Abstract
In most eukaryotes, sphingolipids (SLs) are critical membrane components and signaling molecules. However, mutants of the trypanosomatid protozoan Leishmania lacking serine palmitoyltransferase (spt2−) and SLs grow well, although they are defective in stationary phase differentiation and virulence. Similar phenotypes were observed in sphingolipid (SL) mutant lacking the degradatory enzyme sphingosine 1-phosphate lyase (spl−). This epistatic interaction suggested that a metabolite downstream of SLs was responsible. Here we show that unlike other organisms, the Leishmania SL pathway has evolved to be the major route for ethanolamine (EtN) synthesis, as EtN supplementation completely reversed the viability and differentiation defects of both mutants. Thus Leishmania has undergone two major metabolic shifts: first in de-emphasizing the metabolic roles of SLs themselves in growth, signaling, and maintenance of membrane microdomains, which may arise from the unique combination of abundant parasite lipids; Second, freed of typical SL functional constraints and a lack of alternative routes to produce EtN, Leishmania redirected SL metabolism toward bulk EtN synthesis. Our results thus reveal a striking example of remodeling of the SL metabolic pathway in Leishmania.
Keywords: metacyclogenesis, phosphatidylethanolamine, sphingolipid, sphingosine-1-phosphate lyase, virulence
Introduction
Protozoan parasites of the genus Leishmania cause a spectrum of human diseases prevalent in many tropical and subtropical countries (Cunningham, 2002). Results emerging from the Leishmania genome sequencing have suggested that metabolic pathways are conserved (Berriman et al, 2005; Ivens et al, 2005). Nonetheless, like many other pathogens, Leishmania parasites possess the ability to redirect apparently conserved pathways to fulfill specific needs and enhance their survival. Here, we report one such example involving sphingolipid (SL) and phosphatidylethanolamine (PtE) metabolism in Leishmania major, a causative agent for cutaneous leishmaniasis.
In most eukaryotes, SLs represent a minor but highly dynamic class of membrane lipids, which are particularly abundant in membrane microdomains known as ‘rafts' (Simons and Ikonen, 1997; Simons and Toomre, 2000; van Meer and Lisman, 2002). Complex glyco-SLs (such as gangliosides in animals) and small SL metabolites (sphingomyelins, ceramides, and sphingoid bases) play crucial roles in cell-to-cell recognition, intracellular signaling, modulation of apoptosis and stress response, and regulation of cell growth and differentiation (Kolter et al, 2002; Maceyka et al, 2002; Hakomori, 2003; Ruvolo, 2003). SLs are also involved in protein trafficking and endocytic pathways (Gruenberg, 2001; Ikonen, 2001; Funato et al, 2002). Consistent with their diverse functions, SLs are typically essential in eukaryotes.
Unlike mammals or plants, Leishmania parasites do not synthesize sphingomyelin or glyco-SLs. Instead, the primary SL is inositol phosphorylceramide (IPC), which accounts for 5–10% of total membrane lipids (Kaneshiro et al, 1986; Zhang et al, 2005). Recently, SL functions in L. major were probed using an SL-free mutant generated by deleting an essential subunit gene (SPT2) of serine palmitoyltransferase (SPT), the first enzyme in the de novo SL biosynthesis pathway (Figure 1). This spt2− mutant exhibited several intriguing phenotypes distinct from those seen in mammals and yeast: (1) it completely lacked SLs, yet was viable and replicated as non-infective log-phase promastigotes (procyclics); (2) it retained the ability to form membrane microdomains or ‘rafts'; and (3) upon entry into stationary phase, it showed severe defects in vesicular trafficking, accompanied by a rapid loss of viability and an inability to differentiate into the infective metacyclic stage (normally transmitted by the insect vector to the mammalian host) (Zhang et al, 2003; Denny et al, 2004).
Figure 1.
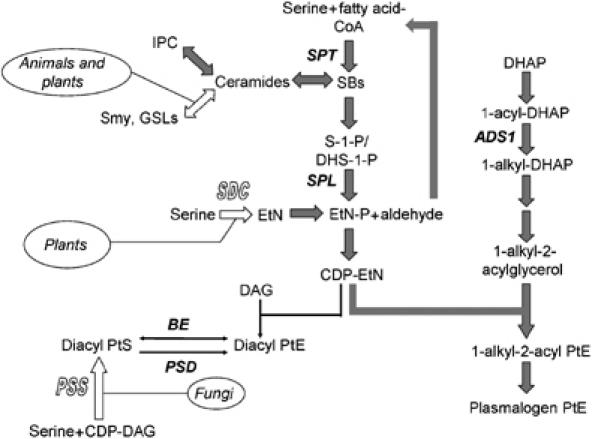
Synthesis of SLs and PtE in eukaryotes. Open block arrows represent pathways not present in Leishmania. Filled block arrows (gray) represent dominant metabolic routes in Leishmania promastigotes. ADS1: 1-alkyl dihydroxyacetonephosphate synthase; SDC: serine decarboxylase (not present in Leishmania); PSS: phosphatidylserine synthase (not present in Leishmania); BE: base exchange enzyme (phosphatidyl serine synthase 2; PSS2); PSD: phosphatidylserine decarboxylase; Smy: sphingomyelin; GSL: glycosylsphingolipid; DAG: diacylglycerol; DHAP: dihydroxyacetonephosphate.
Although it was remarkable that Leishmania could replicate normally in the absence of SLs, there remained a potential role for SLs in differentiation and virulence. To further probe this pathway, we have generated an anticipated ‘gain-of-function' mutant (spl−), defective in the degradation of SLs, by deleting the gene encoding for sphingosine-1-phosphate lyase (SPL). SPL breaks down phosphorylated sphingoid bases (sphingosine-1-phosphate or S-1-P and dihydrosphingosine-1-phosphate or DHS-1-P) into ethanolamine phosphate (EtN-P) and fatty aldehydes (Figure 1). EtN-P can be used to synthesize PtE, an abundant membrane lipid (Figure 1). However, in many eukaryotes, degradation of SLs by SPL is not the major pathway leading to the production of EtN-P and/or PtE (Figure 1), possibly because of constraints imposed by the role of SLs in critical signaling functions (Merrill, 2002), and the availability of SL-independent pathways for EtN synthesis.
In this study, we show that unlike other eukaryotes, the SL synthetic and degradative pathways demarcated by SPT and SPL have assumed the role of bulk EtN provision, which is essential for Leishmania survival and differentiation in stationary phase. These data allowed us to establish that a remarkable and unprecedented remodeling of the ‘dangerous' SL pathway (Merrill, 2002) has occurred in Leishmania parasites.
Results
Identification and targeted replacement of L. major SPL
A single candidate sphingosine 1-phosphate lyase (SPL) gene was identified by database mining (LmjF30.2350). The predicted L. major SPL protein contained 537 amino acids and showed significant homology to the SPLs from other eukaryotes (35–39% amino-acid identity; Supplementary Figure S1). The LmSPL exhibited a pyridoxal-phosphate binding site motif and other conserved amino-acid residues known to be required for SPL activity (Van Veldhoven et al, 2000) (Supplementary Figure S2).
Gene replacements can be performed readily in L. major (Cruz and Beverley, 1990), and an spl− null mutant was generated by consecutive targeted replacements of the SPL ORF with those encoding G418 (NEO) and hygromycin (HYG) resistance. Candidate spl− mutants were obtained and Southern blots confirmed loss of SPL genes (data not shown). As a control, SPL was reintroduced into the mutant on an episomal expression vector, referred to as spl−/+SPL.
spl− and spt2− promastigotes have similar defects in growth and differentiation
Unexpectedly, the spl− mutant resembled the SL-free spt2− mutant closely. Both were viable and grew at near normal rates in log phase (9.2–9.5 h doubling time for spt2− and spl− versus 7.0 h for wild type (WT; Figure 2A). Second, both mutants reached a lower density) in stationary phase (0.8–1.2 × 107/ml versus 2–3.3 × 107/ml; Figure 2A). Third, upon entry into stationary phase, both mutants showed increased cell death. After 3 days in stationary phase, 30–55% of mutant cells became permeable to propidium iodide, in comparison to only 3–6% of WT (Figure 2B; small differences between the two mutants evident in the experiments shown were not reproducible and/or significant in other experiments). These defects were due solely to the loss of SPL or SPT2 gene, as re-expression of SPL or SPT2 restored the behavior to WT (Figure 2A and B).
Figure 2.
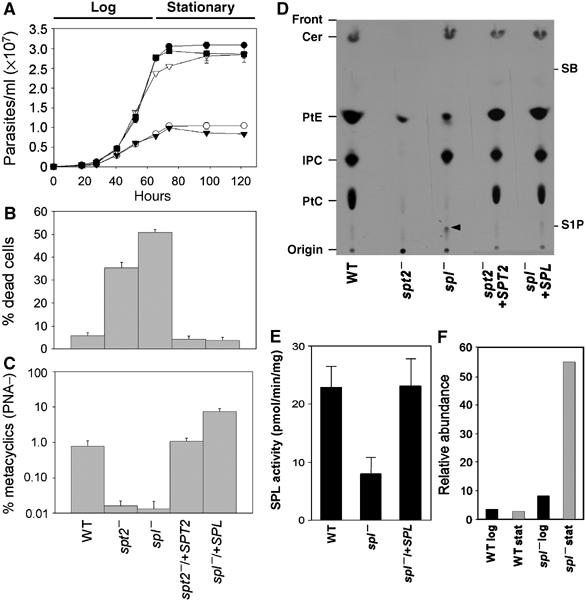
spl− and spt2− mutants have similar defects. (A) Growth in vitro. Promastigotes were inoculated at 1 × 105/ml and densities were measured. •: WT; ○: spt2−; ▾: spl−; ▿: spt2−/+SPT2; ▪: spl−/+SPL. (B) Stationary phase viability. (C) Metacyclogenesis (PNA lectin method). In (A–C), experiments were performed in duplicate or triplicate. Error bars represent s.d. (D) TLC separation of [3-3H]serine-labeled lipids from SPT2 and SPL mutants. The arrowhead indicates a species accumulated in the spl− mutant probably corresponding to sphingosine-1-phosphate. Abbreviations: PtC, phosphatidylcholine; IPC, inositol phosphorylceramide; PtE, phosphatidylethanolamine; SB, sphingosine; S-1-P, sphingosine-1-phosphate. Note: the spot corresponding to PtC (now confirmed by MS analysis) was previously incorrectly labeled as PtS (Zhang et al, 2003). (E) SPL activity assay. The average and standard error of four determinations is shown. The difference between spl− and the other lines is significant at the P<0.05 level. (F) Elevated levels of sphingosine occur in spl− preparations. ESI/MS was performed in the positive-ion mode and the relative abundance of the m/z 274.2 d16:1 sphingosine peak was expressed as its ratio relative to the PtC peak (m/z 830.6). The identity of the sphingosine was confirmed by secondary collisions (not shown).
We examined formation of the infective metacyclic stage, which can be distinguished from the non-infective procyclics (log-phase promastigotes) in morphology, reactivity to lectins such as peanut agglutin (PNA) and/or monoclonal antibodies, density gradient sedimentation, and virulence (Sacks et al, 1985; Späth and Beverley, 2001). When metacyclics were scored by the PNA lectin method, the spl− mutant produced few, if any, viable metacyclics (0.5–1.0% for WT versus <0.02% in the spt2− and spl− mutants; Figure 2C); similar results were seen with the density gradient method (Supplementary Figure S3). Metacyclogenesis was restored by re-expression of SPL and SPT2 genes (Figure 2C and Supplementary Figure S3) to WT levels in the case of spt2−/+SPT2, but to levels reproducibly 3–4-fold greater than WT with spl−/+SPL (Figure 2C). Interestingly, when scored by the density gradient (size) method, metacyclic levels were similar to WT in both complemented mutants (Supplementary Figure S3). The basis of this ‘overshoot' phenomenon for metacyclics scored by the PNA lectin method is unknown, although it appears to be transient, as it disappears after a few passages in culture (data not shown).
SPL activity and phospholipid and sphingolipid synthesis in spl− Leishmania
Microsomal preparations from stationary phase WT, spl−, and spl−/+SPL parasites were assayed for SPL activity (Figure 2E). WT and spl−/+SPL parasites showed low but significant activity (22.9±1.8 and 23.2±4.5 pmol/mg/min), whereas spl− parasites showed lower levels, at most 35% of WT (8.0±3.4 pmol/mg/min; P<0.05). Experience suggests that the low spl− value is close to background in these assays, although the presence of a second residual SPL activity cannot be ruled out.
Log-phase promastigotes were labeled 48 h with [3H]serine and total lipids were extracted and separated by thin-layer chromatography. Labeled lipids were identified by comparison with the migration of standards, and where possible, mass spectrometry after extraction from plates. In WT parasites, serine was abundantly incorporated into both glycerophospholipids (PtE and phosphatidylcholine (PtC) and sphingolipids (ceramides and IPC; Figure 2D). As expected, the spl− mutant synthesized ceramides and IPC, but unexpectedly, PtE and especially PtC synthesis was greatly reduced (Figure 2D). A similar decrease in phospholipid synthesis was seen in the spt2− mutant (Figure 2D; Zhang et al, 2003).
Additionally, spl− showed accumulation of a new species whose mobility was similar to S-1-P (Figure 2D, arrowhead). Attempts to determine its structure by mass spectrometry were unsuccessful, nor were we able to identify sphingoid base 1-phosphates in these samples by mass spectrometry (data not shown). Elevated levels of d16:1 sphingosine were detected in the spl− mutant, especially in stationary phase (Figure 2F); elevated sphingosine levels are also seen in other eukaryotic SPL mutants (Mendel et al, 2003). Notably, d16:1 sphingosine is the form expected from metabolism of the abundant d16:1/18:0 IPC of Leishmania (Zhang et al, 2003). Restoration of SPL expression returned the serine incorporation profile back to the WT pattern (Figure 2D). Thus, SPL deletion specifically leads to a substantial decrease in SPL activity (Figure 2E), and within the limits of the techniques used, accumulation of upstream metabolites such as sphingosine and possibly S-1-P (Figure 2D).
Ethanolamine completely restores the stationary phase defects of spl− and spt2− promastigotes
Given the similar phenotypic consequences of SPL or SPT2 inactivation, we sought common metabolic perturbations. While the effects on SL synthesis differed as expected (Figure 2D), both mutants showed reduced PtE and PtC synthesis (Figure 2D), which returned to WT upon restoration of SPL or SPT2 synthesis (Figure 2D). Phosphatidylserine (PtS) was below the limit of detection by serine labeling or electrospray ionization/mass spectrometry (ESI/MS) of total promastigote lipids (Figure 2D, data not shown) (Zufferey et al, 2003).
Notably, phosphoethanolamine (P-EtN) is the first common downstream metabolite from SPT and SPL (Figure 1). In mammals and fungi, EtN is incorporated into PtE (and then PtC) through the Kennedy pathway (EtN → P-EtN → CDP-EtN → PtE; (Kent, 1995; Storey et al, 2001; Vance, 2003) (Figure 1). This pathway occurs in trypanosomes (Rifkin et al, 1995), and relevant genes are evident in the Leishmania genome (not shown).
Thus spt2− and spl− mutants were tested in media containing EtN (Figure 3A–C). Above 37 μM EtN, their stationary phase defects, including culture density, cell viability, and metacyclogenesis, were reversed completely (Figure 3A–C). Rescue was also seen at similar concentrations of P-EtN, CDP-EtN, and PtE, although potentially these compounds were converted to EtN before and/or after uptake (data not shown). Interestingly, both mutants grown in EtN (>37 μM) exhibited the overshoot phenomenon mentioned earlier, producing more metacyclics than WT, when scored by the PNA lectin method (Figure 2C) but not by the density gradient method (Supplementary Table S1). As before, this effect was transitory and lost over several passages in culture (data not shown).
Figure 3.
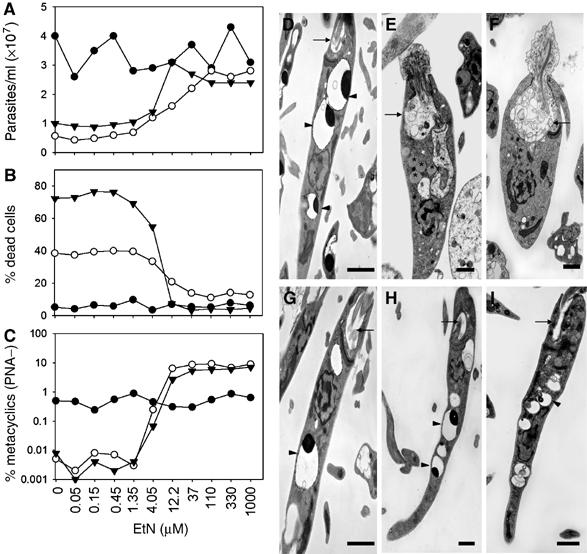
EtN restores the stationary-phase defects of spt2− and spl− mutants. (A–C) Exogenous EtN reverses the defects of spt2− and spl− mutants in growth and differentiation. WT (•), spt2− (○), and spl− (▾) promastigotes were inoculated at 1 × 105/ml in media supplemented with EtN. Three days after entry into stationary phase, culture density, cell viability, and percentage of PNA metacyclics were determined. (D–I) Transmission EM images of WT (D, G), spt2− (E, H), and spl− (F, I) promastigotes in late stationary phase. Cells were grown in the absence (D–F) or presence (G–I) of 0.5 mM EtN. Arrows indicate flagellar pockets (D–I), arrowheads indicate acidocalcisomes (D, G–I), and asterisks indicate lipid inclusions accumulated in spt2− (E) and spl− promastigotes (F). Bars=1 μm.
In transmission electron microscopy (EM) analysis, spl− was less slender in shape, lacked mature acidocalcisomes, and had enlarged flagellum pockets filled with membranous structures (Figure 3D–F) similar to spt2− (Zhang et al, 2003). Compared with the spt2− mutant, spl− accumulated fewer lipid inclusions and MVB-like vesicles (Figure 3E and F). Importantly, growth in the presence of EtN restored all of these features to normal (Figure 3G–I).
The stationary phase defects of the spt2− and spl− mutants could be rescued through the expression of a serine decarboxylase from Arabidopsis thaliana, which directly converts serine into EtN (Rontein et al, 2001) (Figure 1 and Supplementary Figure S4; as with the EtN studies, an overshoot was seen when metacyclics were scored by the PNA lectin method). Provision of several EtN derivatives failed to rescue the stationary phase defects of either mutant, including N-methylethanolamine, N,N-dimethylethanolamine, choline, and propanolamine (3-amino-1-propanol; 4–500 μM). This showed that choline deficiency was not solely responsible for the defects in spt2− and spl− mutants (data not shown).
EtN is required for log-phase promastigote growth
Given the importance of EtN in phospholipid synthesis and cellular viability, we were surprised that only stationary phase defects were observed in the mutants. Standard culture media contain 10% fetal bovine serum (FBS), which contains sphingoid bases, ceramides, and phospholipids including PtE, suggesting the possibility to salvage these compounds and conversion into EtN. Thus, we tested parasites adapted for growth in serum-free medium supplemented with 0.4% bovine serum albumin. Under these conditions, WT grew to densities greater than 107/ml, with a doubling time of ∼24 h (versus 7 h in medium plus 10% FBS). In contrast, neither the spt2− nor spl− mutants were able to replicate in defined medium, unless EtN was provided (Figure 4). Thus, EtN is essential for growth, but unlike other organisms, the primary source of EtN derives from de novo SL synthesis and degradation.
Figure 4.
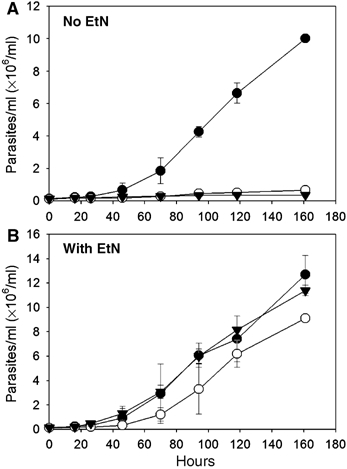
EtN is essential for promastigote growth. Promastigotes (•: WT; ○: spt2−; ▾: spl−) were inoculated at 2 × 105/ml in serum-free medium in the absence (A) or presence (B) of 1 mM EtN and densities were measured over time. Experiments were performed in duplicate and error bars represent s.d.
WT promastigotes contain elevated amount of plasmalogen PtE during metacyclogenesis
As the EtN defect of the two SL mutants manifested only in stationary phase, we compared the abundance of PtE between log- and stationary-phase growth. In L. major, the majority of PtEs are 1-O-alkenyl-2-acyl-PtE, or plasmalogen PtE (80–90%), accompanied by low levels of 1,2-diacyl-PtE and 1-acyl-2-lyso-PtE (Zhang et al, 2003; Zufferey et al, 2003). The two dominant species of plasmalogen PtE are 1-O-octadec-1′-enyl-2-octadecadienoyl-sn-glycero-3-phosphoethanolamine (p18:0/18:2-PtE) and 1-O-octadec-1′-enyl 2-octadecenoyl sn-glycero-3-phosphoethanolamine (p18:0/18:1-PtE). We measured the cellular level of plasmalogen PtE (p18:0/18:2-PtE plus p18:0/18:1-PtE) by semiquantitative electrospray mass spectrometry.
When grown in the absence of EtN, WT plasmalogen PtE levels rose 2–3-fold as cells progressed from log to stationary phase (0.7–1.5 × 108 to 2.0–2.9 × 108 molecules/cell; Figure 5A–C). In log phase, the PtE levels of both the spt2− and spl− mutants were initially similar to WT, but decreased progressively during growth, ultimately falling to 10-fold less than WT in stationary phase (Figure 5A–C). Notably, EtN addition restored the mutants' plasmalogen PtE levels to that of WT (Figure 5C). These data suggest that although some depletion of EtN occurs during growth, the inability to undergo the stationary phase increase in plasmalogen PtE levels probably accounts for the stationary-phase specificity of EtN deficiency in the mutant lines.
Figure 5.
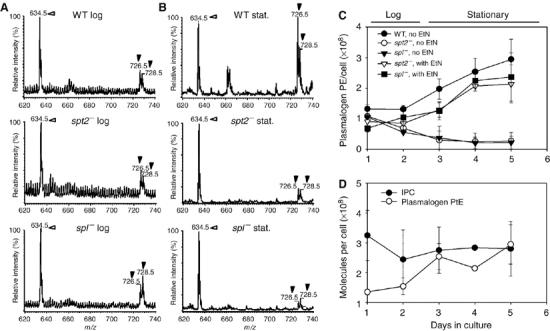
WT but not spl− or spt2− promastigotes contain increased levels of plasmalogen PtE during stationary phase. (A, B) Negative-ion ESI/MS spectra of plasmalogen PtE in promastigote lipids. WT, spl− parasites or spt2− parasites were grown in the absence of EtN. Total lipids from log- (A) or stationary-phase (B) parasites were extracted and analyzed by negative-ion ESI/MS. Before extraction, a PtE standard (14:0/14:0-PtE at m/z 634.5, indicated by open arrowheads) was added as an internal standard to each sample (15 μg per 108 cells). Filled arrowheads indicate plasmalogen PtE species (at m/z 726.5 and 728.5). (C) Promastigotes were inoculated at 105/ml in the absence or presence of 0.5 mM EtN. Cellular plasmalogen PtE levels were then determined. (D) WT promastigotes were inoculated at 105/ml in the absence of EtN, and IPC and plasmalogen PtE levels were determined over time. Data were collected from duplicate experiments, and error bars represent s.d.
In contrast to plasmalogen PtE, IPC remained relatively constant throughout the growth cycle in WT parasites, declining slightly to 80% in stationary phase (Figure 5D). Given its abundance, catabolism of IPC (via S-1-P) may contribute to the increase in plasmalogen PtE during metacyclogenesis.
Viable spl− and spt2− metacyclics are fully virulent
Previously we showed that spt2− promastigotes were highly attenuated in infections of macrophages and susceptible mice (Zhang et al, 2005). Similar results were obtained with the spl− mutant in both assays (Figure 6A and data not shown). However, when both mutants were grown with EtN or transfected with the SDC gene from A. thaliana, their ability to induce progressive lesions in susceptible mice returned to WT (Figure 6B–D and Supplementary Figure S4). Thus, whereas EtN was required for full metacyclic differentiation and virulence, SLs were not.
Figure 6.
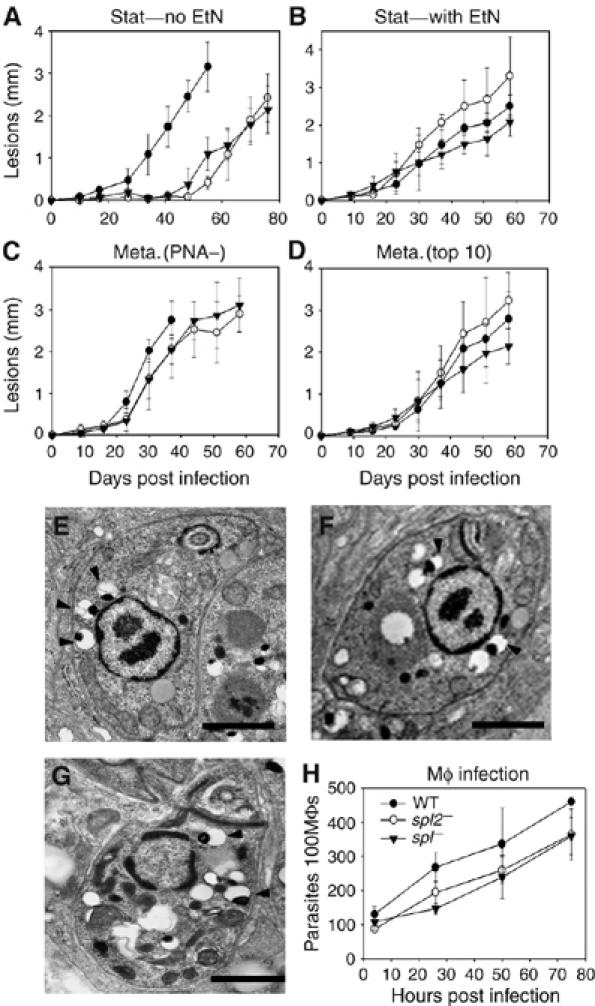
Virulence studies of spt2− and spl− mutants. (A–D) Mouse footpad infections with WT (•), spt2− (○), and spl− (▾) promastigotes. In (A) and (B), stationary phase promastigotes grown in the absence (A) or presence (B) of 0.5 mM EtN were inoculated. In (C) and (D), metacyclics grown in the presence of 0.5 mM EtN prepared by the PNA lectin method (C) or the density gradient method (D) were inoculated, and lesions sizes monitored. Error bars represent s.d. (E–H) Amastigotes of WT (E), spt2− (F), and spl− (G) were isolated from infected Balb/c mice and subjected to transmission EM analysis. Arrowheads indicate acidocalcisomes. Bars=1 μm. In (H), amastigotes were used to infect murine peritoneal macrophages (Mφs) at a ratio of two amastigotes per macrophage. Error bars represent s.d.
Even when grown in the absence of EtN, after an initial delay of 4–6 weeks, both mutants induced lesions that progressed as rapidly as WT thereafter (Figure 6A). Typically this ‘delayed lesion' phenotype arises when parasites are compromised in the initial phases of infection (macrophage entry, survival, and differentiation), but not in their ability to survive and replicate thereafter as amastigotes (Späth et al, 2000; Zhang et al, 2005). Previously we showed that WT and spt2− amastigotes salvaged SLs from the host, which were then converted into parasite-specific IPCs, and were morphologically normal (Zhang et al, 2005). Similarly, recovered spl− amastigotes appeared normal in shape, size, and the abundance of acidocalcisomes (Figure 6E–G). The virulence of these spl− and spt2− amastigotes was also comparable to WT in macrophage infections (Figure 6H). Thus, neither SPT2-dependent SL synthesis nor SPL-dependent degradation is required for parasite survival in macrophage or animal hosts.
SLs are not required for the metacyclic-specific relocalization of lipophosphoglycan to lipid rafts
The ability to generate healthy metacyclic spt2− and spl− parasites, when grown in EtN, enabled further tests of potential SL roles beyond that as a conduit for EtN synthesis. Previously we showed that the abundant surface glycoconjugate lipophosphoglycan (LPG) undergoes a stage-specific relocalization into lipid rafts and detergent-resistant membrane (DRM) fractions; in log-phase promastigotes, LPG does not localize to DRMs, whereas in metacyclic parasites, LPG is abundant in DRMs (Zhang et al, 2003; Zufferey et al, 2003). These and other data suggest that membrane organization differs considerably between the two developmental stages, and recently it was proposed that SLs are required for both membrane reorganization and LPG relocalization (Denny and Smith, 2004). To test this, we prepared DRMs from WT and mutant Leishmania grown in the presence of EtN. Despite the absence of SLs, spt2− parasites relocalized LPG from a non-DRM compartment (Figure 7A, 4°C soluble fraction) in log phase to a DRM compartment in metacyclics (Figure 7B, 4°C insoluble fraction). Similar results were seen in the spl− mutants (Figure 7). Thus, neither SLs nor SLs metabolism via SPT and SPL are required for redistribution of LPG during promastigote development.
Figure 7.
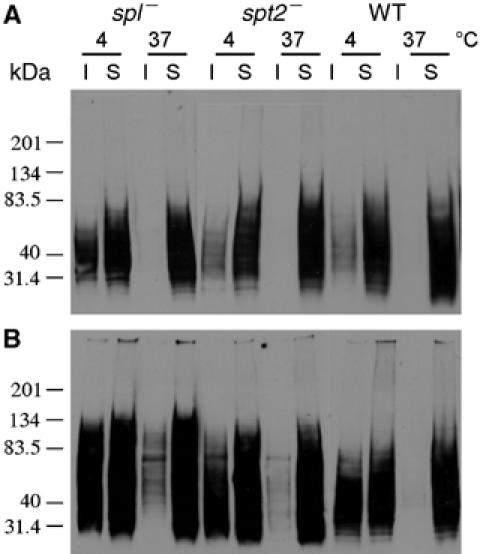
SLs are not required for the redistribution of LPG during promastigote development. Log (A) and metacyclic (B, isolated from stationary cultures grown in the presence of 0.5 mM EtN) promastigotes were extracted with 1% Triton and resubjected to Western blot analysis with the anti-LPG monoclonal antibody WIC79.3. I, insoluble; S, soluble.
One minor phenotype associated specifically with altered SL metabolism independent of EtN was found: spt2− parasites appear ‘rounded' in shape relative to WT, as do spl− mutants (Figure 8). ‘Round cells' were quantified as those with the length of the long axis less than twice the length of the short axis. In stationary phase, round cells comprised 82% in spt2− and 79% in spl−, versus 13% for WT; this was reduced to 7.8% (spt2−) and 5.0% (spl−) in the presence of EtN. However in log-phase cells, EtN had little effect on the shape of spt2− (36% round without and 44% round with EtN) or spl− (27% round without EtN, 30% with EtN) mutants. Similar results were obtained when EtN was synthesized through the expression of SDC (Figure 8). As expected, SBs restored the shape of spt2− (18% round in log and 8.8% in stationary phase) but not spl− (29% round in log and 71% in stationary phase; Figure 8) mutants. Therefore, SLs contribute in some way to the maintenance of cell shape in log (but not stationary) phase, apart from their role in EtN synthesis.
Figure 8.
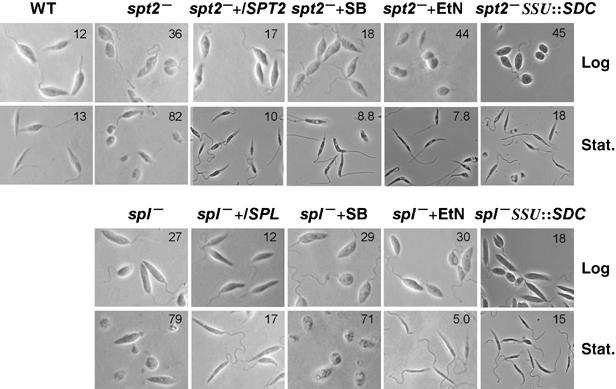
EtN reverses the morphological defects of spl− and spt2− promastigotes in stationary but not in log phase. Images show WT, spt2−, spl−, spt2−/+SPT2, spl−/+SPL, spt2− SSU∷SDC, and spl− SSU∷SDC promastigotes in log or stationary phase (day 3). Percentages of round cells are indicated in each panel.
Discussion
Here we probed the role of SL metabolism in Leishmania promastigotes in two mutants defective in seemingly opposing steps: an SL-null spt2− mutant lacking de novo SL synthesis, versus an spl− mutant unable to degrade SL metabolites. Surprisingly, both had similar phenotypes: neither could grow in completely defined medium, whereas in standard culture media, both were viable during log phase, but rapidly lost viability in stationary phase and failed to differentiate into the infective metacyclic forms, compromising virulence. We identified EtN deficiency as the common problem, and showed that these defects were completely reversed by exogenous EtN. As the deletion of the SPT2 or SPL genes was sufficient to induce auxotrophy for EtN, our data prove that a major role of SL metabolism in Leishmania promastigotes is to provide EtN, essential for both growth and differentiation of promastigotes. In a later section, we discuss the implications of this finding to pathway evolution and function.
We showed that deletion of the Leishmania SPL greatly reduced SPL activity, to a residual level approaching the limits of our assay (Figure 2E). There is a formal possibility that Leishmania possess a second SPL activity, from a gene undetectable thus far by database mining. Regardless, even if present, the level of this hypothetical activity is insufficient to provide enough EtN for growth or differentiation (Figures 2, 3 and 4), and thus it would not alter the conclusions drawn in our work.
The availability of these two mutants allowed for the first time assessment of the specific role of SLs in Leishmania development, by examining SL-null mutants grown in the presence of EtN. Clearly, SL deficiency had little negative effect on differentiation, metacyclic-specific membrane reorganization (Figure 7), membrane trafficking (data not shown) (Zhang et al, 2005), localization of more than a dozen membrane protein or lipid markers for ‘rafts', or the DRM protein profiles (K Zhang and S M Beverley, in preparation). One phenotype attributable to SL deficiency specifically was an altered shape of spt2− mutants in log but not stationary growth phase (Figure 8), which could reflect changes in permeability and/or rigidity of membrane. In contrast, in other eukaryotes, SLs and SL metabolites play crucial roles in many cellular functions, including the formation of DRM rafts, membrane trafficking, cell-to-cell recognition, regulation of growth and differentiation, stress response, intracellular signaling, apoptosis, and modulation of immune response, making perturbations of SL metabolism in these creatures highly deleterious. The availability of a healthy eukaryotic model lacking sphingolipids (spt2− L. major promastigotes) thus offers a superb platform in which complex SL pathways from other organisms could be studied in a simpler, more defined and viable biological context.
How is Leishmania able to tolerate complete SL deficiency with minimal effect? Previously we proposed that the unique properties of the parasite membrane may provide some redundancy and buffer of membrane perturbations (Zhang et al, 2003, 2005). Specifically, WT L. major membranes contain abundant levels of ergosterol, ether phospholipids, and sphingolipids in the form of IPC, in a combination that appears to be evolutionarily distinctive (for example, fungi make ergosterol but not ether phospholipids, whereas mammals do not synthesize ergosterol). All three of these lipid species show preferential localization to ‘lipid raft' membrane microdomains (Pike et al, 2002; Silvius, 2003; Wang and Silvius, 2003), and perturbation of their synthesis can lead to alterations in microdomains (Ilangumaran and Hoessli, 1998; Nagafuku et al, 2003; Rodemer et al, 2003). We believe it is likely that this parasitic lipid triumvirate renders Leishmania relatively resistant to perturbation, as seen for loss of SLs here or other phospholipids (ads1− mutant) (Zufferey et al, 2003). In light of this, perhaps a more accurate characterization of our findings with the SL-deficient mutant grown in the presence of EtN is that we are probing the unique, non-redundant roles of SLs in this organism.
The pros and cons of depending upon the SL pathway for EtN
Owing to their critical roles in most eukaryotes in signaling pathways and other essential functions, sphingolipids have been termed ‘dangerous' metabolites, as their levels must be precisely controlled and regulated (Merrill, 2002). This is a task of considerable complexity, as SLs and their metabolites, whose relative concentrations vary over many orders of magnitude, can show distinct and sometimes opposing actions. Perhaps because of these perils, SLs are typically not a major source of EtN in most eukaryotes (Hannun et al, 2001). Instead, SL-independent pathways are used as the primary route to synthesize EtN and/or PtE (Figure 1). For example, plants make EtN through decarboxylation of serine by SDC (Rontein et al, 2001); animals make PtE through salvage and the CDP-EtN and/or base exchange/PtS decarboxylase (PSD) pathway (Kent, 1995; Voelker, 1997; Dobrosotskaya et al, 2002); and fungi synthesize the majority of their PtE through decarboxylation of PtS (Trotter and Voelker, 1995; Birner et al, 2001), synthesized de novo from serine and CDP-DAG by PtS synthase (PSS; Figure 1).
In contrast, Leishmania and other trypanosomatids lack SDC and PSS genes or activities (Figure 1), and although their genomes encode two genes showing similarity to the eukaryotic phosphatidyl serine/ethanolamine base exchange enzymes PSS2 and PSD (LmjF14.1200 and Lmj35.4590), our studies show that their contribution to PtE synthesis is negligible. In addition, Leishmania promastigotes show no essential non-redundant function for SLs in trafficking or maintenance of membrane microdomains, and as yet no role in signaling, thus allowing the SL pathway to bear the responsibility of bulk provision of EtN with relative impunity.
What led Leishmania to uniquely emphasize the SL → EtN option? Previous studies showed that the majority of PtE in Leishmania consist of plasmalogen PtE (Zhang et al, 2003; Zufferey et al, 2003), whereas in most organisms, PtEs are a mix of diacyl and alkylacyl PtEs (Nagan and Zoeller, 2001). In mammals, there is some evidence that plasmalogen PtEs may be more dependent on direct synthesis via CDP-EtN: whereas both EtN-phospholipids may arise from CDP-EtN through the action of CDP-EtN:diacylglycerol ethanolamine phosphotransferase (EPT), diacyl PtE species may be somewhat buffered from this depletion owing to the presence of the combined activities of the base-exchange enzymes PSS1 and PSS2 and PSD in most eukaryotes (Polokoff et al, 1981; Nagan and Zoeller, 2001). Why Leishmania chose to rely heavily on plasmalogen PtE is not immediately evident. Their trypanosome relatives synthesize high levels of both diacyl and plasmalogen PtEs, although plasmalogen PtE may also be dominant in the insect procyclic form (Patnaik et al, 1993; Guther et al, 2006).
In Leishmania, plasmalogen PtE levels rise dramatically in stationary phase, accompanied by an increased requirement for EtN synthesis (Figure 5). This suggests that EtN plays a critical role as parasites undergo differentiation in stationary phase to the infective metacyclic stage. Plasmalogen PtE exhibits different physical properties compared with diacyl PtE (Lohner, 1996), including an ability to promote rapid membrane fusions (Glaser and Gross, 1994), as well as displaying a propensity to form and/or interact with membrane microdomains as noted earlier. Potentially, these unique membrane properties play an important role during metacyclogenesis, a time marked by greatly increased membrane trafficking and remodeling. PtE is required for the formation of protein–GPI anchors, which are particularly abundant virulence factors in protozoa including Leishmania (Menon et al, 1993; Ilgoutz and McConville, 2001). The activity of translation factors can be modified by conjugation to EtN (Whiteheart et al, 1989), and developmental roles for this are readily envisaged. The onset of stationary phase is marked by an increase in macroautophagy, where a key step involves conjugation of PtE to ATG8 proteins, and macroautophagy mutants show interesting defects in metacyclogenesis and infectivity (Wang et al, manuscript in preparation; (Ichimura et al, 2000; Besteiro et al, 2006). It is also possible that EtN itself may play a role directly. Any and/or all of these roles may be reasonably implicated in the EtN requirement for stationary-phase differentiation of promastigotes to the metacyclic form, and thus play critical roles in parasite virulence.
The role of SLs and EtN in the amastigote stage
In contrast to the problems encountered during metacyclogenesis, spt2− and spl− amastigotes appeared normal and fully infective (Figure 6; Zhang et al, 2005), and SPT2 and SPL mRNAs and/or proteins are downregulated in WT amastigotes (Zhang et al, 2003, 2005; data not shown). These findings imply that amastigotes do not need EtN, or more likely, that amastigotes acquire EtN from the mammalian host, either directly or through hydrolysis of host PtEs. Purified amastigotes of several Leishmania species contain host-derived lipids, including sphingolipids (McConville and Blackwell, 1991; Schneider et al, 1993; Zhang et al, 2005), and phospholipids including PtE are taken up in vitro (Araujo-Santos et al, 2003). Thus it seems likely that amastigotes acquire both SL and EtN from the host, and extrapolating from the normality of both spt2− and spl− amastigotes, these occur independently. In the future, tests of the amastigote requirements for both SLs and EtN will require the generation of mutants, where these requirements cannot be bypassed by salvage.
Although the synthetic pathways of SLs and PtE are largely conserved among eukaryotes, Leishmania parasites have evolved novel ways of utilizing and emphasizing these to fulfill their specific needs. This transition would not have been predicted from genomic comparisons, as from this perspective Leishmania appears as a typical eukaryote. Potentially, this type of genomically ‘cryptic' metabolic reprogramming may represent an important strategy underlying the biodiversity of many organisms, and especially for opportunistic parasites that often rely on host pathways for essential metabolites.
Materials and methods
Cloning and sequencing of the L. major SPL gene
A 1614-bp ORF that is similar to the SPL genes in other species was identified from the L. major Genomic Database (annotated as LmjF30.2350). The gene was PCR-amplified, cloned into the expression vector pXGBSD as pXGBSD-SPL (B4974), and its sequence confirmed and submitted to GenBank as AY770983.
Leishmania culture and genetic manipulation
WT L. major LV39 clone 5 (Rho/SU/59/P), spt2− (Δspt2∷HYG/Δspt2∷PAC), and spt2−/+SPT2 (Δspt2∷HYG/Δspt2∷PAC/+ pXG-SPT2) cells were grown as described (Zhang et al, 2003). Promastigotes were cultured in complete M199 medium with 10% FBS (Kapler et al, 1990a). Cell density was measured using a Coulter counter (Z1, Beckman) and viability was measured by flow cytometry after staining with 0.5 μg/ml of propidium iodide using a Becton Dickinson FACSCalibur. Metacyclics were isolated and quantified using the PNA or density centrifugation methods (Sacks and Perkins, 1984; Späth and Beverley, 2001).
SPL coding regions were sequentially replaced by a neomycin (NEO) and a hygromycin (HYG) marker to generate the spl− (Δspl∷NEO/Δspl∷NEO/Δspl∷HYG) mutant. Chromosome 30 in LV39c5 is trisomic (E Brooke-Powell and S M Beverley, unpublished data) and fortuitously replacement of the third SPL copy occurred by gene conversion with the NEO allele (Gueiros-Filho and Beverley, 1996). To restore SPL expression, spl− mutant clones were transfected with pXGBSD-SPL, referred to as spl−/+SPL (Δspl∷NEO/Δspl∷NEO/Δspl∷HYG/+ pXGBSD-SPL). Transfections were performed as described (Kapler et al, 1990b). Several spl− mutants were characterized and showed similar phenotypes; data for clone #C6 are presented here.
Supplements (EtN, P-EtN, choline, etc,) were provided in log (1–5 × 105 cells/ml) or late log (5–9 × 106 cells/ml) phase. The A. thaliana SDC ORF (AF389349) was cloned in the BglII site of pIR1-Phleo (pIR1-Phleo-SDC, B5198). This was integrated into the small ribosomal subunit site of spl− and spt2− to generate spl− SSU∷SDC and spt2− SSU∷SDC. To test EtN requirements, promastigotes were grown in serum-free medium (M199 supplemented with 0.4% bovine serum albumin and no FBS) with 1 mM EtN. After five consecutive passages, promastigotes were inoculated into serum-free media at 2 × 105/ml±1 mM EtN.
SPL assay, metabolic labeling, and lipid analysis
Promastigotes were inoculated in M199/10% FBS at 1.0 × 105/ml and labeled with [3-3H]serine (34 μCi/mmol and 1.4 μCi/ml) for 48 h at 26°C. Total lipids were extracted from late log-phase cultures and analyzed by TLC as described (Zhang et al, 2003), using a different solvent system (chloroform/methanol/water, 65:25:4 by volume). Detergent extraction and Western blot analysis of LPG were performed as described (Zhang et al, 2003). For microsomal preparations, stationary-phase promastigote was washed three times with PBS and suspended in 200 mM potassium phosphate, pH 7.2, 20 mM EDTA, 11% glycerol, 1 mM pyridoxal-5-phosphate, 15 μg/ml each of chymostatin, leupeptin, antipain, pepstain, 2 mM AEBSF, 10 mM NaF, and 5 mM 1,10-phenanthroline. Samples were sonicated, debris removed by centrifugation (2000 g, for 5 min), and microsomes were collected by centrifugation (90 min × 100 000 g) and suspended in the buffer above. SPL activity was assayed as described (Van Veldhoven and Mannaerts, 1991; Mendel et al, 2003). The average measured product c.p.m. in this assay for WT, spl−, spl−/+SPL, and boiled extract control preparations were 2284, 1729, 2261, and 1419, respectively.
Phase-contrast microscopy and transmission EM
Promastigotes were attached to polylysine-coated cover-slips, followed by fixation with 3.7% formaldehyde in PBS. Phase-contrast images were obtained using an Olympus AX70 microscope. Percentages of ‘round cells', defined as cells with length/width ratios less than 2:1, were determined after examinations of 200–300 cells. Transmission EM of stationary-phase promastigotes (3 days after reaching the maximal density) and amastigotes harvested from infected mice was performed as described (Zhang et al, 2005).
Semiquantitative analysis of sphingosine, plasmalogen PtE, and IPC by electrospray ionization mass spectrometry
Cellular levels of plasmalogen PtE and IPC in promastigote samples were determined using a similar protocol as previously described (Zhang et al, 2005). Briefly, 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (14:0/14:0-PtE) and 1,2-dipalmitoyl-sn-glycero-1-phosphoinositol (16:0/16:0-PtI) were added to promastigote samples at 15 μg per 108 cells each as internal controls. Total lipids were then extracted and subjected to ESI/MS analysis in the negative-ion mode. Abundance of plasmalogen PtE and IPC species relative to the internal standards was estimated from the ESI mass spectra. Similar protocol was used for the measurement of sphingosine in promastigotes samples.
Mouse footpad infection and macrophage infections
Promastigote virulence was evaluated in vivo by mouse footpad infections (Titus et al, 1991). After three passages, late stationary-phase promastigotes (3 days at constant maximal density) and metacyclics (isolated from stationary-phase promastigotes using the PNA method or the density centrifugation method, as described) were resuspended in DMEM and injected into the footpads of 5–6 female BALB/c mice (8-week-old) at 106 cells/mouse (stationary phase) or 2 × 105 cells/mouse (metacyclics). Lesion sizes were measured with a Vernier caliper and parasite numbers in the infected foot pads were determined by limiting dilution assays (Titus et al, 1985). Amastigotes were harvested from infected BALB/c mice and infections of murine peritoneal macrophages were performed as previously described (Zhang et al, 2005).
Supplementary Material
Supplementary Figures and Tables
Acknowledgments
We thank Wandy Beatty for the EM work, Dr Andrew Hanson for providing the A. thaliana SDC gene, E Brooke-Powell for preliminary data, A Capul, D Dobson, T Vickers, and D Scott for comments on this manuscript, and everyone in the SMB laboratory for insightful discussions. This work was funded by National Institutes of Health (NIH) grants AI31078 (SMB) and GM66954 (JS). JP was supported in part by a WU/HHMI Summer Undergraduate Research Fellowship funded by an undergraduate biological sciences education program grant from the Howard Hughes Medical Institute to Washington University. PK was supported by National Institutes of Health grant T32 GM00706. Mass spectrometric analyses were performed at the Washington University Medicine Department MS center, which was supported by United States Public Health Service grants P41-RR00954, P60-DK20579, RO1-69455, and P30-DK56341.
References
- Araujo-Santos JM, Gamarro F, Castanys S, Herrmann A, Pomorski T (2003) Rapid transport of phospholipids across the plasma membrane of Leishmania infantum. Biochem Biophys Res Commun 306: 250–255 [DOI] [PubMed] [Google Scholar]
- Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Bohme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UC, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens A, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DM, Morgan GW, Mungall K, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reitter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CM, Tait A, Tivey AR, Van Aken S, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, Melville SE, El-Sayed NM (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309: 416–422 [DOI] [PubMed] [Google Scholar]
- Besteiro S, Williams RA, Morrison LS, Coombs GH, Mottram JC (2006) Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J Biol Chem 281: 11384–11396 [DOI] [PubMed] [Google Scholar]
- Birner R, Burgermeister M, Schneiter R, Daum G (2001) Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol Biol Cell 12: 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A, Beverley SM (1990) Gene replacement in parasitic protozoa. Nature 348: 171–173 [DOI] [PubMed] [Google Scholar]
- Cunningham AC (2002) Parasitic adaptive mechanisms in infection by Leishmania. Exp Mol Pathol 72: 132–141 [DOI] [PubMed] [Google Scholar]
- Denny PW, Goulding D, Ferguson MA, Smith DF (2004) Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol 52: 313–327 [DOI] [PubMed] [Google Scholar]
- Denny PW, Smith DF (2004) Rafts and sphingolipid biosynthesis in the kinetoplastid parasitic protozoa. Mol Microbiol 53: 725–733 [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB (2002) Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science 296: 879–883 [DOI] [PubMed] [Google Scholar]
- Funato K, Vallee B, Riezman H (2002) Biosynthesis and trafficking of sphingolipids in the yeast Saccharomyces cerevisiae. Biochemistry 41: 15105–15114 [DOI] [PubMed] [Google Scholar]
- Glaser PE, Gross RW (1994) Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry 33: 5805–5812 [DOI] [PubMed] [Google Scholar]
- Gruenberg J (2001) The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol 2: 721–730 [DOI] [PubMed] [Google Scholar]
- Gueiros-Filho FJ, Beverley SM (1996) Selection against the dihydrofolate reductase-thymidylate synthase (DHFR-TS) locus as a probe of genetic alterations in Leishmania major. Mol Cell Biol 16: 5655–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guther ML, Lee S, Tetley L, Acosta-Serrano A, Ferguson MA (2006) GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, and are not the only components of the surface coat. Mol Biol Cell 17: 5265–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S (2003) Structure, organization, and function of glycosphingolipids in membrane. Curr Opin Hematol 10: 16–24 [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM (2001) Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 40: 4893–4903 [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y (2000) A ubiquitin-like system mediates protein lipidation. Nature 408: 488–492 [DOI] [PubMed] [Google Scholar]
- Ikonen E (2001) Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 13: 470–477 [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Hoessli DC (1998) Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 335 (Part 2): 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgoutz SC, McConville MJ (2001) Function and assembly of the Leishmania surface coat. Int J Parasitol 31: 899–908 [DOI] [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Muller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O'neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schafer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, Myler PJ (2005) The genome of the kinetoplastid parasite, Leishmania major. Science 309: 436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro ES, Jayasimhulu K, Lester RL (1986) Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J Lipid Res 27: 1294–1303 [PubMed] [Google Scholar]
- Kapler GM, Coburn CM, Beverley SM (1990a) Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol 10: 1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler GM, Zhang K, Beverley SM (1990b) Nuclease mapping and DNA sequence analysis of transcripts from the dihydrofolate reductase-thymidylate synthase (R) region of Leishmania major. Nucleic Acids Res 18: 6399–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C (1995) Eukaryotic phospholipid biosynthesis. Annu Rev Biochem 64: 315–343 [DOI] [PubMed] [Google Scholar]
- Kolter T, Proia RL, Sandhoff K (2002) Combinatorial ganglioside biosynthesis. J Biol Chem 277: 25859–25862 [DOI] [PubMed] [Google Scholar]
- Lohner K (1996) Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids 81: 167–184 [DOI] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstien S, Spiegel S (2002) Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta 1585: 193–201 [DOI] [PubMed] [Google Scholar]
- McConville MJ, Blackwell JM (1991) Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem 266: 15170–15179 [PubMed] [Google Scholar]
- Mendel J, Heinecke K, Fyrst H, Saba JD (2003) Sphingosine phosphate lyase expression is essential for normal development in Caenorhabditis elegans. J Biol Chem 278: 22341–22349 [DOI] [PubMed] [Google Scholar]
- Menon AK, Eppinger M, Mayor S, Schwarz RT (1993) Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. EMBO J 12: 1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH Jr (2002) De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem 277: 25843–25846 [DOI] [PubMed] [Google Scholar]
- Nagafuku M, Kabayama K, Oka D, Kato A, Tani-ichi S, Shimada Y, Ohno-Iwashita Y, Yamasaki S, Saito T, Iwabuchi K, Hamaoka T, Inokuchi J, Kosugi A (2003) Reduction of glycosphingolipid levels in lipid rafts affects the expression state and function of glycosylphosphatidylinositol-anchored proteins but does not impair signal transduction via the T cell receptor. J Biol Chem 278: 51920–51927 [DOI] [PubMed] [Google Scholar]
- Nagan N, Zoeller RA (2001) Plasmalogens: biosynthesis and functions. Prog Lipid Res 40: 199–229 [DOI] [PubMed] [Google Scholar]
- Patnaik PK, Field MC, Menon AK, Cross GA, Yee MC, Butikofer P (1993) Molecular species analysis of phospholipids from Trypanosoma brucei bloodstream and procyclic forms. Mol Biochem Parasitol 58: 97–105 [DOI] [PubMed] [Google Scholar]
- Pike LJ, Han X, Chung KN, Gross RW (2002) Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088 [DOI] [PubMed] [Google Scholar]
- Polokoff MA, Wing DC, Raetz CR (1981) Isolation of somatic cell mutants defective in the biosynthesis of phosphatidylethanolamine. J Biol Chem 256: 7687–7690 [PubMed] [Google Scholar]
- Rifkin MR, Strobos CA, Fairlamb AH (1995) Specificity of ethanolamine transport and its further metabolism in Trypanosoma brucei. J Biol Chem 270: 16160–16166 [DOI] [PubMed] [Google Scholar]
- Rodemer C, Thai TP, Brugger B, Gorgas K, Just W (2003) Targeted disruption of ether lipid synthesis in mice. Adv Exp Med Biol 544: 355–368 [DOI] [PubMed] [Google Scholar]
- Rontein D, Nishida I, Tashiro G, Yoshioka K, Wu WI, Voelker DR, Basset G, Hanson AD (2001) Plants synthesize ethanolamine by direct decarboxylation of serine using a pyridoxal phosphate enzyme. J Biol Chem 276: 35523–35529 [DOI] [PubMed] [Google Scholar]
- Ruvolo PP (2003) Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 47: 383–392 [DOI] [PubMed] [Google Scholar]
- Sacks DL, Hieny S, Sher A (1985) Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol 135: 564–569 [PubMed] [Google Scholar]
- Sacks DL, Perkins PV (1984) Identification of an infective stage of Leishmania promastigotes. Science 223: 1417–1419 [DOI] [PubMed] [Google Scholar]
- Schneider P, Rosat JP, Ransijn A, Ferguson MA, McConville MJ (1993) Characterization of glycoinositol phospholipids in the amastigote stage of the protozoan parasite Leishmania major. Biochem J 295: 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius JR (2003) Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta 1610: 174–183 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Späth GF, Beverley SM (2001) A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol 99: 97–103 [DOI] [PubMed] [Google Scholar]
- Späth GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM (2000) Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci USA 97: 9258–9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey MK, Clay KL, Kutateladze T, Murphy RC, Overduin M, Voelker DR (2001) Phosphatidylethanolamine has an essential role in Saccharomyces cerevisiae that is independent of its ability to form hexagonal phase structures. J Biol Chem 276: 48539–48548 [DOI] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA (1985) A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 7: 545–555 [DOI] [PubMed] [Google Scholar]
- Titus RG, Muller I, Kimsey P, Cerny A, Behin R, Zinkernagel RM, Louis JA (1991) Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol 21: 559–567 [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Voelker DR (1995) Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J Biol Chem 270: 6062–6070 [DOI] [PubMed] [Google Scholar]
- van Meer G, Lisman Q (2002) Sphingolipid transport: rafts and translocators. J Biol Chem 277: 25855–25858 [DOI] [PubMed] [Google Scholar]
- Van Veldhoven PP, Gijsbers S, Mannaerts GP, Vermeesch JR, Brys V (2000) Human sphingosine-1-phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochim Biophys Acta 1487: 128–134 [DOI] [PubMed] [Google Scholar]
- Van Veldhoven PP, Mannaerts GP (1991) Subcellular localization and membrane topology of sphingosine-1-phosphate lyase in rat liver. J Biol Chem 266: 12502–12507 [PubMed] [Google Scholar]
- Vance JE (2003) Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog Nucleic Acid Res Mol Biol 75: 69–111 [DOI] [PubMed] [Google Scholar]
- Voelker DR (1997) Phosphatidylserine decarboxylase. Biochim Biophys Acta 1348: 236–244 [DOI] [PubMed] [Google Scholar]
- Wang TY, Silvius JR (2003) Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys J 84: 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart SW, Shenbagamurthi P, Chen L, Cotter RJ, Hart GW (1989) Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha. J Biol Chem 264: 14334–14341 [PubMed] [Google Scholar]
- Zhang K, Hsu FF, Scott DA, Docampo R, Turk J, Beverley SM (2005) Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol 55: 1566–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Showalter M, Revollo J, Hsu FF, Turk J, Beverley SM (2003) Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J 22: 6016–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, Smith DF, Turco SJ, Ferguson MA, Beverley SM (2003) Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem 278: 44708–44718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Tables


