Abstract
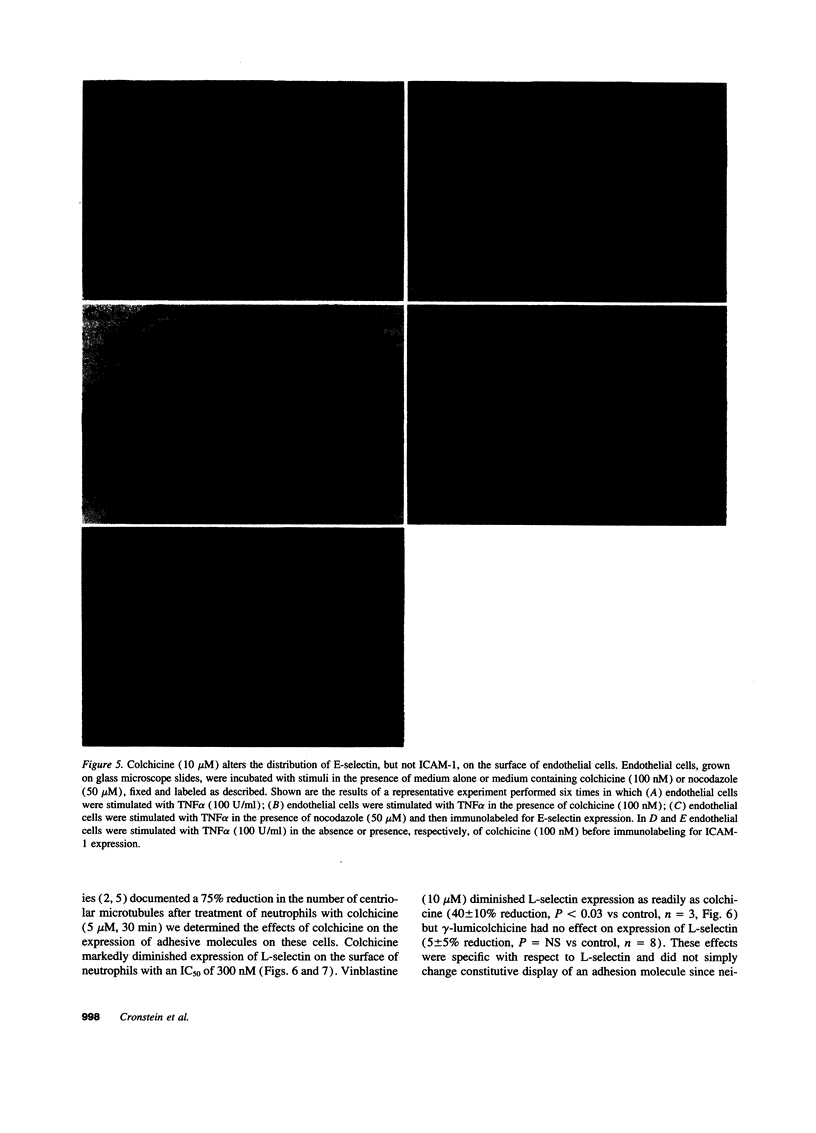
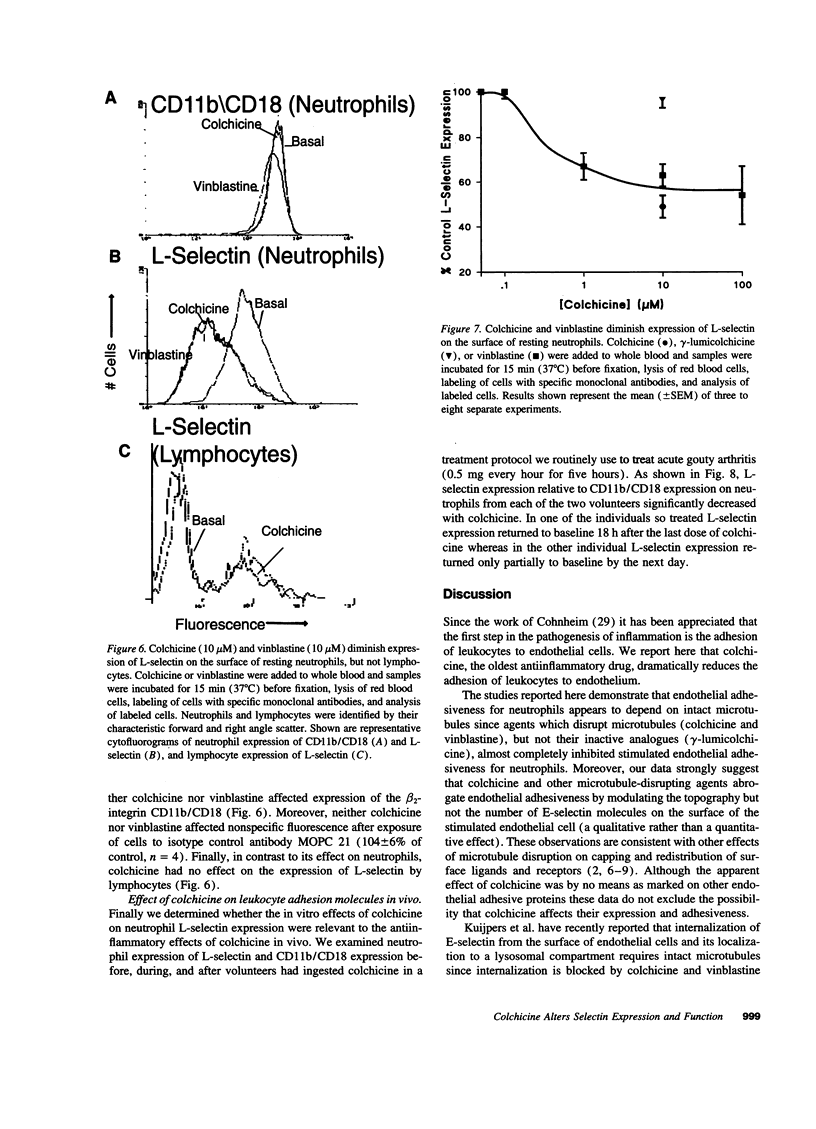
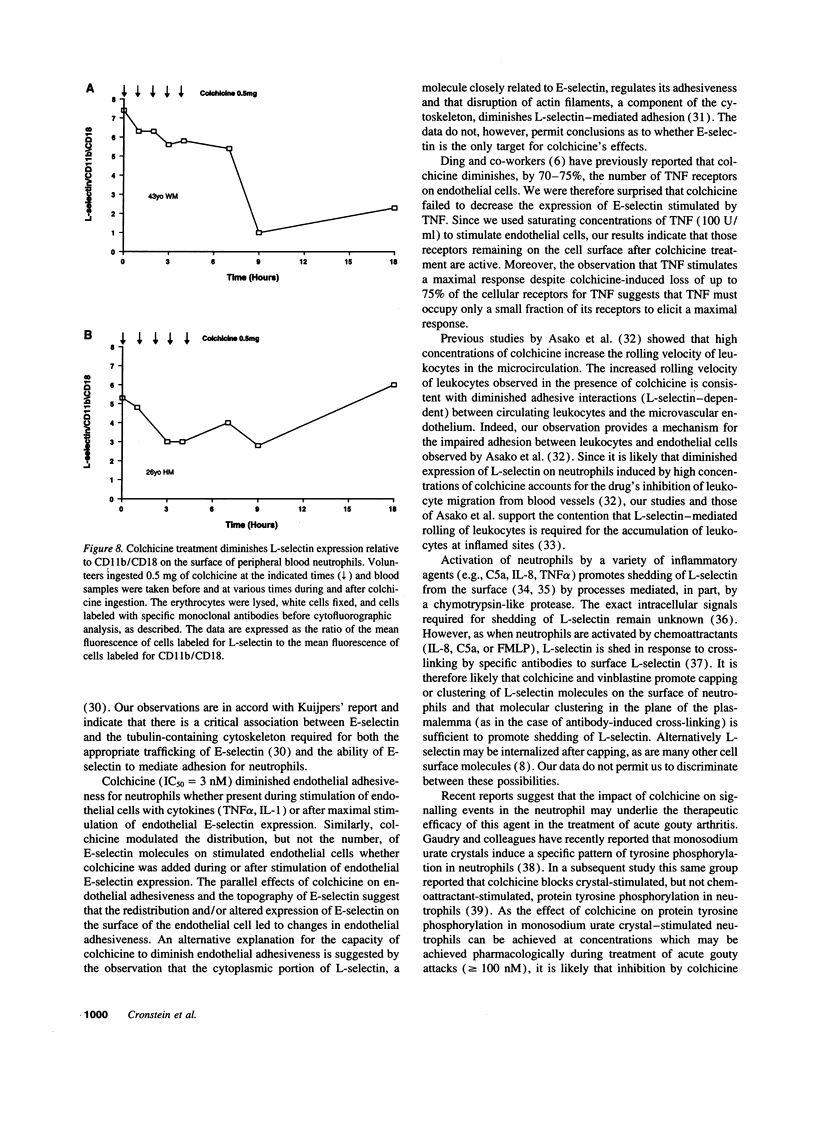
Since colchicine-sensitive microtubules regulate the expression and topography of surface glycoproteins on a variety of cells, we sought evidence that colchicine interferes with neutrophil-endothelial interactions by altering the number and/or distribution of selectins on endothelial cells and neutrophils. Extremely low, prophylactic, concentrations of colchicine (IC50 = 3 nM) eliminated the E-selectin-mediated increment in endothelial adhesiveness for neutrophils in response to IL-1 (P < 0.001) or TNF alpha (P < 0.001) by changing the distribution, but not the number, of E-selectin molecules on the surface of the endothelial cells. Colchicine inhibited stimulated endothelial adhesiveness via its effects on microtubules since vinblastine, an agent which perturbs microtubule function by other mechanisms, diminished adhesiveness whereas the photoinactivated colchicine derivative gamma-lumicolchicine was inactive. Colchicine had no effect on cell viability. At higher, therapeutic, concentrations colchicine (IC50 = 300 nM, P < 0.001) also diminished the expression of L-selectin on the surface of neutrophils (but not lymphocytes) without affecting expression of the beta 2-integrin CD11b/CD18. In confirmation, L-selectin expression was strikingly reduced (relative to CD11b/CD18 expression) on neutrophils from two individuals who had ingested therapeutic doses of colchicine. These results suggest that colchicine may exert its prophylactic effects on cytokine-provoked inflammation by diminishing the qualitative expression of E-selectin on endothelium, and its therapeutic effects by diminishing the quantitative expression of L-selectin on neutrophils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asako H., Kubes P., Baethge B. A., Wolf R. E., Granger D. N. Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation. 1992 Feb;16(1):45–56. doi: 10.1007/BF00917514. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Eberle M. A., Gruber H. E., Levin R. I. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2441–2445. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993 Feb;36(2):147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Porteu F., Sanchez E., Nathan C. F. Downregulation of tumor necrosis factor receptors on macrophages and endothelial cells by microtubule depolymerizing agents. J Exp Med. 1990 Mar 1;171(3):715–727. doi: 10.1084/jem.171.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry M., Roberge C. J., de Médicis R., Lussier A., Poubelle P. E., Naccache P. H. Crystal-induced neutrophil activation. III. Inflammatory microcrystals induce a distinct pattern of tyrosine phosphorylation in human neutrophils. J Clin Invest. 1993 Apr;91(4):1649–1655. doi: 10.1172/JCI116373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G. A., Harvey E. A., Ferreira S. H., Vane J. R. The effects of antiinflammatory drugs on the production of prostaglandins in vivo. Adv Prostaglandin Thromboxane Res. 1976;1:105–110. [PubMed] [Google Scholar]
- Hoffstein S., Goldstein I. M., Weissmann G. Role of microtubule assembly in lysosomal enzyme secretion from human polymorphonuclear leukocytes. A reevaluation. J Cell Biol. 1977 Apr;73(1):242–256. doi: 10.1083/jcb.73.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell Immunol. 1991 Jan;132(1):201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Kammer G. M., Smith J. A., Mitchell R. Capping of human T cell specific determinants: kinetics of capping and receptor re-expression and regulation by the cytoskeleton. J Immunol. 1983 Jan;130(1):38–44. [PubMed] [Google Scholar]
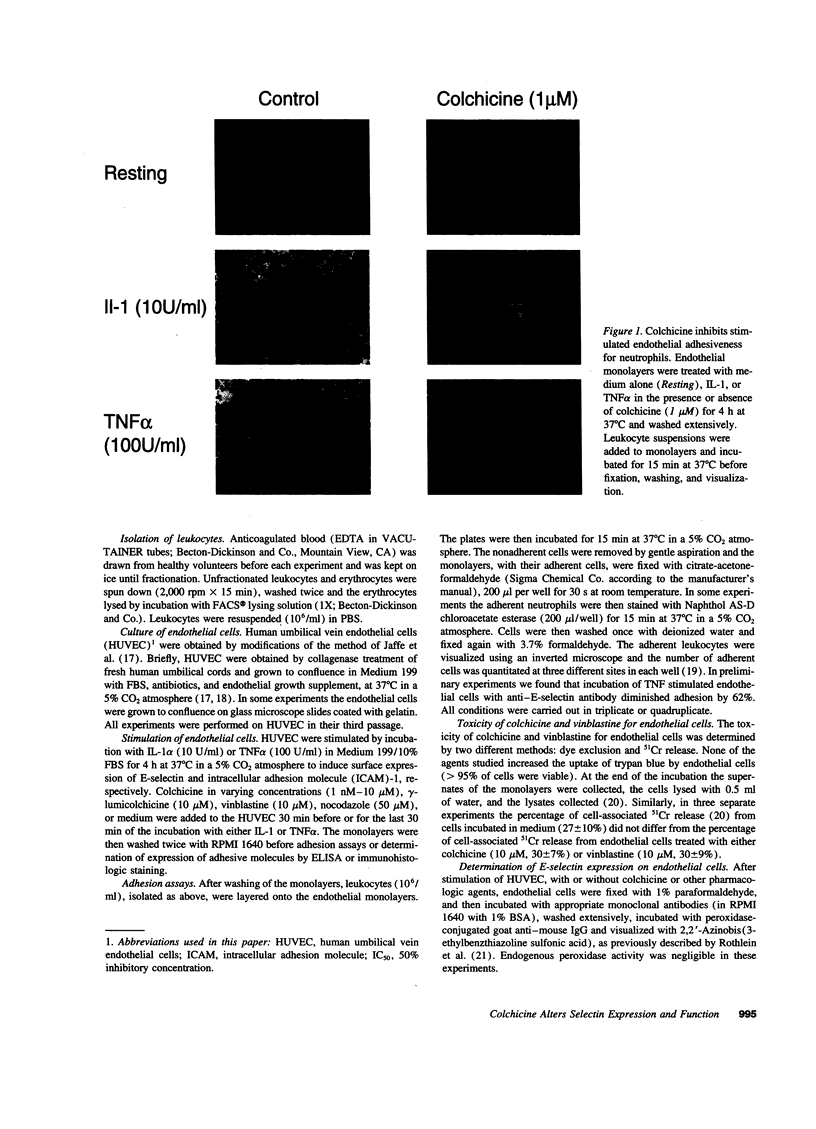
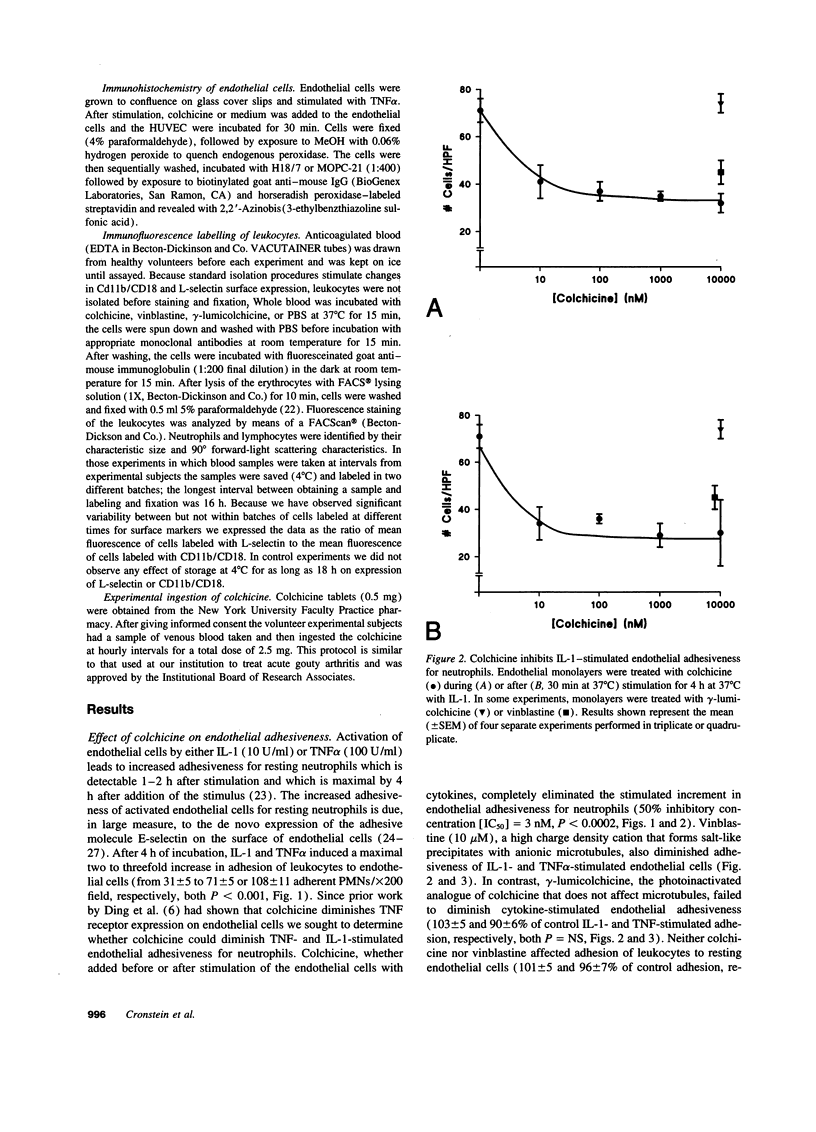
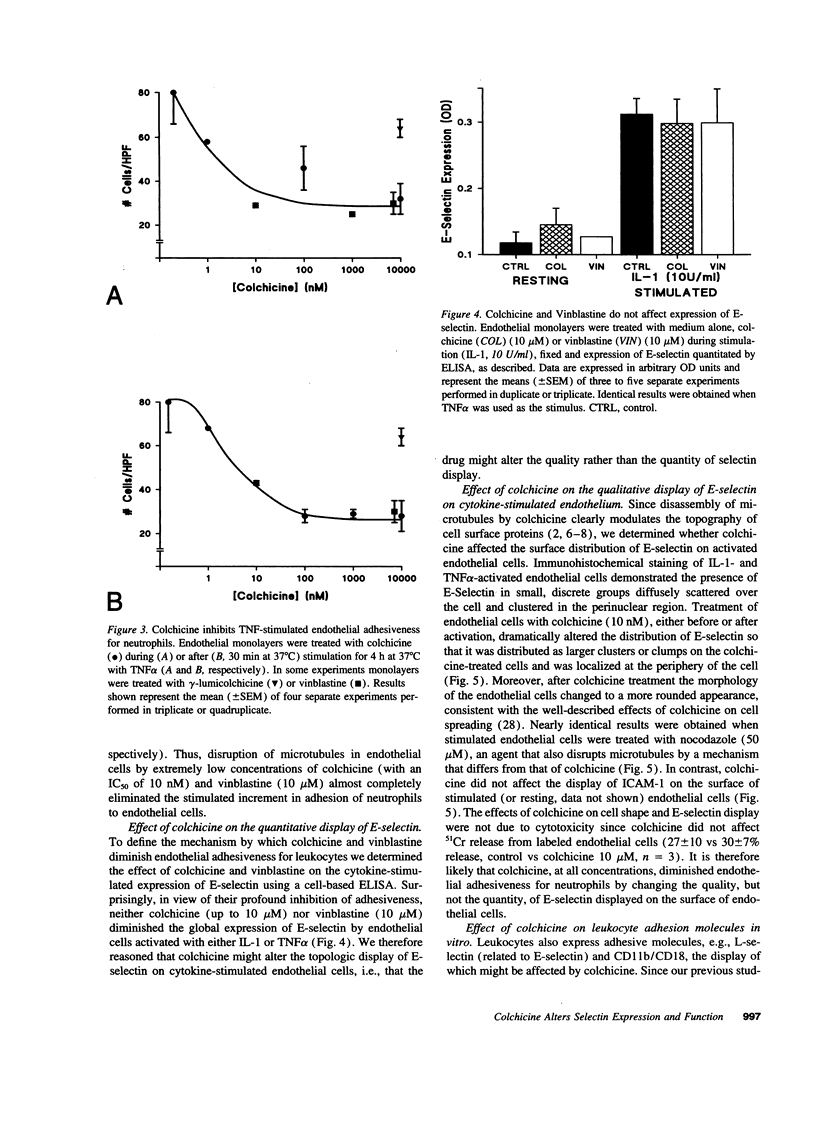
- Kansas G. S., Ley K., Munro J. M., Tedder T. F. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J Exp Med. 1993 Mar 1;177(3):833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T. W., Raleigh M., Kavanagh T., Janssen H., Calafat J., Roos D., Harlan J. M. Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process. J Immunol. 1994 May 15;152(10):5060–5069. [PubMed] [Google Scholar]
- Leiber D., Jasper J. R., Alousi A. A., Martin J., Bernstein D., Insel P. A. Alteration in Gs-mediated signal transduction in S49 lymphoma cells treated with inhibitors of microtubules. J Biol Chem. 1993 Feb 25;268(6):3833–3837. [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Palecanda A., Walcheck B., Bishop D. K., Jutila M. A. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992 May;22(5):1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Reibman J., Haines K. A., Gude D., Weissmann G. Differences in signal transduction between Fc gamma receptors (Fc gamma RII, Fc gamma RIII) and FMLP receptors in neutrophils. Effects of colchicine on pertussis toxin sensitivity and diacylglycerol formation. J Immunol. 1991 Feb 1;146(3):988–996. [PubMed] [Google Scholar]
- Reibman J., Haines K. A., Rich A. M., Cristello P., Giedd K. N., Weissmann G. Colchicine inhibits ionophore-induced formation of leukotriene B4 by human neutrophils: the role of microtubules. J Immunol. 1986 Feb 1;136(3):1027–1032. [PubMed] [Google Scholar]
- Rich A. M., Hoffstein S. T. Inverse correlation between neutrophil microtubule numbers and enhanced random migration. J Cell Sci. 1981 Apr;48:181–191. doi: 10.1242/jcs.48.1.181. [DOI] [PubMed] [Google Scholar]
- Roberge C. J., Gaudry M., de Médicis R., Lussier A., Poubelle P. E., Naccache P. H. Crystal-induced neutrophil activation. IV. Specific inhibition of tyrosine phosphorylation by colchicine. J Clin Invest. 1993 Oct;92(4):1722–1729. doi: 10.1172/JCI116759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Rudolph S. A., Greengard P., Malawista S. E. Effects of colchicine on cyclic AMP levels in human leukocytes. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3404–3408. doi: 10.1073/pnas.74.8.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Whittaker J., Hammond V. A., Alberti K. G. Effects of colchicine on insulin binding to isolated rat hepatocytes. Biochem Biophys Res Commun. 1981 Dec 15;103(3):1100–1106. doi: 10.1016/0006-291x(81)90921-9. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Modulation of lymphocyte receptor mobility by concanavalin A and colchicine. Ann N Y Acad Sci. 1975 Jun 30;253:455–469. doi: 10.1111/j.1749-6632.1975.tb19221.x. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giovine F. S., Malawista S. E., Thornton E., Duff G. W. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J Clin Invest. 1991 Apr;87(4):1375–1381. doi: 10.1172/JCI115142. [DOI] [PMC free article] [PubMed] [Google Scholar]