann M., Berg M., McVicar D. W., Witthuhn B. A., Silvennoinen O. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994 Nov 11;266(5187):1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993 Dec 17;262(5141):1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Schindler C., Kashleva H., Pernis A., Pine R., Rothman P. STF-IL-4: a novel IL-4-induced signal transducing factor. EMBO J. 1994 Mar 15;13(6):1350–1356. doi: 10.1002/j.1460-2075.1994.tb06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K. Interferon-activated signal transduction to the nucleus. Curr Opin Cell Biol. 1994 Apr;6(2):253–259. doi: 10.1016/0955-0674(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter U., Texido G., Hofstetter H. Expression of human lymphocyte IgE receptor (Fc epsilon RII/CD23). Identification of the Fc epsilon RIIa promoter and its functional analysis in B lymphocytes. J Immunol. 1989 Nov 1;143(9):3087–3092. [PubMed] [Google Scholar]
- Vercelli D., Geha R. S. Regulation of IgE synthesis in humans. J Clin Immunol. 1989 Mar;9(2):75–83. doi: 10.1007/BF00916934. [DOI] [PubMed] [Google Scholar]
- Wegenka U. M., Buschmann J., Lütticken C., Heinrich P. C., Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993 Jan;13(1):276–288. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegenka U. M., Lütticken C., Buschmann J., Yuan J., Lottspeich F., Müller-Esterl W., Schindler C., Roeb E., Heinrich P. C., Horn F. The interleukin-6-activated acute-phase response factor is antigenically and functionally related to members of the signal transducer and activator of transcription (STAT) family. Mol Cell Biol. 1994 May;14(5):3186–3196. doi: 10.1128/mcb.14.5.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Xu L., Rothman P. IFN-gamma represses epsilon germline transcription and subsequently down-regulates switch recombination to epsilon. Int Immunol. 1994 Apr;6(4):515–521. doi: 10.1093/intimm/6.4.515. [DOI] [PubMed] [Google Scholar]
- Xu M. Z., Stavnezer J. Regulation of transcription of immunoglobulin germ-line gamma 1 RNA: analysis of the promoter/enhancer. EMBO J. 1992 Jan;11(1):145–155. doi: 10.1002/j.1460-2075.1992.tb05037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A., Kikutani H., Tanaka T., Sato R., Barsumian E. L., Suemura M., Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988 Nov 18;55(4):611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]