Abstract
The plant cuticle composed of cutin, a lipid-derived polyester, and cuticular waxes covers the aerial portions of plants and constitutes a hydrophobic extracellular matrix layer that protects plants against environmental stresses. The botrytis-resistant 1 (bre1) mutant of Arabidopsis reveals that a permeable cuticle does not facilitate the entry of fungal pathogens in general, but surprisingly causes an arrest of invasion by Botrytis. BRE1 was identified to be long-chain acyl-CoA synthetase2 (LACS2) that has previously been shown to be involved in cuticle development and was here found to be essential for cutin biosynthesis. bre1/lacs2 has a five-fold reduction in dicarboxylic acids, the typical monomers of Arabidopsis cutin. Comparison of bre1/lacs2 with the mutants lacerata and hothead revealed that an increased permeability of the cuticle facilitates perception of putative elicitors in potato dextrose broth, leading to the presence of antifungal compound(s) at the surface of Arabidopsis plants that confer resistance to Botrytis and Sclerotinia. Arabidopsis plants with a permeable cuticle have thus an altered perception of their environment and change their physiology accordingly.
Keywords: cutin, cuticle, environmental stresses, lipid biosynthesis, pathogen defence
Introduction
The cuticle covers the epidermal cell wall of aerial tissues forming a boundary between the plant and its environment. It represents a primary barrier minimizing water and solute loss and protecting the plants against various abiotic and biotic stresses, but may also act in a number of developmental processes, such as delimitation of organs as well as trichome and stomata formation (Goodwin and Jenks, 2005; Nawrath, 2006; Riederer, 2006). The cuticle is composed of epicuticular and intracuticular waxes and a structural polymer, cutin, that are laid down in a well-organized manner (Jeffree, 2006). Waxes are a mixture of very long-chain fatty acids and their derivatives, and in some species, triterpenes and β-diketones (Kunst and Samuels, 2003; Jetter et al, 2006). Cutin is a polyester that is, in many plant species, formed of C16 and C18 ω-hydroxylated fatty acids that may carry additional hydroxy or/and epoxy groups in the mid-chain position (Kolattukudy, 2001). However, the cutin of Arabidopsis has an unusual composition as it consists of high amounts of C16 and C18 dicarboxylic acids (Bonaventure et al, 2004; Xiao et al, 2004; Franke et al, 2005). The large amounts of monomers only carrying carboxyl groups in the Arabidopsis cutin require other monomers rich in hydroxy groups in order to form polyesters. Glycerol might be such a monomer as it has recently been found to be a cutin monomer in several plant species (Graça et al, 2002).
Although a number of mutants potentially affected in the deposition of the cuticular polyesters have been characterized in recent years (Yephremov and Schreiber, 2005; Nawrath, 2006), changes in the amount and composition of cuticular polyesters have been determined only for a few mutants. The Arabidopsis mutant attenuation of type three genes (att1) having only 30% of the wild-type (WT) cuticular polyester encodes the cytochrome P450-dependent monooxygenase CYP86A2 (Xiao et al, 2004). The Arabidopsis mutant lacerata (lcr) carries a mutation in the homologue CYP86A8 and has a pleiotropic phenotype, including a permeable cuticle and a number of developmental disorders, such as organ fusions (Wellesen et al, 2001). Both mutants are most likely affected in cutin monomer biosynthesis as cytochrome P450s of the CYP86A type have been shown to have fatty acid hydroxylase activity (Benveniste et al, 1998). The organ fusion mutant hothead (hth) that exhibits a 30% reduction in dicarboxylic acids in its polyesters indicates the involvement of an oxidoreductase-dependent pathway for the formation of dicarboxylic acids in Arabidopsis (Krolikowski et al, 2003; Kurdyukov et al, 2006a). The extracellular α/β hydrolase BODYGUARD (BDG) may be involved in the formation of the polyester itself (Kurdyukov et al, 2006b). The bdg mutant accumulates more ester-bound cutin monomers than WT in the outer extracellular matrix surface, but is unable to form a functional continuous cuticular membrane. A potential function of bdg in both hydrolysis and synthesis of the polyester has been hypothesized as a number of phenotypes of bdg, including structural aspects of the polyester, resemble those observed in transgenic Arabidopsis plants expressing a fungal cutinase (Sieber et al, 2000; Kurdyukov et al, 2006b). WAX2 and LONG-CHAIN FATTY ACID SYTHETASE 2 (LACS2) are also enzymes potentially involved in cutin biosynthesis. LACS2 is an epidermis-specific long-chain acyl-CoA synthetase, whose absence, as that of WAX2, leads to altered properties and ultrastructure of the cuticular membrane (Schnurr et al, 2004). The LACS2 protein expressed in Escherichia coli has a preference for hydroxylated fatty acids, in accordance with the idea that LACS2 plays an important role in cutin biosynthesis (Schnurr et al, 2004).
The proposed function of the cuticle as a diffusion barrier has been intensively studied. Intercuticular waxes seem to play an important role in sealing the cuticle and are thought to be densely packed in the spaces of the cutin polyester that serves as a scaffold (Goodwin and Jenks, 2005). This model would explain why some mutants affected in cutin deposition are strongly disturbed in their permeability barrier (Sieber et al, 2000; Kurdyukov et al, 2006b). Although the cuticle forms a diffusion barrier, molecules can diffuse through the cuticle. Lipophilic non-ionic molecules diffuse through the hydrophobic cuticle and their diffusion kinetics are different from those of charged molecules that travel in an apolar path, while H2O diffuses in both routes (Schreiber, 2005; Riederer and Friedmann, 2006; Burghardt and Riederer, 2006).
Phytopathogenic fungi often secrete cutinases during their initial contact with a plant that liberates cutin monomers serving as signals for the induction of cutinases necessary for penetration and for a number of differentiation processes in fungi. For instance, germination and appressorium formation in Magnaporte grisea and formation of the appressorial tube in Erysiphe graminis are induced by cutin monomers (Kolattukudy et al, 1995; Francis et al, 1996; Gilbert et al, 1996). However, a crucial role of cutinases in the invasion of a plant was not always found. For example, deletion of the cutinases and cutinolytic lipases in the necrotrophic fungus Botrytis cinerea did not hinder the pathogen to enter intact plant tissues (Reis et al, 2005).
Very little is known about the significance of cutin for plant defence (Yephremov and Schreiber, 2005; Nawrath, 2006). The Arabidopsis mutant att1 was identified by its attenuating effect on virulence gene induction in phytopathogenic bacteria and its increased disease susceptibility toward bacterial infection (Xiao et al, 2004). In contrast, cutinase-expressing Arabidopsis plants and the bdg mutant were found to be strongly resistant to the necrotrophic fungus B. cinerea because of a multifactorial defence mechanism (Chassot et al, 2007). This resistance phenomenon has been seen in relation to earlier findings that cutin monomers may induce defence responses in plants (reviewed in Chassot and Métraux, 2005).
A forward genetic screen in Arabidopsis made it possible to dissect the potential pathway linking cutin or cutin monomers to resistance to B. cinerea. A mutant, botrytis resistant1 (bre1), displaying strong resistance to infection by B. cinerea, was also found to be defective in its cuticular membrane. bre1 carries a mutation in the LACS2 gene that was previously shown to be involved in cuticle development (Schnurr et al, 2004), and was here found to be important for cutin biosynthesis. The study of bre1/lacs2 and a number of other Arabidopsis mutants impaired in cutin monomer biosynthesis demonstrated that the increase in permeability of the cuticular membrane directly correlates with the amount of antifungal compounds released to the plant surface and Botrytis resistance. An interrupted cuticular membrane allowed the diffusion of signals and effector molecules across the cuticle resulting in an arrest of infection by Botrytis and Scleotiorum Sclerotinia.
Results
Isolation and characterization of the bre1 mutant
A total of 13 000 plants of an M2 population of EMS-mutagenized Arabidopsis plants were screened in parallel for two phenotypes: resistance to B. cinerea and increased cuticular permeability. One mutant of nearly normal size was strongly resistant to infection with B. cinerea strain (BMM) and was called bre1. Infection sites on bre1 leaves remained usually symptom free, in contrast to those on Col leaves (less than 2% of outgrowing lesions) (Figure 1A–C). In addition, bre1 displays increased cuticle permeability compared with WT as determined by Calcofluor staining. Calcofluor white fluoresces when it binds to β-glucans, such as cellulose in the cell wall. bre1 showed an extensive staining in all parts of the plant. In particular, the staining of cotyledons, the second rosette leaf pair, as well as most parts of the flower was distinctly different in bre1 compared with WT plants (Figure 1F, I, and L). All phenotypes were inherited in a recessive manner.
Figure 1.
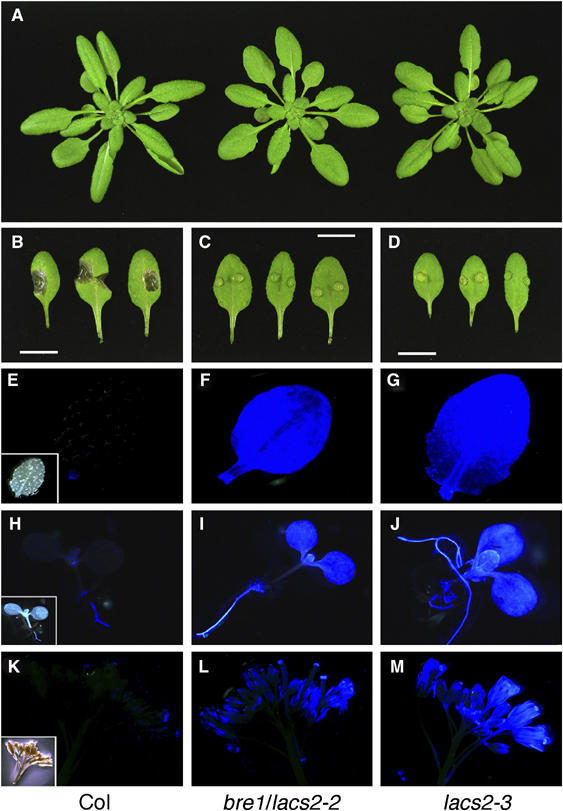
Phenotypes of bre1/lacs2-2 and lacs2-3 mutant isolates. (A) Morphology of the rosette of Col-0, lacs2-2, and lacs2-3. (B–D) Macroscopic evaluation of symptoms 3 days after inoculation with B. cinerea of Col-0, bre1/lacs2-2, and lacs2-3. Scale bar: 1 cm. (E–M) Calcofluor white-stained organs of Col-0, bre1/lacs2-2, and lacs2-3, viewed under UV light. Col-0 pictures are shown with the corresponding pictures in bright-field microscopy as inset. Leaves (E–G), young seedlings (H–J), and the tip of a flowering inflorescence (K–M) are shown.
BRE1 has a mutated LACS2 gene
BRE1 was mapped to the middle of chromosome 1 close to LACS2 (Lukowitz et al, 2000), which has been previously identified to be important for cuticle development (Schnurr et al, 2004). Arabidopsis plants (accession Col-0) carrying a T-DNA insertion in the LACS2 gene were obtained that were also resistant to B. cinerea (quantitative data shown in Figure 6B), stained strongly with Calcofluor, and had no other obvious morphological phenotypes (Figure 1A, D, J, and M) (Rosso et al, 2003). Complementation analysis between bre1 and the LACS2 T-DNA insertion line revealed that BRE1 is indeed LACS2, as both mutants did not complement each other in the F1 generation (Supplementary Figure 1). We therefore named bre1 lacs2-2 and the T-DNA insertion line lacs2-3.
Figure 6.
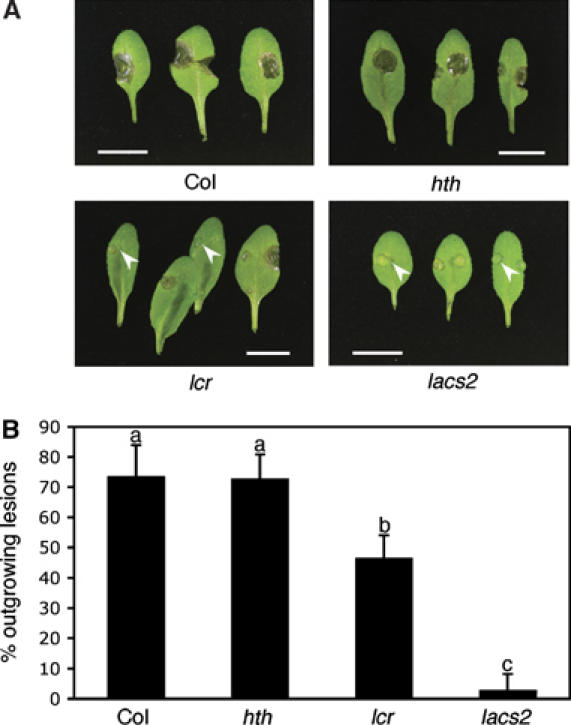
Resistance to Botrytis of mutants having differences in their cuticular polyesters. (A) Macroscopic symptoms 3 days after inoculation with B. cinerea (BMM) on leaves of different genotypes. White arrowheads indicate examples of symptom-free inoculation sites. Scale bar: 1 cm. (B) Number of outgrowing lesions developed 3 days after inoculation with B. cinerea (BMM) in different genotypes (n=4; ±s.e.; different letters indicate significance at P<0.005 using ANOVA). The experiment was repeated twice with similar results.
Sequence analysis revealed mutations in LACS2-2 leading to the expression of a truncated cDNA (Supplementary Figure 2A and C). Mutations accompanied by insertion of the T-DNA in LACS2-3 lead to an RNA-null allele (Supplementary Figure 2B and C). As lacs2-2 and lacs2-3 had very similar phenotypes during the initial characterization, only data for lacs2-3 are presented in the following studies.
The cuticle of the lacs2 mutant
The ultrastructure of the cuticular membrane of the lacs2-3 mutant was analyzed by transmission electron microscopy (TEM). The distinct osmium-dense cuticular membrane representing insoluble lipid-derived polymers such as cutin, visible at the epidermal outer extracellular matrix of WT plants, could not be seen at the leaf epidermis of lacs2-3 (Figure 2A and B). Interestingly, the remaining outer epidermal extracellular matrix sometimes had a distinct laminated structure (Figure 2C). Growing plants on a large scale revealed that lacs2-3 plants very rarely formed organ fusions (one organ fusion in less than 1% of plants). Ultrastructural analysis of such organ fusions revealed that both epidermal cell walls are directly fused without any osmium-dense layer in between them (Figure 2D).
Figure 2.
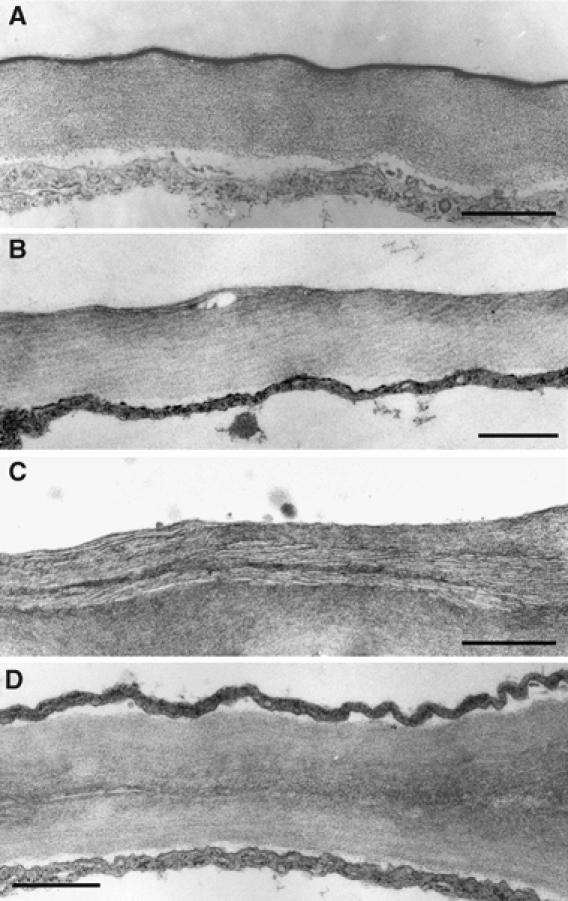
Ultrastructure of the epidermal extracellular matrix of rosette leaves. Lower epidermal extracellular matrix of Col-0 (A) and lacs2-3 (B, C) leaves. Extracellular matrix at the point of fusion between the lower epidermis of one leaf and the upper epidermal cell layer of another leaf in lacs2-3 (D). Scale bar: 500 nm (A) and 800 nm in (B–D).
The cuticular membrane of lacs2-3 was not visible as an electron-opaque layer, indicating that the osmium-dense, insoluble lipids might be largely missing. Thus, the amount and composition of ester-bound lipids were analyzed from plant material after complete elimination of soluble lipids. Analysis of residue-bound lipids of lacs2-3 in comparison with Col-0 plants revealed that a number of ester-bound monomers were reduced in the lacs2-3 mutant (Figure 3A and B). The total amount of ω-hydroxylated fatty acids and their derivatives was reduced 4- to 5-fold in lacs2-3 compared with Col-0 (Figure 3A). The impact of LACS2 mutation on the amount of dicarboxylic acids, characteristic of Arabidopsis cutin, was particularly strong, and the amount was reduced to 15–20% of WT amount. ω-Hydroxylated fatty acids and their dihydroxy fatty acids were reduced to less than 50%. Thus, lacs2 has a strongly reduced content of cuticular polyester.
Figure 3.
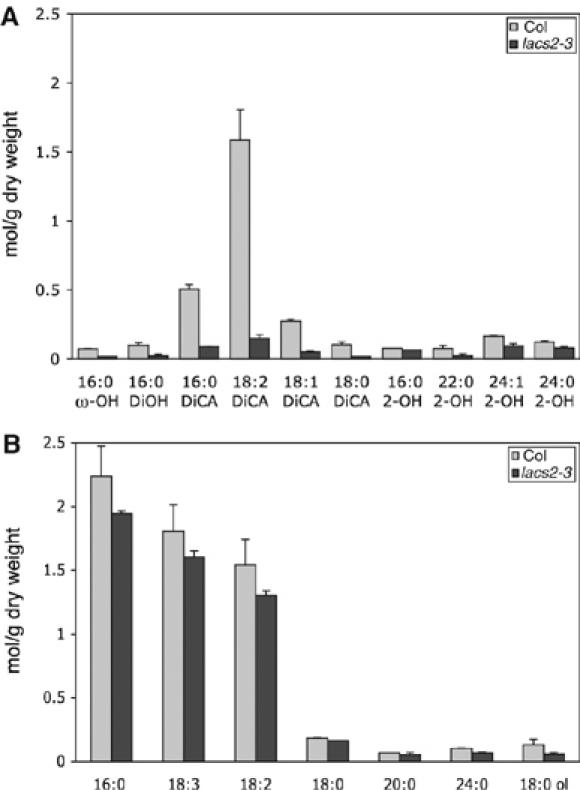
Composition of aliphatic monomers of leaf polyesters. Large rosette leaves of 5- to 6-week-old plants of Col-0 and lacs2-3 were analyzed. (A) Hydroxylated fatty acids and their derivatives and (B) fatty acids and fatty alcohols. DiOH, dihydroxy carboxylic acid; DiCA, dicarboxylic acid (n=3; ±s.e.; the experiment was repeated once with similar results).
Permeability of the cuticle of lacs2 and other cuticle mutants
An intact cuticular membrane is important to reduce water loss from the plant under drought conditions (Goodwin and Jenks, 2005; Burghardt and Riederer, 2006). Water loss was taken as a measure of cuticular permeability and was determined in fully expanded but not yet bolting rosettes excised at the level of the hypocotyls. As shown in Figure 4, the rate of water loss from lacs2-3 is significantly higher than that of Col-0 plants. As the stomata of both Col-0 and lacs2-3 plants were well closed during the experiment (Supplementary Figure 3), lacs2-3 is likely to have a higher cuticular transpiration than Col-0.
Figure 4.
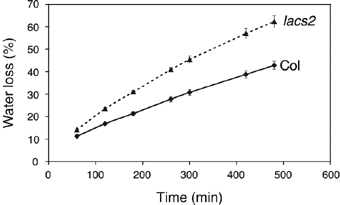
Cuticular transpiration. Loss of water from 5- to 6-week-old rosettes of Col-0 and lacs2-3 plants was measured at the indicated time points (n=4–6; ±s.e.; experiment was repeated twice with similar results).
Sensitivity to xenobiotics such as herbicides also reflects the permeability of the cuticular membrane. Permeability of the upper surface of Arabidopsis leaves was specifically determined to avoid the problem of molecule exchange via stomata, as these openings are mostly positioned on the abaxial side of leaves. Besides WT and lacs2-3 plants, two other mutants, hth and lcr, reported to have a permeable cuticle were included in this experiment (Lolle et al, 1998; Wellesen et al, 2001). lacs2-3 as well as lcr showed severe damage at a lower concentration of BASTA® compared with hth and WT Col-0 plants (Table I).
Table 1.
Sensitivity to BASTA® of hth, lcr, and lacs in comparison with WT Col-0
| Basta (nl/ml) | Col | hth | lcr | lacs2 |
|---|---|---|---|---|
| 1 | +++++ | +++++ | +++++ | +++++ |
| 10 | +++++ | +++++ | ++++ | ++++ |
| 20 | ++++ | ++++ | +++ | +++ |
| 40 | ++++ | ++++ | ++ | ++ |
| 60 | +++ | +++ | +/− | +/− |
| +++++: fully green; ++++: chlorotic tips or spots; +++: whole leaves chlorotic, some dying; ++: all expanded leaves died, but vigorous regrowth; +: poor or late regrowth; +/−: dead or regrowth after 10 days only. | ||||
Permeability of the cuticle of lacs2 and other mutants was further assessed with toluidine blue applied in the form of droplets on the adaxial sites of the leaves (Tanaka et al, 2004; Kurdyukov et al, 2006b). As toluidine blue can be directly applied to plant tissues without solvent treatment, permeability of a functional cuticular membrane can be investigated by this staining method, in contrast to Calcoflour staining. Two hours after the application of toluidine blue solution, Col-0 leaves showed a small stained spot covering less than 10% of the area on which the droplet was applied, whereas lacs2 leaves showed dark blue staining on 75–100% of the area. Interestingly, the lcr leaves showed staining on approximately 25–50% of the droplet area, whereas hth had a staining pattern similar to that of Col-0 (Figure 5A). A shorter incubation time, that is, 30 min, resulted in a similar pattern, but the staining was weaker (data not shown). Leaves of lacs2-3 were already stained after only 5 min and the whole area underneath droplets was stained after 30 min (Figure 5B).
Figure 5.
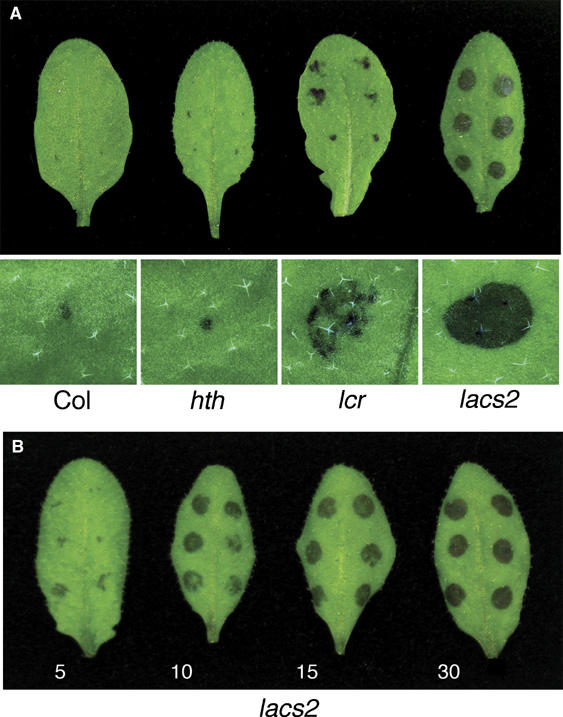
Permeability of leaf cuticles of mutants having an altered cuticular polyester. (A) Droplets of a toluidine blue solution were incubated on the leaves for 2 h and then washed with water. An overview of typically stained leaves from each genotype is given in the top panel. A microscopic view of the typical staining underneath a droplet that was incubated for 2 h on leaves from each genotype is given below its overview. (B) Toluidine blue droplets were incubated on lacs2-3 leaves for the time points indicated below each leaf and then briefly rinsed with water. Typically stained leaves are presented. A colour version of this figure is available at the EMBO Journal online.
Taken together, our data demonstrate that the lacs2-3 has a highly permeable cuticle that is even more permeable than that of lcr, in comparison with WT Col-0, whereas an increased cuticular permeability of hth leaves could not be confirmed in our experimental settings.
Resistance to B. cinerea of mutants having altered cuticular permeability
Resistance of lacs2 plants to Botrytis was compared with other mutants affected in cuticle formation. The visual phenotypes of the leaves 3 days after inoculation are depicted in Figure 6A. The number of lesions that were larger than the original infection site (outgrowing lesions) is given as a quantitative measure of the resistance level (Figure 6B). The lacs2-3 mutant exhibited a strong resistance to infection with B. cinerea strain BMM, as described above, with less than 5% outgrowing lesions in comparison with WT Col-0 (75% outgrowing lesions). A significant increase in the resistance to B. cinerea was also observed in lcr (45% outgrowing lesions), whereas no increase in resistance was observed in hth. In addition, infection sites could be observed in lcr that did not develop any disease symptoms, similar to the symptom-free infection sites in the lacs2-3 mutant (Figure 6A). The frequency of symptom-free lesions in lcr was significantly lower than that in the lacs2-3 mutant (30 and 95%, respectively). Similar results were obtained with B. cinerea strain BO5.10.
Analysis of leaf diffusates for antifungal compounds
The permeability of the cuticle correlated with the resistance to B. cinerea (Figures 5A and 6) but not with PDF1.2 and camalexin production that have often been correlated to Botrytis resistance (Penninckx et al, 1998; Berrocal-Lobo et al, 2002; Coego et al, 2005; Kliebenstein et al, 2005) (Supplementary Figure 4). In the search for antimicrobial compounds at the surface of the plant that might arrest the infection at an early stage, droplets of 1/4 strength potato dextrose broth (PDB) were incubated on the leaves so that antifungal substances could diffuse into them. The activity of these diffusates was analyzed in two assays: (1) spores of B. cinerea were germinated in vitro in the presence or absence of diffusates and the length of the hyphae was measured (in vitro assay). (2) Botrytis spores were also tested for their ability to infect WT plants in the presence or absence of diffusates (in vivo assay).
The results of in vitro assay are presented in Figure 7A. The 1/4 PDB diffusate from lacs2-3 collected after 18 h of incubation inhibited the development of Botrytis hyphae, whereas the diffusates from Col-0, lcr, and hth did not. After 44 h, the 1/4 PDB diffusates collected from lcr and lacs2-3 were active, whereas these from hth and Col-0 were not. The growth-inhibiting effect of diffusate from lacs2-3 was much stronger than that from lcr, whereas the 1/4 PDB diffusates from hth and Col-0 were not active. Results of the in vivo test are presented in Figure 7B. Botrytis spores that were mixed with 1/4 PDB diffusates, collected after 18 or 44 h from lacs2-3 plants, were unable to infect Col-0 plants (0% outgrowing lesions). Spores incubated with 1/4 PDB diffusates from lcr collected after 44 h could not infect Col-0 plants (0% outgrowing lesions), but when collected after 18 h could still infect Col-0 normally (85% outgrowing lesions). Spores incubated with 1/4 PDB diffusates from hth or Col-0 had no significant reduction in infection capability (70–85% outgrowing lesions).
Figure 7.
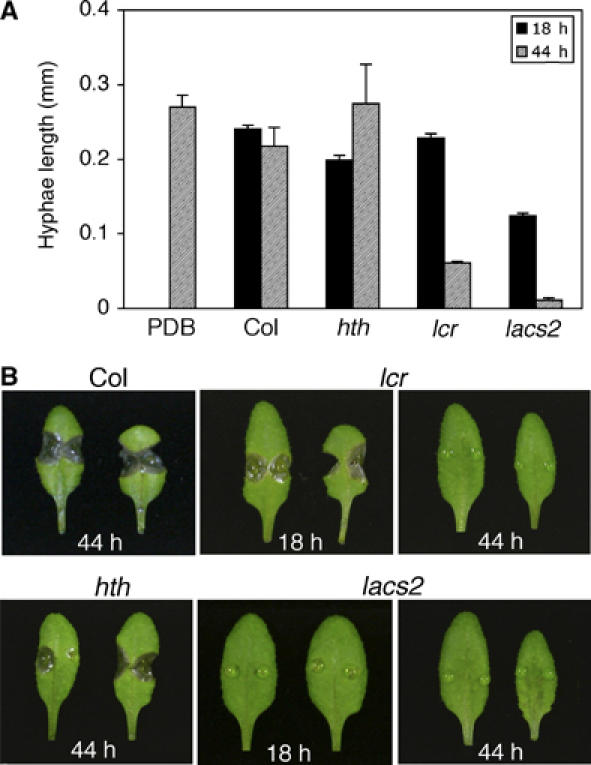
Analysis of PDB diffusates for the presence of growth-inhibiting activities against B. cinerea. (A) Germination and growth of B. cinerea (BMM) in vitro in the presence of leaf diffusates collected at 18 h (grey bars) and 44 h (black bars) from plants of different genotypes. Three independent experiments were undertaken and six representative pictures were evaluated ±s.d. (B) B. cinerea (BMM) spores were incubated on WT Col-0 leaves in the presence of 1/4 PDB diffusates collected from leaves of different genotypes at indicated time points. Typical symptoms 3 days after inoculation are presented.
Both tests clearly indicate that the surface of plants with an increased cuticle permeability releases antifungal compounds against Botrytis and that the activity depends on the time of diffusion. Comparison of the in vitro and in vivo tests suggests that a reduction of 50% and more in the growth of the hyphae in vitro leads to an arrest in infection in vivo.
Characterization of antifungal compounds and their induction
The antifungal compounds present in the 1/4 PBD diffusate obtained from lacs2-3 plants after an incubation time of 42 h were characterized by testing their in vitro activity on Botrytis spores after different treatments: the active compound(s) were resistant to lyophilization and heat treatment and could not be extracted into ethyl acetate, hexane, or chloroform, indicating that they are of polar nature.
The antifungal activity of the lacs2 diffusate could be diminished by treatment with a lipase from Mucor miehei. The germlings of Botrytis were four to eight times longer than without lipase treatment of the diffusate (Figure 8 and Supplementary Figure 5). Depending on the PDB diffusate preparation, 12–60% of growth of the germlings in 1/4 PDB treated with lipase could be restored. Similar results were obtained with a lipase from Candida antarctica that had higher specific activity (data not shown). Proteinase K treatment has only a small effect on the activity of PDB diffusates (Supplementary Figures 5 and 8). Germlings were only 2–3 times longer than when incubated in untreated PDB diffusate. Between 5 and 18% of the germling length grown in 1/4 PDB treated with proteinase K could be restored (Figure 8).
Figure 8.
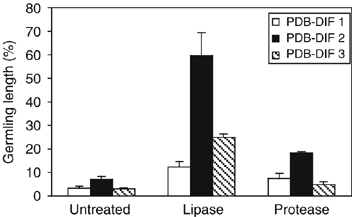
Analysis of the activity of different PDB diffusates from lacs2 after enzymatic digestions. Germination and growth of B. cinerea (BMM) in the presence of different 1/4 PDB diffusates (PDB DIF) from lacs2 that had undergone no treatment (control), treatment with lipase from M. miehei at 32 U/μl (lipase), or proteinase K (protease) are shown. Lengths of the germlings grown in treated 1/4 PDB diffusates from lacs2 were normalized to those grown in treated 1/4 PDB (100%). Three to four representative pictures were evaluated ±s.d.
These results indicate that the lacs2 diffusate most likely contains several components. Differences in the efficiency of the treatments may reflect differences in the composition of diffusates that may result from slightly different induction kinetics of the compounds. One of the prominent active compounds of the PDB diffusate from lacs2 plants may be a lipid or a compound that contains a linkage to an acyl group that is important for its activity. This lipase-sensitive compound is of relatively low molecular weight, as it could be filtrated through a membrane with a molecular weight cutoff of 3000 Da (data not shown).
Interestingly, only PDB itself—even when adjusted to different pH—induced the antifungal compounds in lacs2-3 plants and not the major components of PDB (sucrose and starch), different pH, different ion concentrations, and osmotic potential (Table II). The inducer is, therefore, most likely a minor component of PDB that acts as an elicitor to induce antifungal compounds inhibiting the growth of the hyphae of B. cinerea and S. sclerotiorum, but not of Alternaria brassicicola and Plectosphaerella cucumerina (Supplementary Figure 6). In accordance with this observation, lacs2-3 also showed increased resistance to infection by Sclerotinia (Figure 9). Interestingly, the resistance of lacs2 to infection by Sclerotinia could be further increased by a 20-h preincubation period of the future inoculation sites with 1/4 strength PDB, again demonstrating the importance of PDB-mediated induction for the defence response observed in permeable cuticle mutants (Figure 9).
Table 2.
In vitro hyphal growth of Botrytis in the presence of various diffusates
| Col-0 | lacs2 | |
|---|---|---|
| 1/4 PDB | +++ | − |
| 1/4 PDB pH 4.0 | +++ | − |
| 1/4 PDB pH 8.0 | +++ | − |
| Glucose (5 g/l) | +++ | +++ |
| Starch (1 g/l) | +++ | +++ |
| 1 M NaCl | + | + |
| 150 mM NaCl | +++ | +++ |
| 300 mM mannitol | +++ | +++ |
| 0.1 M Na-acetate buffer pH 4.0 | − | +/− |
| 0.1 M K-phosphate buffer pH 8.0 | − | +/− |
| H2O | +++ | +++ |
| H2O/Botrytis (5 × 104 spores/ml) | +++ | +++ |
| +: more than 90% hyphal growth in comparison with 1/4 PDB; +/−: reduced growth of hyphae with abnormal morphology; −: less than 10% growth of hyphae. | ||
Figure 9.
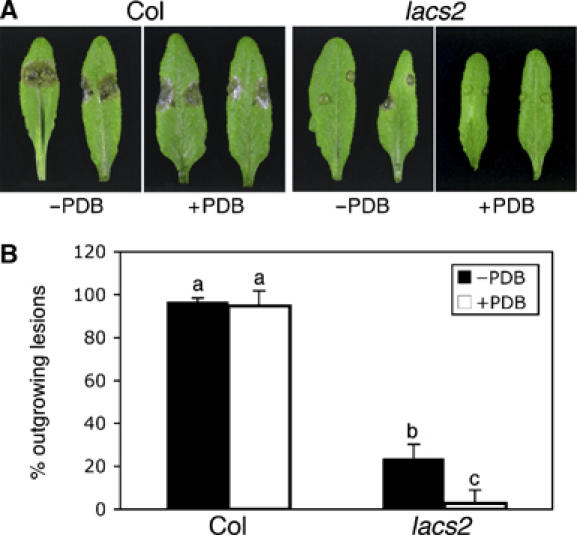
Influence of PDB preincubation on the resistance of lacs2 to Sclerotinia. (A) Macroscopic symptoms 3 days after inoculation with S. sclerotiorum on leaves of Col-0 and lacs2 without (−PDB) and with preincubation (+PDB) with 1/4 PDB for 20 h. (B) A number of outgrowing lesions developed 3 days after inoculation with S. sclerotiorum in Col-0 and lacs2 plants without (−PDB) and with preincubation (+PDB) with 1/4 PDB for 20 h (n=6; ±s.e.; different letters indicate a significance at P<0.005 using ANOVA). The experiment was repeated with equivalent results.
Discussion
bre1/lacs2 is a cuticle mutant
Here, we describe the isolation of two new alleles of lacs2 using a screening based on resistance to the fungal pathogen B. cinerea. The LACS2 gene has previously been identified to be involved in cuticle formation (Schnurr et al, 2004).
Several methods were used to demonstrate an increased permeability of the native cuticle in the lacs2-3 mutant; these methods include cuticular transpiration rate, herbicide sensitivity, and staining of leaves with toluidine blue (Figures 5 and 6; Table I). These studies corroborated and extended the findings that lacs2-1 may have an increased permeability of the cuticle, as previously shown by increased chlorophyll leaching (Schnurr et al, 2004).
Ultrastructural analysis revealed that the cuticular membrane of lacs2-3 was not visible at the outer extracellular matrix of the epidermis (Figure 3B). Previous findings showed a cuticular membrane of reduced thickness in the lacs2-1 mutant (Schnurr et al, 2004). The differences in the appearance of the cuticular membrane in TEM may result from different growth conditions, developmental stage of the organ, and microscopic techniques. The surface of the epidermis collapsed in a very similar way when prepared for SEM in both lacs2-1 and lacs2-3 mutants (data not shown) (Schnurr et al, 2004). Furthermore, the amount of cuticular waxes increased in lacs2-3, as reported for the lacs2-1 mutant (data not shown) (Schnurr et al, 2004).
Developmental phenotypes described for lacs2-1 were weaker under our growth conditions, that is, the rosettes were only slightly smaller and leaf deformations and/or organ fusions between leaves were only rarely observed (Schnurr et al, 2004). When organs were fused, the ultrastructure of the fusion was similar to that in other mutants defective in the cuticular membrane (Figure 2d) (Lolle et al, 1998; Wellesen et al, 2001; Yephremov and Schreiber, 2005; Nawrath, 2006; Kurdyukov et al, 2006a, 2006b). Obvious macroscopic developmental phenotypes were thus different between the different lacs2 alleles and most likely depended on the environmental conditions under which the plants were grown. In addition, our studies were performed in the Col-0 background, whereas lacs2-1 is in the WS background (Schnurr et al, 2004).
LACS2 is necessary for the biosynthesis of the cutin polyester
Analysis of the monomer composition of residue-bound polyesters of LACS2 revealed that ω-hydroxylated fatty acids and their derivatives, including ω-hydroxy carboxylic acids, dihydroxy carboxylic acids, and dicarboxylic acids, were overall reduced to 20–25% of WT Col-0 amounts. This strong reduction in the typical cutin monomers of Arabidopsis coincided with a complete loss of insoluble lipids that can be normally visualized as a distinct osmium-dense layer present on the outer epidermal extracellular matrix (Figures 2B and 3A) (Bonaventure et al, 2004; Franke et al, 2005). The reduction in hydroxylated fatty acids corroborates studies on LACS2 expressed in E. coli showing a preference for ω-hydroxy palmitic acid over palmitic acid (Schnurr et al, 2004). Interestingly, the reduction in dicarboxylic acids in the polyester (to 85%) was higher than that of 2-hydroxy acids, dihydroxy acids, and ω-hydroxy acid, suggesting that dicarboxylic acids may be the preferred substrate of LACS2. Reduction in 2-hydroxy acids was not always significant. Comparisons of the polyester composition between total extracted plant material and cuticles of Arabidopsis had previously shown that only 20–25% of 2-hydroxy acids detected in total extracted leaves are present in the cuticle, whereas 75–100% of ω-hydroxylated fatty acid derivatives are in the cuticle (Franke et al, 2005). Thus, a small reduction in total 2-hydroxy acids may still represent significant changes in cuticular 2-hydroxy acids. Further studies on the LACS2 enzyme using a broad range of hydroxylated fatty acids and their derivatives will be necessary to determine the substrate specificity more accurately.
The broad impact of LACS2 on polyester composition is interesting, indicating that LACS2 may be important for a general step in cutin biosynthesis. Nothing is known about the transport mechanism of cutin monomers and the assembly of the cutin polyester. Other isoenzymes of long-chain fatty acid-CoA synthetases are however involved in the CoA activation of fatty acids before their transfer to glycerol during lipid biosynthesis (Shockey et al, 2002). Therefore, an attractive idea is that LACS2 is essential for activation of cutin monomers for their assembly into cutin domains, potentially containing glycerol or other molecules rich in hydroxy groups still inside the cell, that are then transported across the membrane to the outermost layer of the cell wall for their final polymerization.
The high permeability of the cuticle of lacs2-3 can thus be attributed to the large reduction in the cutin polyester. The slight increase in cuticular waxes of lacs2 that has also been observed in transgenic Arabidopsis plants expressing a fungal cutinase and in the bdg mutant might represent a compensation reaction to improve inadequate cuticular properties (Sieber et al, 2000; Schnurr et al, 2004; Kurdyukov et al, 2006b). The results obtained with lacs2 support the idea that the cutin polyester could serve as a scaffold in which intracuticular waxes are packed to seal the cuticle (Goodwin and Jenks, 2005).
Role of cuticle in plant pathogen interactions
The characterization of lacs2 as a cutin biosynthetic mutant that is strongly resistant to Botrytis and Sclerotinia is per se a counter-intuitive result. In the past, the cuticle has been seen as a barrier that protects plants from invading pathogens. However, lacs2 did not show an altered resistance or susceptibility to a number of pathogens, such as the adapted and non-adapted powdery mildew isolates Golovinomyces orontii, Erysiphe pisi, and Blumeria graminis f. sp hordei (Supplementary Figure 7) as well as certain necrotrophic fungi such as A. brassicicola (data not shown) and P. cucumerina (Supplementary Figure 8) (Thomma et al, 1999; Berrocal-Lobo et al, 2002; Consonni et al, 2006). Similarly, cutinase-expressing Arabidopsis plants did not show an altered susceptibility to E. cichoracearum, B.g. hordei, Hyaloperonospora parasitica (NOCO), and Phytophthora brassicae (Sieber et al, 2000; Chassot and Métraux, 2005). The cuticle of Arabidopsis is therefore not a major barrier to prevent pathogen invasions.
As cutinase-expressing plants as well as the Arabidopsis mutant bdg were both immune to infection by Botrytis, it had been speculated that cutin monomers or the action of cutinase/hydrolase that are released into the surface of these plants may be relevant for the resistance to Botrytis (Chassot and Métraux, 2005; Chassot et al, 2007). However, studies presented here show that the phenotype common for all mutants exhibiting an increased resistance to Botrytis is an increased permeability of the cuticle (Lolle et al, 1998; Sieber et al, 2000; Wellesen et al, 2001; Schnurr et al, 2004; Kurdykow et al, 2006a). Most likely a direct effect of the permeable cuticle, such as an altered transpiration rate or stomata closure, does not confer resistance to Botrytis; resistance is probably due to an indirect mechanism.
A permeable cuticle allows the induction of antifungal compounds
Detailed studies on the permeability of the cuticle by using toluidine blue evidenced a direct correlation between increased permeation through the cuticle into the apoplast (Figure 6A) to the appearance of antifungal compounds active against Botrytis in leaf diffusates (Figure 8) and the degree of resistance to B. cinerea (Figure 7).
lacs2-3 showed a very high permeability of the cuticle (Figure 6), a rapidly appearing strong antifungal activity in leaf diffusates (Figure 8), and very strong resistance to B. cinerea (Figure 7). lcr has a cuticle of intermediate permeability that is not as high as that of lacs2-3 plants (Figure 6A). Antifungal activities of lcr could only be identified at late time points of the incubation period (Figure 8). In addition, lcr had an intermediate level of resistance to Botrytis that was clearly lower than that of lacs2-3 plants (Figure 7). The hth mutant did not show any increased cuticle permeability in our experimental settings, no antifungal activities (Figure 8), and no resistance to Botrytis (Figure 7), in accordance with the hypothesis that an increased permeability of the cuticle is necessary for the presence of antifungal compounds and Botrytis resistance.
Toluidine blue diffused through the cuticular membrane of lacs2 and lcr within less than 1 h, whereas antifungal compounds appear only much later on the surface of lacs2 and lcr (18–44 h), indicating that the production of these antifungal compounds has to be induced. Induction studies with different solutions evidenced that the inducer of Botrytis and Sclerotinia resistance in lacs2 plants is most likely a minor component present in PDB. Molecules acting as elicitors could be, for example, cell-wall degradation products of potato, such as oligosaccharides that have been shown to elicit defence responses (Côté and Hahn, 1994; Ridley et al, 2001). The fact that pretreatment with PDB increases resistance of lacs2 to Sclerotinia indicates that the induction of defence responses is a critical step for this type of resistance.
PDB-induced defence responses include production of antifungal compounds that are active against certain necrotrophic fungi. Antifungal compounds have also been found in cutinase-expressing plants and in the bdg mutant (Chassot et al, 2007). PDB diffusates collected from lacs2 plants could be partially inactivated by treatment with lipases, indicating that an acyl group is necessary for the activity of some of its components. Oxylipins are potent antimicrobial compounds and Botrytis is sensitive to some of them (Prost et al, 2005). Oxylipins of plant origin have also been shown to activate biological responses in fungi, showing that oxylipins can be transferred from a plant to a fungus (Tsitsigiannis and Keller, 2006). However, the inactivation of the active compound by lipases is somewhat surprising as Botrytis secretes lipases (Reis et al, 2005). Inactivation may thus also result from an additional activity, that is, an esterase activity, at high enzyme concentrations. Only the determination of the chemical structure of this antimicrobial compound will solve this enigma. In addition, the PDB diffusate from lacs2 lost some activity on treatment with proteinase K. A large number of peptides and antimicrobial proteins are encoded in the Arabidopsis genome, among which are 300 defensin-like genes (Garçia-Olemedo et al, 1998; Silverstein et al, 2005). Antimicrobial peptides may thus contribute to the described resistance response. A specific sensitivity of a pathogen to different antimicrobial compounds has been found in a number of cases and could explain why lacs2 is resistant only to Botrytis and Sclerotinia and not to other necrotrophs (Thomma et al, 1999; Prost et al, 2005).
A number of different genes conferring Botrytis resistance when overexpressed in WT plants were induced in cutinase-expressing plants and in bdg (Chassot et al, 2007). Although the genes characterized by Chassot et al were not induced in lacs2 plants under our experimental settings (data not shown), it is well possible that additional defence mechanisms, that is, strengthening of the cell wall, contribute to the resistance to Botrytis upon induction with PDB, explaining why the effect of the PDB diffusate on Botrytis resistance in vivo seems to be stronger than in vitro (50% reduction in hyphal length in vitro results in 100% resistance).
The basis of the resistance described here in plants having a permeable cuticle thus lies in a better perception of elicitors from PDB leading to a number of defence responses, including the release of antifungal compounds, that arrest the invasion of certain necrotrophic pathogens. Most likely both the elicitor and the antifungal compounds are restricted in their diffusion across the cuticle, resulting in the observation that resistance to Botrytis is directly correlated to permeability of the cuticle of different Arabidopsis mutants. Botrytis resistance in permeable cuticle mutants is therefore an example of the importance of the timing of defence responses for the outcome of a plant–pathogen interaction (Tao et al, 2003). The cuticle is usually seen as a protective layer because it slows down interactions between the plant and its environment. We present here an example where this reduced diffusion rate is to the disadvantage of the plant, an aspect of cuticle biology that has not been reported before.
Materials and methods
Plant material
Plants were grown on a pasteurized soil mix under a 12 h light and 12 h dark cycle, with an average night temperature of 16°C (up to 90% humidity) and a day temperature of 20°C (60% humidity). WT plants are Arabidopsis accession Col-0, obtained from the Arabidopsis Biological Research Center (Columbus, OH). The mutants lcr (lcr1) and hth (hth-12), both in the Col-0 background, were obtained from Alexander Yephremov (MPI für Züchtungsforschung, Cologne) (Wellesen et al, 2001; Kurdyukov et al, 2006b). EMS-mutagenized M2 seeds accession Col-0 were provided by the laboratories of Chris Somerville, Carnegie Institution of Washington, Stanford, USA and Fred Ausubel, Massachusets General Hospital, Boston, USA. The T-DNA insertion line in the LACS2 gene (At1g49430) is the GABI-Kat line 368C02 (Rosso et al, 2003).
Inoculation with B. cinerea and S. sclerotiorum
B. cinerea strains BMM and BO5.10 were provided by Brigitte Mauch-Mani, University of Neuchatel, Neuchatel, Switzerland and Jan van Kan, Wageningen University, Wageningen, The Netherlands, respectively. S. sclerotiorum was provided by Henk-Jan Schoonbeek, University of Fribourg, Fribourg, Switzerland. Both pathogens were grown on 1 × PDA (potato dextrose agar; Difco). Conidia of Botrytis were harvested in water and filtered through glass wool. A liquid culture of S. sclerotiorum in 1/2 PDB was prepared from 2 cm2 quickly growing hyphae and incubated for 2 days at 20°C under constant agitation. A Sclerotinia suspension containing 1 ml of mycelium/10 ml 1/2 PDB was prepared by disruption of the mycelium by a short treatment in a Polytron. Plants were inoculated with 5-μl droplets of a suspension of 5 × 104 spores/ml in 1/4 PDB of Botrytis (Difco) or of the hyphal preparation of Sclerotinia and kept under 100% humidity. Control plants were inoculated with 1/4 PDB (mock). The growth of both pathogens was quantified by monitoring the presence of lesions and their diameter 2–4 days after inoculation. The level of resistance was estimated by the potential of the pathogen to cause soft rot symptoms extending beyond the inoculation site (outgrowing lesions).
Assessment of cuticle permeability
For staining with Calcofluor white, plant material was decolorized in 95% ethanol, equilibrated in 0.2 M NaPO4 (pH 9.0) for 1 h, and incubated for 1–3 min in 0.5% Calcofluor in 0.2 M NaPO4 (pH 9.0). Tissues were viewed under a Leica MZ16 fluorescence microscope equipped with a UV filter. Toluidine blue staining was adapted from a previously described protocol (Tanaka et al, 2004). Tissues were incubated with 5-μl droplets of a 0.025% solution of toluidine blue in 1/4 PDB for different time periods (5 min to 2 h).
Herbicide sensitivity was tested by spraying BASTA® on the adaxial side of the leaves of 4- to 5-week-old rosettes at concentrations of 5, 10, 20, 30, 40, 50, and 60 μl/ml. Plants were kept under a transparent lid for 1 day and evaluated 1 and 2 weeks after the treatment. Water loss from 6-week-old rosettes excised at the hypocotyl was measured by weighing plants at different time points. 100% equals the total water content of a plant.
Analysis of residue-bound lipids
Monomers were liberated by hydrolysis with sodium methoxide of total extracted leaf material of 6-week-old Arabidopsis plants according to an established protocol (Bonaventure et al, 2004). Trimethylsilyl derivatives were analyzed by gas chromatography using an HP-5MS column (J&W Scientific) either in a unit from Hewlett Packard (model 5890) coupled to a flame ionization detector or in a unit from Agilent (Agilent 6890N Network GC system) linked to a mass spectrometer (Agilent 5973 Network Mass Selective Detector) using the following temperature profile: after 2 min at 90°C, the temperature was increased at a rate of 8°C/min to 290°C and held for 10 min.
Characterization of leaf diffusates
Droplets (5 μl) of 1/4 PDB were incubated on 5-week-old plants under 100% humidity. For the in vitro test, 9 μl of PDB diffusate was mixed with 3 μl of B. cinerea spores to a final concentration of 5 × 104 spores/ml and placed on a microscope glass slide. Fungal growth was observed under the microscope after incubation under high humidity conditions for ±14 h (in vitro test). Diffusates without PDB were adjusted to 1/4 PDB before carrying out the in vitro test. Microscopic pictures were evaluated with the help of WinRhizo software (www.regent.qc.ca/products/rhizo/Rhizo.html). For the in vivo test, 15–20 5-μl droplets of the same mixture were directly applied on WT plants and formation of lesions was evaluated after 3 days.
For characterization of 1/4 PDB diffusate, 20 μl of diffusate was incubated in 32 U/μl of the lipase from M. miehei (Sigma), 3.2 U/μl of the lipase from C. antarctica (Sigma), or 0.035 U/μl of proteinase K at 37°C for 15 min. Molecular weight was estimated by using a Microcon® 3 (Millipore Corp.) column followed by the in vitro growth test.
Transmission electron microscopy
Samples were fixed in 2% glutaraldehyde, 2% paraformaldehyde, 0.3 mM calcium chloride in 0.1 M cacodylate buffer, precontrasted with 2% uranyl acetate in 10% ethanol, and then dehydrated in a graded series of ethanol and propylene oxide. Specimens were embedded in a mixture of epon/araldite. Ultrafine sections of 50–70 nm were cut on a Reichert ultracut microtome with a Diatome diamond knife and mounted on either copper or nickel parallel bar grids. The grids were then contrasted with 2% aqueous uranyl acetate for 10 min and saturated lead citrate for 10 min. The samples were observed with a Phillips CM12 transmission electron microscope.
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Alexander Yephremov for the seeds from lcr and hth and Linda Grainger and Luis Carraça for technical help. We thank Alexander Yephremov, Gustavo Bonaventure, and Rochus Franke for helpful discussions and Gustavo Bonaventure, Antony Buchala, and Karen Osmont for critically reading the manuscript. This work was supported by SNF (grant no. 3100A0-109405/1 to CN and 3100A0-104224/1 to JPM).
References
- Benveniste I, Tijet N, Adas F, Philipps G, Salaun JP, Durst F (1998) CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450-dependent fatty acid omega-hydroxylase. Biochem Biophys Res Commun 243: 688–693 [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J 49: 920–930 [DOI] [PubMed] [Google Scholar]
- Burghardt M, Riederer M (2006) Cuticular transpiration. In Biology of the Plant Cuticle, Riederer M, Müller C (eds) pp 292–311. Oxford: Blackwell Publishing [Google Scholar]
- Chassot C, Métraux JP (2005) The cuticle as source of signals for plant defense. Plant Biosystems 139: 28–31 [Google Scholar]
- Chassot C, Nawrath C, Métraux JP (2007) Cuticular defects lead to full immunity to a major plant pathogen. Plant J 49: 972–980 [DOI] [PubMed] [Google Scholar]
- Chen XB, Goodwin SM, Boroff VL, Liu XL, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15: 1170–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coego A, Ramirez V, Gil MJ, Flors V, Mauch-Mani B, Vera P (2005) An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OFCATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. Plant Cell 17: 2123–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni C, Humphry ME, Hartmann HA, Livaja M, Durner J, Westphal L, Vogel J, Lipka V, Kemmerling B, Schulze-Lefert P, Somerville SC, Panstruga R (2006) Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat Genet 38: 716–720 [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG (1994) Oligosaccharins: structures and signal transduction. Plant Mol Biol 26: 1379–1411 [DOI] [PubMed] [Google Scholar]
- Francis SA, Dewey FM, Gurr SJ (1996) The role of cutinase in germling development and infection by Erysiphe graminis f sp hordei. Physiol Mol Plant Pathol 49: 201–211 [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochemistry 66: 2643–2658 [DOI] [PubMed] [Google Scholar]
- Garçia-Olemedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P (1998) Plant defence peptides. Biopolymers 47: 479–491 [DOI] [PubMed] [Google Scholar]
- Gilbert RD, Johnson AM, Dean RA (1996) Chemical signals responsible for appressorium formation in the rice blast fungus Magnaporthe grisea. Physiol Mol Plant Pathol 48: 335–346 [Google Scholar]
- Goodwin SM, Jenks MA (2005) Plant cuticle function as a barrier to water loss. In Plant Abiotic Stress, Jenks MA, Hasegawa PM (eds) pp 14–36. Oxford, UK: Blackwell Publishing Inc. [Google Scholar]
- Graça J, Schreiber L, Rodrigues J, Pereira H (2002) Glycerol and glyceryl esters of w-hydroxyacids in cutins. Phytochemistry 61: 205–215 [DOI] [PubMed] [Google Scholar]
- Jeffree CE (2006) The fine structure of the plant cuticle. In Biology of the Plant Cuticle, Riederer M, Müller C (eds) pp 11–125. Oxford, UK: Blackwell Publishing [Google Scholar]
- Jetter R, Kunst L, Samuels AL (2006) Composition of plant cuticular waxes. In Biology of the Plant Cuticle, Riederer M, Müller C (eds) pp 145–181. Oxford, UK: Blackwell Publishing [Google Scholar]
- Kliebenstein DJ, Rowe HC, Denby KJ (2005) Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J 44: 25–36 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (2001) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71: 1–49 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Rogers LM, Li DX, Hwang CS, Flaishman MA (1995) Surface signaling in pathogenesis. Proc Natl Acad Sci USA 92: 4080–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolikowski KA, Victor JL, Wagler TN, Lolle SJ, Pruitt RE (2003) Isolation and characterization of the Arabidopsis organ fusion gene HOTHEAD. Plant J 35: 501–511 [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42: 51–80 [DOI] [PubMed] [Google Scholar]
- Kurdyukov S, Faust A, Nawrath C, Bär S, Voisin D, Franke R, Schreiber L, Saedler H, Métraux J-P, Yephremov A (2006b) The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 18: 321–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdyukov S, Faust A, Trenkamp S, Bär S, Franke R, Efremova N, Tietjen K, Schreiber L, Saedler H, Yephremov A (2006a) Genetic and biochemical evidence for involvement of HOTHEAD in the synthesis of long-chain alpha-, omega-dicarboxylic acids in the formation of extracellular matrix. Planta 224: 315–329 [DOI] [PubMed] [Google Scholar]
- Lolle SJ, Hsu W, Pruitt RE (1998) Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics 149: 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol 123: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C (2006) Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol 9: 281–287 [DOI] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Métraux J-P, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ, Kift N, Carbonne F, Griffiths G, Esquerré-Tugayé M-T, Rosahl S, Castresana CHamberg M, Fournier J (2005) Evaluation of the antimicrobial activities of plant oxylipins support their involvement in defence against pathogens. Plant Physiol 139: 1902–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis H, Pfiffi S, Hahn M (2005) Molecular and functional characterization of a secreted lipase from Botrytis cinerea. Mol Plant Pathol 6: 257–267 [DOI] [PubMed] [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonides-related signaling. Phytochemistry 57: 929–967 [DOI] [PubMed] [Google Scholar]
- Riederer M (2006) Introduction: biology of the plant cuticle. In Biology of the Plant Cuticle, Riederer M, Müller C (eds) pp 1–10. Oxford, UK: Blackwell Publishing [Google Scholar]
- Riederer M, Friedmann A (2006) Transport of lipophilic non-electrolytes across the cuticle. In Biology of the Plant Cuticle, Riederer M, Müller C (eds) pp 250–279. Oxford, UK: Blackwell Publishing [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53: 247–259 [DOI] [PubMed] [Google Scholar]
- Schnurr J, Shockey J, Browse J (2004) The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16: 629–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L (2005) Polar paths of diffusion across plant cuticles: new evidence for an old hypothesis. Ann Bot 95: 1069–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey J, Fulda M, Browse J (2002) Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129: 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Metraux JP, Nawrath C (2000) Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12: 721–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein KA, Graham MA, Paape TD, VandenBosch KA (2005) Genome organization of more than 300 defensin-like genes in Arabidopsis. Plant Physiol 138: 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis. Plant J 37: 139–146 [DOI] [PubMed] [Google Scholar]
- Tao Y, Xie ZY, Chen WQ, Glazebrook J, Chang HS, Han B, Zhu T, Zou GZ, Katagiri F (2003) Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Nelissen I, Eggermont K, Broekaert WF (1999) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19: 163–171 [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, Keller NP (2006) Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol Microbiol 59: 882–892 [DOI] [PubMed] [Google Scholar]
- Wellesen K, Durst F, Pinot F, Benveniste I, Nettesheim K, Wisman E, Steiner-Lange S, Saedler H, Yephremov A (2001) Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different robes of fatty acid omega-hydroxylation in development. Proc Natl Acad Sci USA 98: 9694–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao FM, Goodwin SM, Xiao YM, Sun ZY, Baker D, Tang XY, Jenks MA, Zhou JM (2004) Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J 23: 2903–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yephremov A, Schreiber L (2005) The dark side of the cell wall: molecular genetics of plant cuticle. Plant Biosystems 139: 74–79 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures


