Abstract
Copper is an essential but potentially toxic trace element. In Drosophila, the metal-responsive transcription factor (MTF-1) plays a dual role in copper homeostasis: at limiting copper concentrations, it induces the Ctr1B copper importer gene, whereas at high copper concentrations, it mainly induces the metallothionein genes. Here we find that, despite the downregulation of the Ctr1B gene at high copper concentrations, the protein persists on the plasma membrane of intestinal cells for many hours and thereby fills the intracellular copper stores. Drosophila may risk excessive copper accumulation for the potential benefit of overcoming a period of copper scarcity. Indeed, we find that copper-enriched flies donate a vital supply to their offspring, allowing the following generation to thrive on low-copper food. We also describe two additional modes of copper handling: behavioral avoidance of food containing high (⩾0.5 mM) copper levels, as well as the ability of DmATP7, the Drosophila homolog of Wilson/Menkes disease copper exporters, to counteract copper toxicity. Regulated import, storage, export, and avoidance of high-copper food establish an adequate copper homeostasis under variable environmental conditions.
Keywords: copper toxicity, Ctr1, metallothioneins, MTF-1, trace element, taste recognition
Introduction
Copper is an essential component of several important enzymes involved in respiration, oxidative stress protection, pigmentation and iron homeostasis. Most of these enzymatic reactions rely on the ability of copper to undergo redox transitions between the Cu(I) and Cu(II) state. This important trait of copper is at the same time a threat to the organism, as copper can catalyze the generation of reactive oxygen species (ROS) via the Fenton reaction (Halliwell and Gutteridge, 1990; Puig and Thiele, 2002). Eukaryotic organisms from yeast to humans use elaborate systems to regulate copper homeostasis, consisting of copper importers, copper chaperones, transcription factors, small metal binding proteins called metallothioneins and copper exporters (O'Halloran and Culotta, 2000; Puig and Thiele, 2002; Mercer and Llanos, 2003; Balamurugan and Schaffner, 2006). Studies in yeast have identified the high-affinity copper transporters yCtr1 and yCtr3 (Dancis et al, 1994; Pena et al, 2000). Homologs of these were identified in mammals (Ctr1) and in Drosophila (Ctr1A, B and C) (Lee et al, 2000; Zhou et al, 2003). A null mutant of Ctr1 in the mouse is lethal, whereas a mutation of Ctr1B in Drosophila results in a pigmentation defect and lethality under conditions of copper scarcity (Kuo et al, 2001; Lee et al, 2001; Zhou et al, 2003).
The low-copper phenotype in the fly is reminiscent of the phenotype of a mutation in the metal-responsive transcription factor-1 (MTF-1) (Egli et al, 2003). We have recently shown that Ctr1B transcription is activated by dMTF-1 under normal conditions and to a greater extent upon copper depletion, but repressed when copper is abundant (Selvaraj et al, 2005). In contrast, when copper is in excess, MTF-1 activates transcription of the genes for metallothioneins, small, cysteine-rich metal scavenger proteins. The genes for Ctr1B and the metallothioneins are therefore both target genes of the same transcription factor but regulated in an opposite manner (Selvaraj et al, 2005). Whereas Drosophila Ctr1B is regulated at the transcriptional level, the human and yeast Ctr1 homologs appear to be post-translationally regulated. Copper excess stimulates rapid endocytosis and/or degradation of both yCtr1 and hCtr1 (Ooi et al, 1996; Petris et al, 2003; Guo et al, 2004), apparently to prevent overaccumulation and toxicity of copper. In a different study, hCtr1 remained stable and functional even after copper exposure (Eisses et al, 2005). Another transport system is able to maintain adequate activity under both low and high copper conditions. At normal or low copper, the related mammalian copper exporters ATP7A and ATP7B, also referred to as Menkes and Wilson P-type ATPases, respectively, transfer copper from the cytoplasm to the trans-Golgi network (TGN). At high copper, both exporters translocate from the Golgi to the cell surface, where they function to export copper from the cell (Petris et al, 1996). The importance of the latter process is illustrated by mutations in ATP7B, which causes Wilson's disease, where patients accumulate toxic amounts of copper in the liver (Bull et al, 1993; Yamaguchi et al, 1993). In spite of recent progress in our understanding of copper homeostasis, many questions remain concerning how copper levels are sensed and how they feed back to coordinate copper uptake, export or copper sequestration. In the fruit fly Drosophila, overall copper levels of the organism can vary at least by 20-fold depending upon the copper concentration in the diet (H Yepiskoposyan, A Simons and W Schaffner, unpublished). Apparently under high copper conditions, sequestration is a major mechanism ensuring a remarkable tolerance to copper. This tolerance depends on the copper sequestrating ability of metallothioneins and the transcription factor MTF-1, which activates transcription of the metallothionein loci from insects to mammals (Heuchel et al, 1994; Zhang et al, 2001). Accordingly, metallothionein or MTF-1 mutants are highly sensitive to elevated copper concentrations (Egli et al, 2006a). In Drosophila larvae, metallothionein expression and the sites of copper accumulation coincide, especially in the cytoplasm of so-called copper cells of the midgut and also in the posterior midgut (Filshie et al, 1971; Tapp, 1975; Lauverjat et al, 1989; Marchal-Segault et al, 1990; Egli et al, 2006a). Noteworthy, copper cells display a characteristic orange copper luminescence caused by a copper–metallothionein complex (Egli et al, 2006a).
Here, we report that in spite of a downregulation of Ctr1B transcripts in high copper, the protein persists for many hours in a functional state. In Drosophila larvae, Ctr1B remains at the plasma membrane of intestinal cells and imports copper to high levels, causing a metal-stress response and the induction of metallothioneins. Taken at face value, this property indicates a flawed regulation of copper homeostasis. However, we provide evidence that transient copper accumulation in larvae can balance fluctuating copper availability in the food and helps to overcome intermittent periods of copper starvation, and is even used to supply copper to the following generation. Thus, the regulation of Ctr1B seems to reflect a trade-off between copper excess causing copper toxicity and the prospect of copper starvation, which severely delays development. We also show two additional levels of regulation, which help to limit copper accumulation: copper export via DmATP7, and a behavioral avoidance of food containing 0.5 mM or more copper by larvae.
Results
Metallothionein transcription as a sensitive indicator of heavy metal concentration and distribution
Drosophila larvae that are grown on copper-supplemented food contain high levels of metallothioneins, whereas metallothionein expression is low when larvae are grown on copper-depleted food (Figure 1A). Likewise, larvae that are constantly fed on zinc, cadmium or mercury containing media show a dose-dependent increase in metallothionein expression (Balamurugan et al, 2004). The tissue specificity of metal distribution coincides with metallothionein expression, for example, the so-called copper cells of the larval midgut accumulate high levels of copper and strongly express metallothionein genes. The endogenous metallothionein promoters or metallothionein-EYFP (enhanced yellow fluorescent protein) reporter constructs are thus sensitive indicators of metal transport processes within the intact organism.
Figure 1.
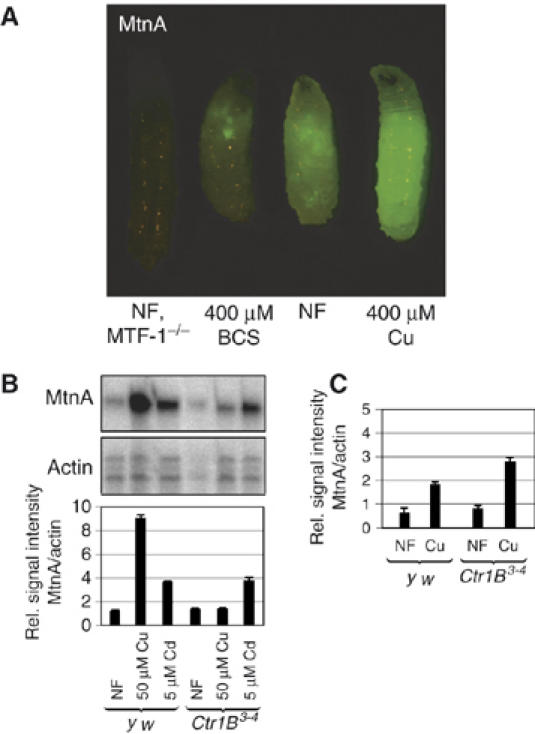
A metallothionein-EYFP reporter as a sensitive indicator of metal content and transport processes. (A) Expression of reporter transgene consisting of the MtnA promoter driving expression of EYFP. Shown are third instar Drosophila larvae grown on the indicated type of food. MTF-1 mutant larvae, which lack metallothionein expression, owing to absence of the major transactivator MTF-1 (metal responsive transcription factor 1 or metal regulatory transcription factor 1), also do not show reporter gene expression as measured by fluorescence microscopy. (B) Mutants for the Ctr1B copper importer are deficient in copper-mediated induction of the MtnA promoter. Larvae at the third instar were transferred from normal to cadmium or copper containing food for 6 h and mRNA expression was determined by S1 nuclease mapping. Bars represent the quantification of MtnA mRNA levels normalized with the signal obtained with a probe complementary to actin mRNA. Results are the mean±s.e.m. of three independent experiments. (C) Mutant and ‘wild type' (y w) larvae that were continuously raised until third instar on normal food (NF) or copper containing food. Transcripts were quantified as in panel B.
Ctr1B is the most efficient copper importer in Drosophila larvae
The copper transporter Ctr1B is expressed during larval stages in the posterior midgut and mediates copper uptake from the food. We determined the induction of the metallothionein A (MtnA) promoter by copper and zinc in Ctr1B mutants and in wild-type larvae that were grown on normal food (NF) and transferred for 6 h to food supplemented with copper. Whereas wild-type larvae showed a strong induction of metallothionein transcripts by copper and cadmium, Ctr1B mutant larvae did not display copper-mediated induction, but maintained cadmium-mediated induction of metallothioneins (Figure 1B). This demonstrates that Ctr1B is the major intestinal copper importer of larvae growing on normal food. Interestingly, when larvae were constantly raised in high copper, metallothionein levels were as high in Ctr1B mutants as in wild type (Figure 1C). At first sight, these results seem to contradict each other. However, metallothionein induction in the first experiment relied on an efficient, rapid import of copper into the cell, whereas in the second, copper could accumulate over a period of several days. Our results suggest that under the latter conditions, copper import occurs independent of Ctr1B by less abundant, less efficient or less specific copper importers, possibly Ctr1A, Ctr1C or Malvolio (a Drosophila homolog of Nramp-1) (Rodrigues et al, 1995), ultimately leading to equal levels of Mtn induction in both Ctr1B mutant and wild-type larvae. Consistent with this observation is the report that Ctr1B mutants contain less copper than wild-type flies under normal conditions, but the same amount under copper load (Zhou et al, 2003).
Rapid copper import by Ctr1B causes a metal-stress response
Ctr1B remained active when larvae were transferred from normal food to food containing high copper concentrations, which strongly induced metallothionein gene expression. Thus, we asked whether high expression of Ctr1B would cause a copper stress response even with normal food. Larvae that are grown in the presence of the copper chelator bathocuproine disulfonate (BCS) show high levels of Ctr1B to ensure sufficient copper uptake (Selvaraj et al, 2005). At the same time, transcription of metallothionein genes is low. Indeed, when copper-depleted larvae were transferred to normal food without copper chelator for 6 h, expression of metallothionein genes was strongly induced owing to high Ctr1B expression. This indicates that import of copper by Ctr1B leads to a copper-mediated stress response characterized by metallothionein gene induction (Figure 2A).
Figure 2.
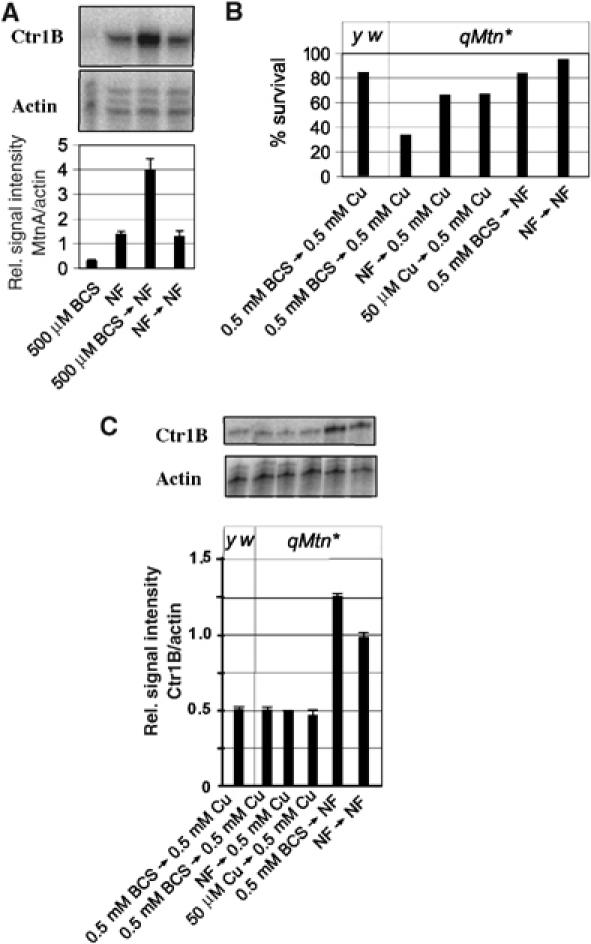
Copper shock: a cellular stress response owing to massive copper import. (A) Drosophila larvae were copper-starved by growth on food containing 0.5 mM copper chelator (BCS) or on normal food (NF) and transferred to NF for 6 h. Shown are the expression levels of metallothionein A (MtnA). Quantification was done as in Figure 1. (B) Wild-type (y w) or mutant flies with inactivation of all four metallothionein genes (qMtn*) were grown for five days on 0.5 mM BCS and 30 third instar larvae were transferred to either normal food or 0.5 mM copper. Shown is the survival to adulthood in percentage. Results are the mean±s.e.m. of three different experiments. (C) Wild-type or flies lacking all four metallothioneins (qMtn*) were first grown in the indicated type of food and allowed to lay eggs. Third instar larvae were transferred to high copper or normal food for 18 h. Total RNA was extracted and transcripts were quantified by S1 nuclease mapping. Actin5c RNA was used as a standard for normalization. Results are the mean±s.e.m. of three independent experiments.
Metallothioneins reduce toxicity of a Ctr1B-mediated copper shock
As Ctr1B appears to import copper to critical levels, we wished to know whether transfer of larvae from low to high copper results in copper toxicity. To this end, we made use of a copper-sensitive ‘quadruple metallothionein' (qMtn*) mutant strain that lacks all four metallothioneins (Egli et al, 2006a). These flies are viable and develop normally in up to 100 μM copper in the food, that is, they tolerate a certain amount of copper excess (although less than 5% of what wild-type larvae tolerate). We transferred copper-depleted or normally fed third instar larvae to food with high copper and scored for survivors that made it to adulthood. Only one-third of qMtn mutant larvae that were copper-depleted and therefore had high starting levels of Ctr1B survived the copper shock. qMtn mutant larvae that were grown in standard or copper-rich food before the transfer, and therefore had lower starting levels of Ctr1B, were more likely to survive (Figure 2B). As copper uptake via importers other than Ctr1B (Ctr1A and Ctr1C) is low under these experimental conditions (Zhou et al, 2003), we conclude that Ctr1B continues to import copper even after normal levels are reached, resulting in copper excess and, especially in the absence of metallothioneins, imminent toxicity. Wild-type control larvae survived a transfer from low to high copper quite well, indicating that metallothioneins play an important role in buffering fluctuating copper availability and uptake (Figure 1B; see also Egli et al, 2006b). We have also measured endogenous Ctr1B transcript levels in metallothionein mutants after transferring early third instar larvae from copper-depleted or normal food to high-copper food. We note that upon copper shock, Ctr1B transcript levels are similarly reduced in wild-type and metallothionein-knockout larvae, indicating that the copper importing activity of Ctr1B is not altered in the latter (Figure 2C). These observations corroborate our previous findings that the copper content of both wild-type and quadruple metallothionein-knockout flies is the same after copper shock (Egli et al, 2006a).
Ctr1B is mostly, if not exclusively, regulated at the transcriptional level
Copper chelation by metallothioneins reduces copper toxicity following a copper shock. In addition, we considered post-translational regulation of Ctr1B, for example, copper-dependent degradation, which was reported for mammalian Ctr1 (Petris et al, 2003). To find whether there was a similar regulation in flies, we transferred larvae from low (250 μM BCS) to high copper (250 μM Cu) and tested mRNA and protein levels of Ctr1B-EGFP. The transcriptional response was rapid: mRNA levels of Ctr1B-EGFP were reduced after merely 2 h, whereas at the same time, metallothionein A mRNA increased in parallel (Figure 3A). In contrast, Ctr1B-EGFP protein levels remained constant even after 18 h of copper load (Figure 3B). In these experiments, we tested the levels of Ctr1B-EGFP fusion construct (AH3) where the EGFP coding region is fused in-frame to the last codon of a complete Ctr1B gene as well as endogenous Ctr1B (not shown). The Ctr1B-EGFP fusion construct was previously shown to be regulated by copper at the transcriptional level and to rescue all known aspects of the Ctr1B mutant phenotype, namely, sensitivity to both copper scarcity and to very high copper concentration (Selvaraj et al, 2005). Nevertheless, AH3 transgenic flies in a Ctr1B wild-type background were not more sensitive to copper shock than wild-type (y w) control larvae, indicating that the fusion construct does not result in a hyperactive allele and excessive copper import (not shown). Taken together, these findings suggest that Drosophila Ctr1B is not degraded in a copper-dependent manner; rather, the amount of Ctr1B is mostly, if not entirely, regulated at the transcriptional level.
Figure 3.
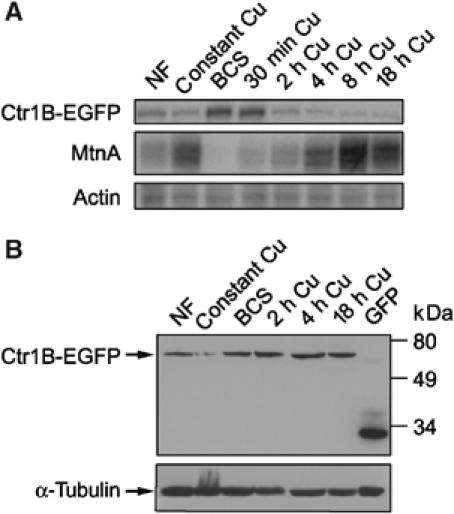
Ctr1B protein but not mRNA levels are stable after transfer of larvae from low to high copper. The different time points indicate the time after removing the larvae from low copper (i.e., BCS containing) food. The concentration of copper or BCS was 250 μM. (A) mRNA levels of MtnA or a Ctr1B reporter construct (Ctr1B-EGFP with the endogenous Ctr1B promoter) determined by S1 nuclease mapping. Note the opposite regulation of the two transcripts by copper availability (see also Selvaraj et al, 2005). (B) Western blot of Ctr1B-EGFP and α-tubulin. Unlike mRNA levels, Ctr1B protein levels are hardly affected by copper load during the first 18 h. NF=normal food.
The Ctr1B-EGFP fusion protein remains on the plasma membrane upon copper shock
Consistent with a role in copper uptake from the food, the Ctr1B-EGFP fusion protein localized to the apical side of the plasma membrane of larval gut cells (Figure 4A). To find out whether Ctr1B undergoes internalization at elevated copper levels as described for mammalian Ctr1 (Petris et al, 2003), we transferred third instar larvae with the Ctr1B-EGFP transgene from 100 μM BCS to 250 μM copper. Even after 24 h, when copper import has led to a strong activation of metallothionein genes, the Ctr1B-EGFP fusion protein persisted at the apical plasma membrane of intestinal cells (Figure 4B). Importantly, these cells also showed the characteristic copper luminescence, indicating that high amounts of intracellular copper were complexed by metallothioneins. Such high amounts of copper are not required for larval development, as larvae that are grown at mild copper depletion (100 μM BCS) also develop normally and maintain the same copper content as controls owing to the upregulation of Ctr1B (see below). These results show that copper does not alter the cellular localization of Ctr1B and provide cytological evidence that Ctr1B continues to import copper from the food beyond the immediate physiological requirements.
Figure 4.
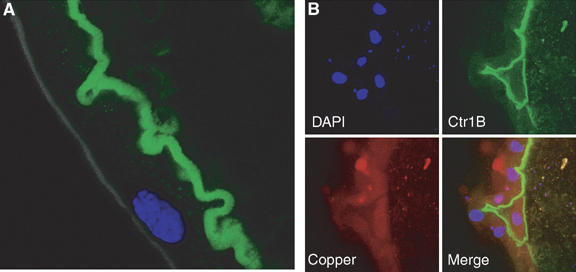
Apical localization of Ctr1B in intestinal cells is not regulated by copper. (A) Localization (apical) of the Ctr1B-EGFP fusion protein on the brush border membrane of an intestinal cell in the posterior midgut. The lumen of the gut is to the right. This larva was raised to the third instar on 100 μM BCS. Blue: DAPI staining of nucleus. (B) Copper shock does not alter the cellular localization of Ctr1B-EGFP. Third instar larvae were transferred from 100 μM BCS to 250 μM copper for 24 h and larval guts were analyzed by confocal microscopy. Copper accumulation is revealed by orange copper luminescence. The lumen of the gut, with some autofluorescent food particles, is to the right.
Copper excess is used to cope with periods of nutritional copper scarcity
To test whether stored copper helps to overcome a period of copper scarcity, we shifted larvae from copper-scarce to copper-supplemented food and then back to copper-scarce food. Growth on normal food until pupariation takes about 5 days, but growth on low copper can delay development by twice the normal time period or even more (Figure 5A). The developmental retardation is most pronounced in rapidly growing larvae. High copper concentrations can also delay growth (see also Egli et al, 2006a). Homeostasis of copper is therefore required to minimize the time span of complete development. To investigate the ability of Drosophila larvae to cope with nutritional copper fluctuations, we transferred 3-day-old second instar larvae from 750 μM BCS (wild type) or 15 μM BCS (Ctr1B mutants) to 250 μM Cu, or to normal food, either for 4 h or for the rest of the larval development, and measured the time needed to reach pupal stage (Figures 5B–D). The short 4 h copper pulse dramatically accelerated development of wild-type larvae by about 4 days compared to constant growth on 750 μM BCS. This result is comparable to growth of larvae shifted to normal food for the rest of development. A shift that was followed by constant growth on copper-enriched food however does not accelerate development (Figure 5B and C). Consistent with the role of Ctr1B as an intestinal copper importer, Ctr1B mutant larvae had great difficulties in exploiting such a transient period of copper abundance and were clearly retarded relative to equally treated wild-type larvae (Figure 5C). These results show that Ctr1B mediates efficient copper import within a short time window, ensuring a sufficient copper supply for the rest of development. This confers a substantial growth advantage when copper becomes scarce, but may also increase the risk of copper-mediated oxidative damage.
Figure 5.
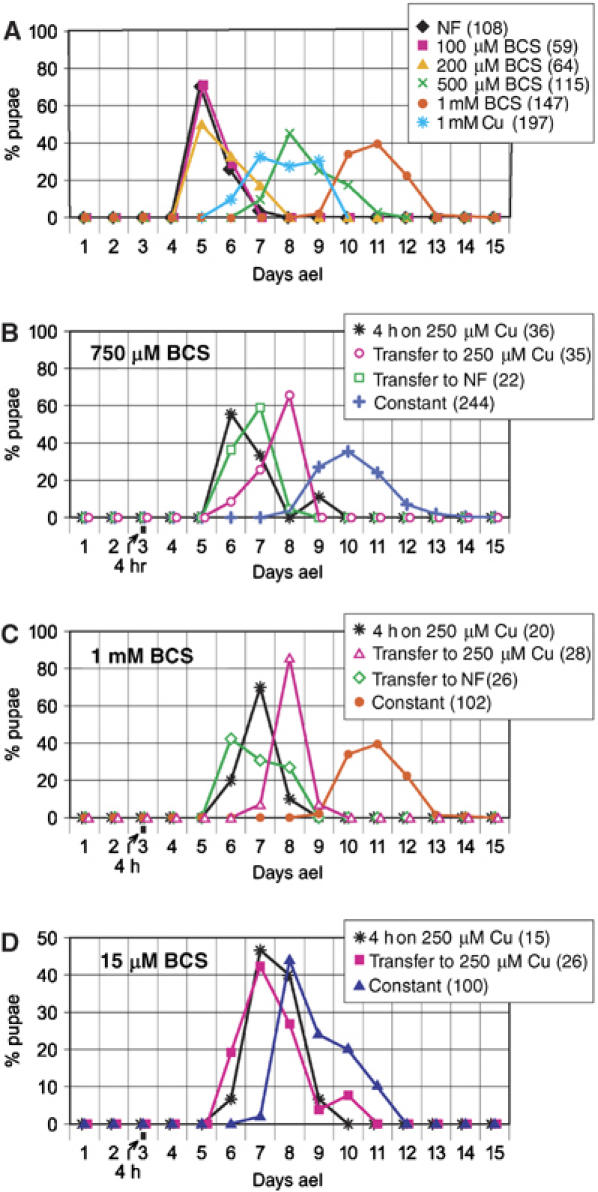
Copper starvation strongly delays development, but is rescued by a short copper pulse. Shown is the percentage of pupae forming on each day, counted after egg laying. The numbers in parentheses indicate the number of pupae in each experiment. (A) Wild-type flies were raised continuously on the indicated type of food. Note the severe delay in development by copper scarcity and also by copper load. (B–D) Larvae were transferred on the third day after egg laying (ael) to normal or copper-enriched food for further development, or kept in high-copper food for just four hours and then transferred back to the type of food indicated in each panel. (B, C) Wild-type Ctr1B+/+ flies, (D) Ctr1B−/− flies.
Accumulation of copper in the body: another key component in Drosophila copper homeostasis
Intestinal cells have a remarkable ability to induce transcription of metallothionein genes and to accumulate and detoxify high amounts of copper. In Drosophila larvae, Ctr1B is expressed in the intestinal cells of the posterior midgut, where metallothionein induction is high upon copper load (this study). To determine the effect of ectopic Ctr1B expression, we have tested a transgene where Ctr1B is under the control of an eye-specific GMR promoter. Such an ectopic (over)expression of Ctr1B resulted in distorted eye development, manifesting itself as ‘rough eyes'. This phenotype critically depends on copper availability: copper scarcity alleviated the rough eye phenotype, whereas copper supplementation of the food aggravated it in a dose-dependent manner (Figure 6A). We also observed that the rough eye phenotype is more severe in flies that were grown in food containing both 20 μM copper and 0.02% hydrogen peroxide as compared to 20 μM copper or 0.02% hydrogen peroxide alone (Figure 6B), probably as a result of radical generation via the Fenton reaction (Halliwell and Gutteridge, 1990; Puig and Thiele, 2002).
Figure 6.
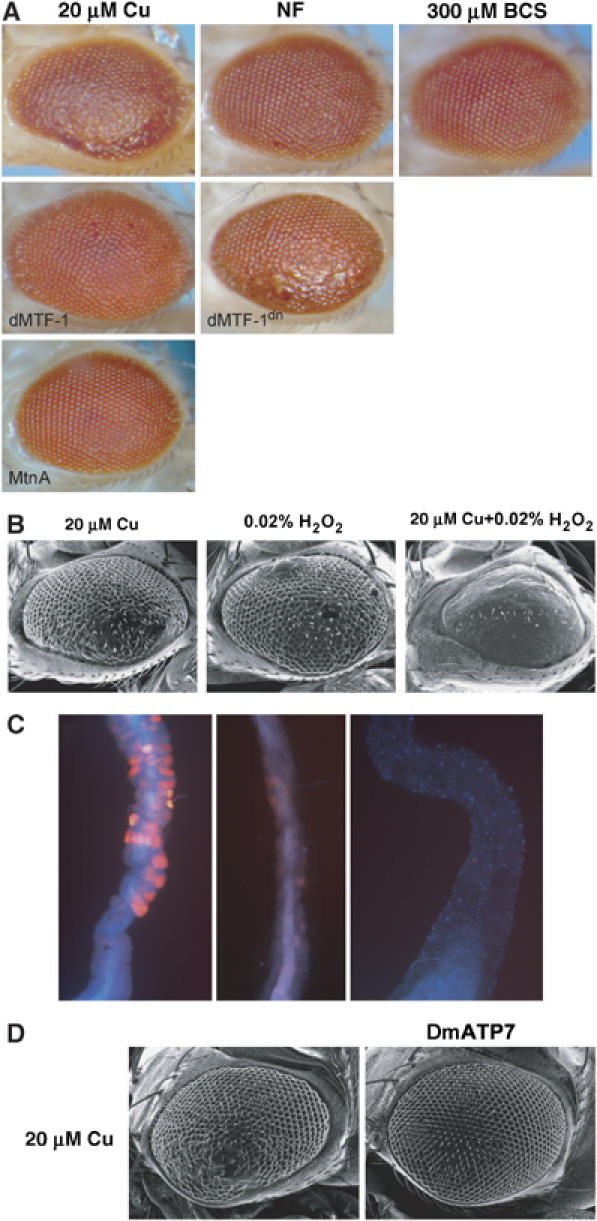
Ectopic copper import interferes with development. (A) Phenotype of flies with ectopic copper import into developing eye imaginal cells. Ctr1B is expressed under the control of the eye-specific GMR promoter in all panels. Shown are the scanning electron micrographs of the Drosophila eye. Flies were grown either in NF or 20 μM copper-supplemented food. 5 day-old flies were fixed in 2% osmium tetroxide for 1 h and then subjected to scanning electron microscopy. MTF-1, MTF-1dn and MtnA designate fly lines with the co-overexpression of additional transgenes (dn=dominant negative form). (B) Synergistic effect of copper and hydrogen peroxide on rough eye phenotype. Flies were grown in food containing either 20 μM copper or 0.02% hydrogen peroxide (H2O2) or both. (C) Decreased copper luminescence in the intestine of larvae following transfer from food containing 250 μM copper to normal food. Shown is the confocal microscopic picture of the Drosophila larval midgut. (D) The phenotype caused by the expression of Ctr1B in the eye is rescued by co-expression of the Drosophila copper exporter DmATP7. Scanning electron micrographs of the eye of a Ctr1B overexpressing fly (left panel) is compared to the one of a Ctr1B and DmATP7 co-overexpresing fly (right panel).
To find out whether overexpression of MTF-1 and metallothioneins affected the rough eye phenotype, we coexpressed Ctr1B with transgenes encoding either metallothionein A or dMTF-1, using the eye-specific GMR promoter. Consistent with their role in copper detoxification, overexpression of metallothionein or dMTF-1 rescued the toxic effects of excessive copper import in the eye, whereas a dominant-negative version of dMTF-1 that maintains DNA binding but lacks activator function yielded a more severe phenotype (Figure 6A). Metallothioneins show their highest expression levels in the gut and are also expressed in the fat body upon copper load, but their expression is below detectable levels in imaginal discs and the brain. In line with this finding, imaginal discs do not accumulate metal under normal conditions (Ballan-Dufrancais, 2002), and may lack an efficient defense system. Therefore, copper distribution as well as the expression of proteins involved in copper homeostasis is tissue-specific. Some tissues, especially larval tissues that do not develop into the adult organism, tolerate high levels of copper and are apparently used to keep copper from the more sensitive tissues. Copper-accumulating tissues probably donate regulated amounts of the stored copper to copper-sensitive tissues during copper scarcity. Indeed, we observed that copper luminescence in the larval midgut that marks sites of copper accumulation disappears within about 10 h upon withdrawal of copper from the food, showing that copper is not irreversibly trapped in copper-binding proteins in intestinal cells (Figure 6C). It remains to be seen what fraction of the copper is transported to other parts of the body and what, if any, is excreted, but it seems safe to state that copper storage is a dynamic process.
Role of copper transporter DmATP7 (Drosophila Menkes/Wilson's disease homolog) in counteracting copper toxicity
Besides the regulation of copper import, copper sequestration by metallothioneins and copper avoidance, copper export also plays a role to defend against copper toxicity. The Drosophila genome encodes a single homolog of the two related mammalian copper exporters ATP7A and ATP7B, also referred to as Menkes and Wilson's disease proteins, respectively (Southon et al, 2004; Norgate et al, 2006). We overexpressed the Drosophila homolog DmATP7 in the Drosophila eye by the UAS-Gal4 system. Overexpression of DmATP7 does not cause an eye phenotype per se but alleviates the toxicity of excessive copper import mediated by Ctr1B (Figure 6D). Detoxification most likely occurs via removal of copper from affected cells, indicating that copper export is yet another defense mechanism against copper excess, similar to the excretion of copper in the liver by the related mammalian Wilson's copper transporter ATP7B. While these experiments were underway, a detailed study on the role of DmATP7 in Drosophila copper homeostasis came to the same conclusions (Norgate et al, 2006).
Copper can be transferred to the next generation
In spite of the mechanisms used by Drosophila to alleviate copper toxicity, in balance it seems best to maximally fill the copper stores, because even the following generation can profit from the supply. To test this hypothesis, Drosophila larvae were raised in copper-supplemented food and the resulting flies were transferred to copper-depleted food for egg deposition. Such offspring grew faster (not shown) and survived better than the offspring of control flies (Figure 7). Strikingly, this beneficial effect extended to a further generation: offspring of second-generation low-copper flies raised again in low copper fared much better if their grandparents had been grown up in copper-supplemented food (Figure 7, lanes G3).
Figure 7.
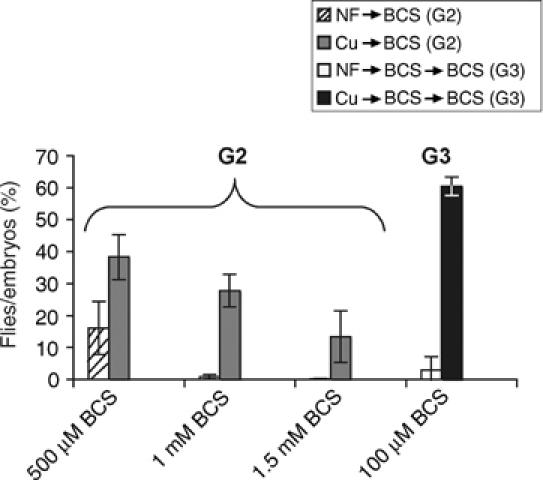
Copper supply to following generation. Drosophila larvae of generation 2 (G2) thrive on copper-starved food if their parents had been raised during larval stage in copper-supplemented food. Controls whose parents grew up in standard food are severely delayed (not shown), and many fail to develop to adulthood, as illustrated by the ratio of flies that eclosed from originally laid eggs on various concentrations of copper chelator (BCS). The beneficial effect of copper transfer to G2 even extends to a further generation (G3) raised again in low-copper food.
Drosophila larvae avoid potentially toxic levels of copper
The ability of Ctr1B to excessively import copper suggests the presence of other mechanisms to protect the organism from copper toxicity. We therefore tested the behavioral response of wild-type larvae to copper. Third instar larvae raised in normal food were placed in a Petri dish and given the choice between two types of semi-solid food (Figure 8A). One-half of the dish contained normal food, whereas food on the other half was supplemented with different copper concentrations or other transition metals. Irrespective of whether larvae were initially placed on the half containing normal food or copper-enriched food, they rapidly redistributed over the whole plate in an initial phase. However, after prolonged crawling, they obviously sensed and avoided the copper-containing half, such that only 10–20% localized to that half after 60 min. While a copper concentration of 0.25 mM copper elicits at most a mild avoidance, a clear avoidance was observed at 0.5 or 1 mM copper. Different anions, either CuCl2 or CuSO4, yielded very similar results (not shown). Drosophila larvae also avoided food with 1 mM silver, but were indifferent to 4 mM zinc, 0.5 mM cadmium or 5 mM iron (Fe3+) (Figure 8B and C). These controls demonstrate that the avoidance reaction is specific to copper, or in general to elements of group Ib (which includes silver) in the periodic table of elements and not due to a difference of osmolarity between the metal-containing half and the normal half. None of the metal concentrations tested affected viability during the 2 h interval of the food-choice test; 4 mM zinc, 0.5 mM copper or 5 mM iron was well tolerated by wild-type larvae even upon constant exposure to these metals, whereas 0.5 mM cadmium or silver was toxic to larvae upon prolonged exposure (not shown). We also tested whether the avoidance of copper and silver depends on the function of either the regulator MTF-1 or the copper importer Ctr1B. However, both MTF-1 and Ctr1B mutant larvae displayed the same copper avoidance as wild type, suggesting that another copper importer or some kind of a copper receptor on the cell surface triggers this behavioral response (data not shown).
Figure 8.
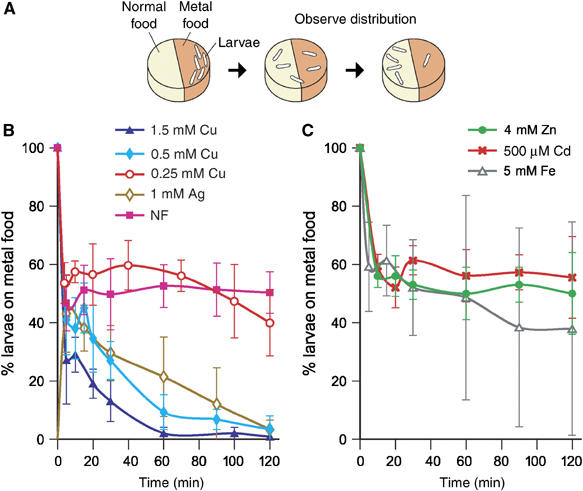
Behavioral avoidance of copper. (A) Groups of 30 larvae were placed on the metal-containing half food in a Petri dish, from where they crawled on the surface of solid food in search of a hole to enter the ‘food cake', thereby given the choice between metal containing and normal food. At the time points indicated, larvae were counted in each half. The colors of the two halves differ in this Figure for didactical purposes only. (B, C) Shown is the percentage of larvae crawling on the metal-containing food as a function of time.
Discussion
Our results imply that copper levels in the food or in the cell do not alter the localization of the Ctr1B copper importer protein in Drosophila. Instead, copper import by Ctr1B is mostly, if not exclusively, regulated at the transcriptional level by copper availability in an MTF-1-dependent manner. This form of regulation is in apparent contrast to yeast Ctr1, which is regulated post-translationally (Ooi et al, 1996). Concerning human Ctr1, one study has shown that it undergoes degradation in response to copper (Petris et al, 2003), whereas another reports that it stably resides on the plasma membrane (Eisses et al, 2005). Interestingly, some other Ctr family members, including hCtr1 and yCtr1, possess ‘Mets motifs' (MxxM or MxM) that are not present in Drosophila Ctr1B and yCtr3, or in paCtr3 of the filamentous fungus Podospora anserina (Ooi et al, 1996; Koch et al, 1997; Pena et al, 2000; Guo et al, 2004). Several studies have shown that these ‘Mets motifs' are pivotal for copper-dependent endocytosis (Guo et al, 2004; Jiang et al, 2005). Consistent with the idea that the ‘Mets motifs' are necessary for copper-stimulated endocytosis and degradation, yCtr3, in contrast to yCtr1, does not undergo copper-dependent endocytosis (Pena et al, 2000). Indeed yCtr3, Ctr1B and paCtr3 are all transcriptionally activated under conditions of copper scarcity by their respective transcription factors Mac1 (which also activates yCtr1), MTF-1 and Grisea (Graden and Winge, 1997; Labbe et al, 1997; Borghouts et al, 2002; Selvaraj et al, 2005). The two other Drosophila copper importers Ctr1A and Ctr1C, whose role in copper homeostasis remains to be elucidated, are not regulated at the transcriptional level but contain Mets-like motifs and may thus be regulated at the protein level by copper (Zhou et al, 2003). In the present study, we show that copper does not trigger endocytosis of Drosophila Ctr1B, and that Ctr1B continues to import copper irrespective of the ambient copper concentration. In agreement with such a differential mode of regulation, Ctr1B is the closest Drosophila homolog of yCtr3, whereas Ctr1A and Ctr1C are more closely related to yCtr1 (Ooi et al, 1996; Koch et al, 1997; Pena et al, 2000; Zhou et al, 2003).
Drosophila has a remarkable ability to accumulate copper. This accumulation is mediated by the major larval copper importer Ctr1B upon an increase of copper levels in the diet, as Ctr1B remains on the brush border membrane of gut cells. Copper accumulation strongly induces transcription of metallothioneins, with subsequent copper sequestration and storage primarily in intestinal cells. The stored copper can serve as a source in periods of copper scarcity, whether in the same or, surprisingly, even in the following generation(s) (Figure 7). Intestinal accumulation of copper also helps to protect other tissues such as brain and imaginal discs, whose development or function might be disrupted by copper load (Rodrigues et al, 1995; Folwell et al, 2006; Egli et al, 2006a). Indeed an increased copper import by ectopic expression of Ctr1B in the eye interferes with its development, resulting in a characteristic ‘rough eye' phenotype.
An important question is whether the seemingly sluggish regulation of Ctr1B that can result in a copper-stress response serves a purpose under natural conditions or whether a more precise regulation is simply not necessary. A sufficient uptake and the ability to store copper may be particularly important in geographical regions where copper is scarce. Copper scarcity results in a strong delay of larval development, a condition that may be of selective disadvantage in a rapidly changing environment. Fluctuations of copper content in the nutrients may be quite common, at least locally: Drosophila had been exposed to high copper levels over the course of the last century as copper-containing sprays are used in vineyards and orchards to combat bacterial and fungal infections (Maroni et al, 1987). A downregulation of Ctr1B upon a change from untreated to copper-treated fruits appears necessary but copper shock can also be controlled by the copper avoidance reaction. Therefore, Drosophila may get by with a sluggish downregulation under most circumstances.
Copper, like other essential trace elements or nutrients such as amino acids or lipids, is a limiting factor for development even when all other nutrients are present in abundance. To avoid such a situation, most organisms have the ability to store nutrients, for example, lipids in fat tissue, sugars as glycogen and iron in ferritins, iron-binding proteins (Harrison and Arosio, 1996). Similarly, metallothioneins can be considered as stores of essential transition metals, notably copper. Ctr1B would simply fill these stores as long as copper is abundant. Because of this potential benefit, we propose that Drosophila allows copper accumulation, which is accompanied by a copper-mediated physiological stress response. The accumulated copper, in spite of its tight binding to metallothioneins, could be made available in periods of copper scarcity by oxidation of metallothioneins in a manner akin to that described for copper-thioneins in mammals (Feldman and Cousins, 1976; Bremner et al, 1978; Liu et al, 2000). The released copper may be distributed to other parts of the body via DmATP7 analogous to mammalian ATP7A, which exports copper from intestinal cells through the basolateral membrane to the bloodstream (Petris et al, 2003; Southon et al, 2004; Norgate et al, 2006). DmATP7, like mammalian ATP7A, is required for proper pigment formation by the copper-containing enzyme tyrosinase (Petris et al, 2003; Norgate et al, 2006). The importance of copper accumulation and distribution is illustrated by the amazing ability of Drosophila to thrive on low-copper food if the parents had been raised during their larval development in copper-supplemented food. We propose that copper accumulation and transfer to the next generation ensures a sufficient copper supply and is of primary importance for the organism, whereas the threat of copper toxicity may be secondary. It remains to be determined which component(s) of the copper homeostasis system is responsible for this trans-generation effect.
The behavioral aspect of copper homeostasis is also intriguing. In Drosophila, copper avoidance, which was also observed in other organisms (Rabin et al, 1985; Sambongi et al, 1999; Lopes et al, 2004; Van Zwieten et al, 2004), occurs at relatively high levels of copper that would lead to a copper-mediated developmental delay of wild-type flies. The threshold of about 0.5 mM copper to trigger the avoidance probably reflects a tradeoff between accumulation to overcome periods of copper scarcity and the prospect of copper-mediated damage. Taken together, regulated copper import with specific chelation and storage, export, and also copper avoidance, establish an adequate homeostasis of this essential but potentially toxic trace metal.
Materials and methods
Fly stocks and genetics
The dMTF-1 (dMTF-1140−1R) and Ctr1B (Ctr1B3–4) mutant alleles, the Ctr1B reporter transgenes and the UAS-Ctr1B transgene used for ectopic expression of Ctr1B were described in previous studies (Egli et al, 2003; Zhou et al, 2003; Selvaraj et al, 2005). As all experiments were carried out in a y w background that is often used for Drosophila experiments, the term ‘wild type' is meant to indicate wild-type status of the relevant genes of copper homeostasis. We used Oregon R y w strain in all our experiments. The full-length as well as the dominant-negative MTF-1 lacking the transcriptional activation domains C-terminal to the zinc fingers was cloned into the widely used pUAST vector. The EP insertion into the DmATP locus was kindly provided by Marcel Zarske and Ernst Hafen. Overexpression of these transgene in the eye was performed by crossing to a GMR-Gal4 transgenic fly. Experimental procedures of copper shock and transfer experiments are indicated in the figure legends.
MtnA-EYFP reporter construct
The MtnA promoter (−446 to +74) was PCR amplified from genomic DNA using the primer pair 5′-CGG GAT CCA GGT ATG GGC TAT TTA GGC C-3′ and 5′-GGG ATG GCC CCA AAG GAT CTG-3′. The PCR product was cloned in pCasper4 vector containing the coding region of EYFP (Thummel and Pirrotta, 1992).
Quantification of metallothionein and Ctr1B transcripts, and metal measurements
Larvae were either continuously raised on the indicated type of food or transferred for 6 h to normal food or metal-supplemented food (for simplicity, the terms or symbols copper, cadmium, silver, zinc and iron are meant to refer to Cu2+, Cd2+, Ag+, Zn2+ and Fe3+, respectively). Only third instar feeding larvae were used for analysis. Total RNA was extracted using the TRIzol reagent (Life Technologies). S1 nuclease mapping of transcripts with 50 μg of total RNA was performed as described previously (Weaver and Weissmann, 1979). The gels were developed using PhosphorImager (Molecular Dynamics) and bands were quantified. The signal from the endogenous actin5c gene was used for normalization of metallothionein transcript levels. Metal measurements were made as described elsewhere (Egli et al, 2006b).
Drosophila protein extracts and Western blot analysis
Transgenic flies harboring the full-length Ctr1B ORF fused to the EGFP-coding sequence (designated as AH3; Selvaraj et al, 2005) were allowed to grow on NF or food supplemented with 250 μM BCS or 250 μM copper. About 30–50 third instar larvae were taken for homogenization in a buffer containing 20 mM Hepes pH 7.5, 100 mM KCl, 5% glycerol, 100 mM EDTA, 0.1% Triton X-100, aprotinin (5 μg/ml), leupeptin (5 μg/ml), 1 mM phenylmethyl sulfonyl fluoride (PMSF) and 2.5 mM dithiothreitol (DTT). The mixture was spun, the supernatant was collected, 50 μg of the extracts was resolved by 11% SDS–PAGE and Western blotting was performed according to standard procedures. The mouse anti-GFP was used at 1:2500 dilution (BD Biosciences, USA). For the GFP control, Drosophila S2 cells were transfected with pAc-GFP expression vector and whole-cell extracts were prepared 72 h after transfection. Blots were visualized using the ECL kit (Amersham Biosciences, Sweden).
Imaging and microscopy
Images were taken with a Leica MZ FLIII fluorescence stereomicroscope and a Nikon COOLPIX950 digital camera for whole larvae. Eyes were photographed using a Leica MZ16 stereomicroscope equipped with a Leica DFC280 camera and scanning electron microscopy images were taken with a JEOL JSM-6360LV scanning electron microscope. Copper cell luminescence and fluorescent protein expression in dissected larval guts were analyzed with a Leica DMRB fluorescence stereomicroscope equipped with the filters A for DAPI and copper cell luminescence and I3 for EGFP and EYFP, respectively. Pictures were taken with a Zeiss Axiocam. Confocal images were taken with a Leica SP1 UV CLSM.
Food choice and copper donation experiments
About 25–40 third instar larvae were placed on 5 cm Petri dishes. These contained two halves consisting of normal food or metal-supplemented food (CuSO4, CuCl2, AgNO3, ZnCl2, FeCl3, CdSO4). One liter of normal food consisted of 15 g agar, 16.6 g sugar (D-glucose), 10 g wheat, 25 g yeast and 330 ml fruit juice (90% apple juice and 10% pear juice). The agar concentration was chosen relatively high to prevent the rapid disappearance of larvae in the substrate.
For copper transfer studies, in each plastic vial, 20–30 wild-type adult flies were placed on either normal food or food containing 1 mM CuSO4 and allowed to lay eggs. The resulting progeny that grew up in normal food or copper-supplemented food was designated as G1NF and G1Cu, respectively. A total of 20–30 adult flies of each G1NF and G1Cu were placed on food containing copper chelator BCS for egg deposition, and were removed from the vials after 12 h. The number of eggs in each vial was determined and 10 days later the eclosed flies in that vial were counted. Similarly, G2 flies raised in BCS were transferred to a new vial with food, allowed to lay eggs and removed after 12 h. Again, the ratio of flies (G3) eclosing per originally deposited eggs was determined.
Acknowledgments
We are grateful to Bruno Schmid and Antonia Manova for technical assistance, to Fritz Ochsenbein for the preparation of figures, to Urs Ziegler (Institute of Anatomy, University of Zurich) for help with the scanning electron microscopy and the confocal microscope, and to Mike Fetchko and Valpuri Sovero for critical reading of the manuscript. This work was supported by grant 3100-064139 from the Swiss National Science Foundation, by the Kanton Zürich, and by grant LSHG-CT-2003-503303 from the project ‘Mechanisms of Gene Integration' (GENINTEG) of the European Union.
References
- Balamurugan K, Egli D, Selvaraj A, Zhang B, Georgiev O, Schaffner W (2004) Metal-responsive transcription factor (MTF-1) and heavy metal stress response in Drosophila and mammalian cells: a functional comparison. Biol Chem 385: 597–603 [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Schaffner W (2006) Copper homeostasis in eukaryotes: teetering on a tightrope. Biochim Biophys Acta 1763: 737–746 [DOI] [PubMed] [Google Scholar]
- Ballan-Dufrancais C (2002) Localization of metals in cells of pterygote insects. Micros Res Tech 56: 403–420 [DOI] [PubMed] [Google Scholar]
- Borghouts C, Scheckhuber CQ, Stephan O, Osiewacz HD (2002) Copper homeostasis and aging in the fungal model system Podospora anserina: differential expression of PaCtr3 encoding a copper transporter. Int J Biochem Cell Biol 34: 1355–1371 [DOI] [PubMed] [Google Scholar]
- Bremner I, Hoekstra G, Davies NT, Young BW (1978) Effect of zinc status of rats on the synthesis and degradation of copper-induced metallothioneins. Biochem J 174: 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW (1993) The Wilson disease gene is a putative copper transporting P-type ATPase similarto the Menkes gene. Nat Genet 5: 327–337 [DOI] [PubMed] [Google Scholar]
- Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD (1994) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 6: 393–402 [DOI] [PubMed] [Google Scholar]
- Egli D, Selvaraj A, Yepiskoposyan H, Zhang B, Hafen E, Georgiev O, Schaffner W (2003) Knockout of ‘metal-responsive transcription factor' MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J 22: 100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D, Yepiskoposyan H, Selvaraj A, Balamurugan K, Rajaram R, Simons A, Mettler S, Vardanyan A, Multhaup G, Georgiev O, Schaffner W (2006a) A family-knockout of metallothioneins reveals its central role in copper homeostasis/detoxification. Mol Cell Biol 26: 2286–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D, Domenech J, Selvaraj A, Balamurugan K, Hua H, Capdevila M, Georgiev O, Schaffner W, Atrian S (2006b) The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification. Genes Cells 11: 647–658 [DOI] [PubMed] [Google Scholar]
- Eisses JF, Chi Y, Kaplan JH (2005) Stable plasma membrane levels of hCTR1 mediate cellular copper uptake. J Biol Chem 280: 9635–9639 [DOI] [PubMed] [Google Scholar]
- Feldman SL, Cousins RJ (1976) Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J 160: 583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filshie BK, Poulson DF, Waterhouse DF (1971) Ultrastructure of the copper-accumulating region of the Drosophila larval midgut. Tissue & Cell 3: 77–102 [DOI] [PubMed] [Google Scholar]
- Folwell JL, Barton CH, Shepherd D (2006) Immunolocalisation of the D. melanogaster Nramp homologue Malvolio to gut and Malpighian tubules provides evidence that Malvolio and Nramp2 are orthologous. J Exp Biol 209: 1988–1995 [DOI] [PubMed] [Google Scholar]
- Graden JA, Winge DR (1997) Copper-mediated repression of the activation domain in the yeast Mac1p transcription factor. Proc Natl Acad Sci USA 94: 5550–5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ (2004) Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem 279: 17428–17433 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186: 1–85 [DOI] [PubMed] [Google Scholar]
- Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275: 161–203 [DOI] [PubMed] [Google Scholar]
- Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W (1994) The transcription factor Mtf-1 is essential for basal and heavy metal-induced metallothionein gene-expression. EMBO J 13: 2870–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Nadas IA, Kim MA, Franz KJ (2005) A Mets motif peptide found in copper transport proteins selectively binds Cu(I) with methionine-only coordination. Inorg Chem 44: 9787–9794 [DOI] [PubMed] [Google Scholar]
- Koch KA, Pena MM, Thiele DJ (1997) Copper-binding motifs in catalysis, transport, detoxification and signaling. Chem Biol 4: 549–560 [DOI] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98: 6836–6841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe S, Zhu ZW, Thiele DJ (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem 272: 15951–15958 [DOI] [PubMed] [Google Scholar]
- Lauverjat S, Ballan-Dufrancais C, Wegnez M (1989) Detoxification of cadmium. Ultrastructural study and electron-probe microanalysis of the midgut in a cadmium-resistant strain of Drosophila melanogaster. Biol Met 2: 97–107 [DOI] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ (2000) Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene 254: 87–96 [DOI] [PubMed] [Google Scholar]
- Lee LW, Prohaska JR, Thiele DJ (2001) Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA 98: 6842–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SX, Fabisiak JP, Tyurin VA, Borisenko GG, Pitt BR, Lazo JS, Kagan VE (2000) Reconstitution of apo-superoxide dismutase by nitric oxide-induced copper transfer from metallothioneins. Chem Res Toxicol 13: 922–931 [DOI] [PubMed] [Google Scholar]
- Lopes I, Baird DJ, Ribeiro R (2004) Avoidance of copper contamination by field populations of Daphnia longispina. Environ Toxicol Chem 23: 1702–1708 [DOI] [PubMed] [Google Scholar]
- Marchal-Segault D, Briancon C, Halpern S, Fragu P, Lauge G (1990) Secondary ion mass spectrometry analysis of the copper distribution in Drosophila melanogaster chronically intoxicated with Bordeaux mixture. Biol Cell 70: 129–132 [DOI] [PubMed] [Google Scholar]
- Maroni G, Wise J, Young JE, Otto E (1987) Metallothionein gene duplications and metal tolerance in natural populations of Drosophila melanogaster. Genetics 117: 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JF, Llanos RM (2003) Molecular and cellular aspects of copper transport in developing mammals. J Nutr 133: 1481S–1484S [DOI] [PubMed] [Google Scholar]
- Norgate M, Lee E, Southon A, Farlow A, Batterham P, Camakaris J, Burke R (2006) Essential roles in development and pigmentation for the Drosophila copper transporter DmATP7. Mol Biol Cell 17: 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran TV, Culotta VC (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275: 25057–25060 [DOI] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD (1996) Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J 15: 3515–3523 [PMC free article] [PubMed] [Google Scholar]
- Pena MM, Puig S, Thiele DJ (2000) Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J Biol Chem 275: 33244–33251 [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JFB, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J (1996) Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J 15: 6084–6095 [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, Thiele DJ (2003) Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278: 9639–9646 [DOI] [PubMed] [Google Scholar]
- Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opi Chem Biol 6: 171–180 [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Lee J (1985) Intragastric copper sulfate produces a more reliable conditioned taste aversion in vagotomized rats than in intact rats. Behav Neural Biol 44: 364–373 [DOI] [PubMed] [Google Scholar]
- Rodrigues V, Cheah PY, Ray K, Chia W (1995) Malvolio, the Drosophila homologue of mouse NRAMP-1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behaviour. EMBO J 14: 3007–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M (1999) Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport 10: 753–757 [DOI] [PubMed] [Google Scholar]
- Selvaraj A, Balamurugan K, Yepiskoposyan H, Zhou H, Egli D, Georgiev O, Thiele DJ, Schaffner W (2005) Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes & Dev 19: 891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southon A, Burke R, Norgate M, Batterham P, Camakaris J (2004) Copper homoeostasis in Drosophila melanogaster S2 cells. Biochem J 383: 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp RL (1975) X-ray microanalysis of the mid-gut epithelium of the fruitfly Drosophila melanogaster. J Cell Sci 17: 449–459 [DOI] [PubMed] [Google Scholar]
- Thummel CS, Pirrotta V (1992) New pCaSpeR p element vectors. Drosophila Infor Serv 71: 150 [Google Scholar]
- Van Zwieten L, Rust J, Kingston T, Merrington G, Morris S (2004) Influence of copper fungicide residues on occurrence of earthworms in avocado orchard soils. Sci Total Environ 329: 29–41 [DOI] [PubMed] [Google Scholar]
- Weaver RF, Weissmann C (1979) Mapping of RNA by a modification of the berk-sharp procedure—5′ termini of 15-S beta-globin messenger-RNA precursor and mature 10-S beta-globin messenger-RNA have identical map coordinates. Nucleic Acids Res 7: 1175–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Heiny ME, Gitlin JD (1993) Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun 197: 271–277 [DOI] [PubMed] [Google Scholar]
- Zhang B, Egli D, Georgiev O, Schaffner W (2001) The Drosophila homolog of mammalian zinc finger factor MTF-1 activates transcription in response to heavy metals. Mol Cell Biol 21: 4505–4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Cadigan KM, Thiele DJ (2003) A copper-regulated transporter required for copper acquisition, pigmentation, and specific stages of development in Drosophila melanogaster. J Biol Chem 278: 48210–48218 [DOI] [PubMed] [Google Scholar]


