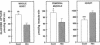Abstract
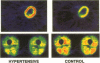
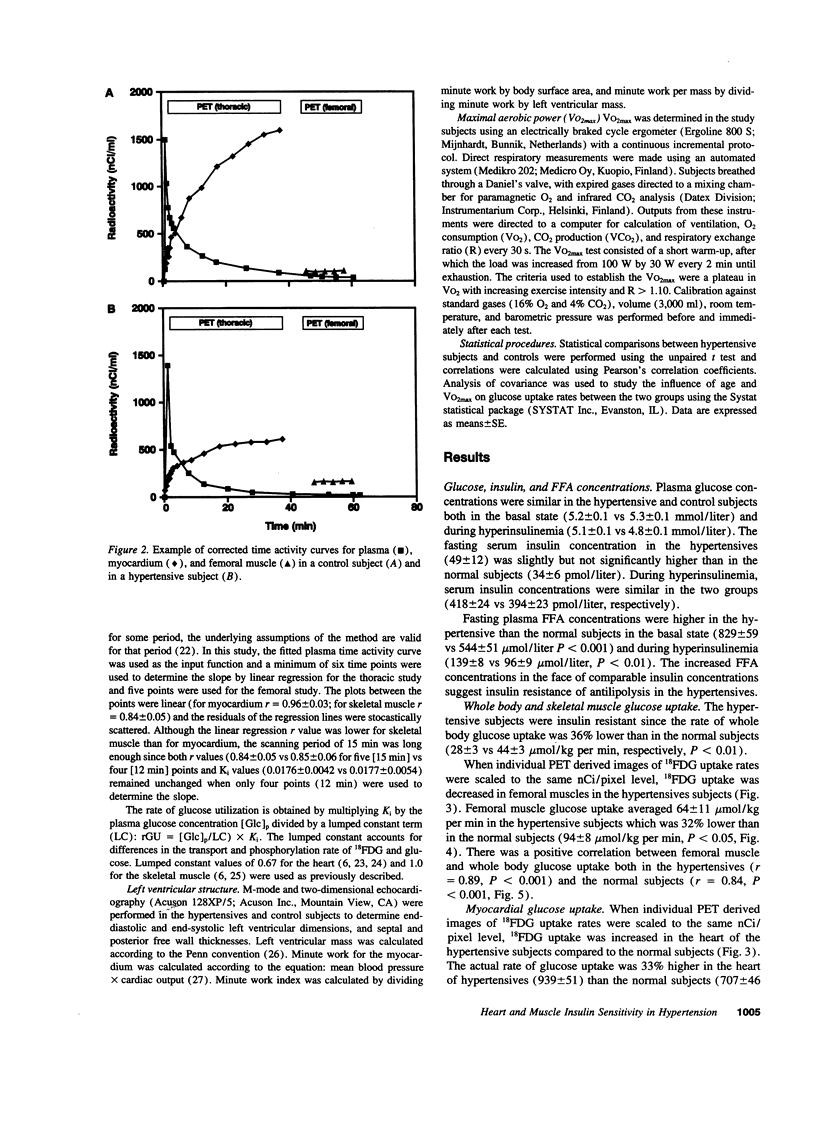
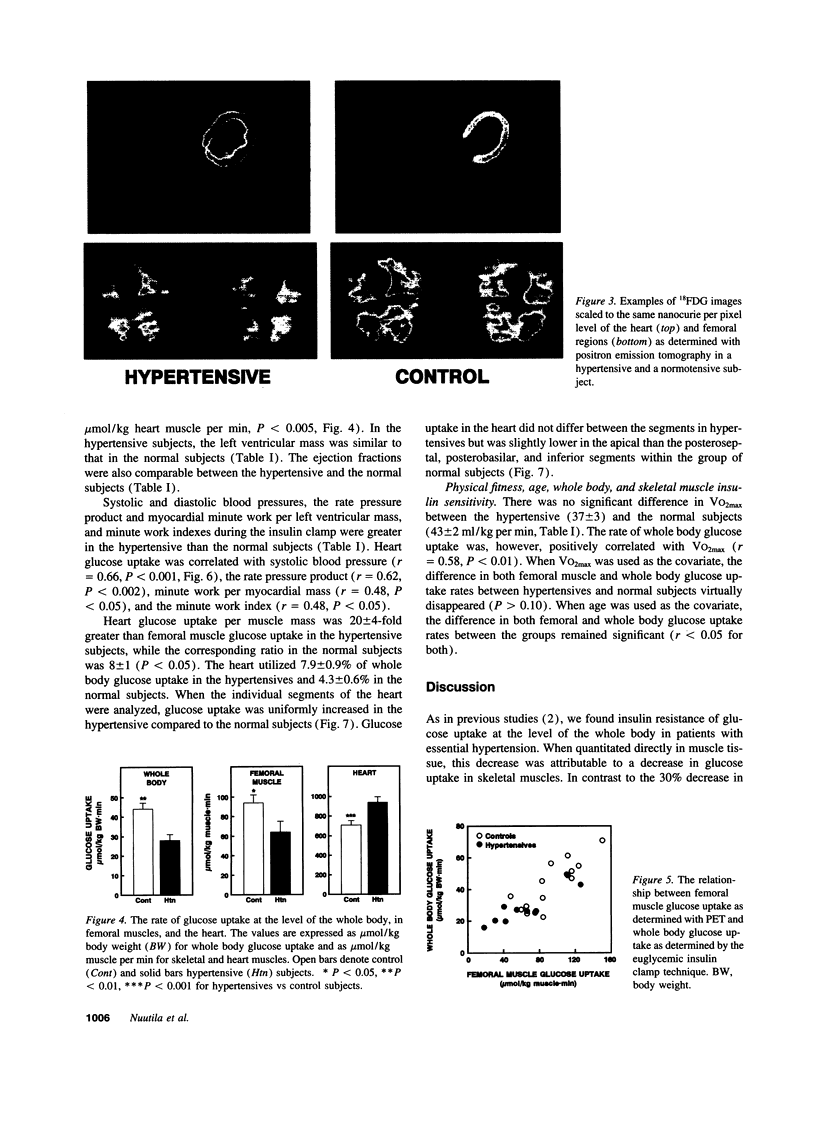
Essential hypertension is characterized by skeletal muscle insulin resistance but it is unknown whether insulin resistance also affects heart glucose uptake. We quantitated whole body (euglycemic insulin clamp) and heart and skeletal muscle (positron emission tomography and 18F-fluoro-2-deoxy-D-glucose) glucose uptake rates in 10 mild essential hypertensive (age 33 +/- 1 yr, body mass index 23.7 +/- 0.8 kg/m2, blood pressure 146 +/- 3/97 +/- 3 mmHg, VO2max 37 +/- 3 ml/kg per min) and 14 normal subjects (29 +/- 2 yr, 22.5 +/- 0.5 kg/m2, 118 +/- 4/69 +/- 3 mmHg, 43 +/- 2 ml/kg per min). Left ventricular mass was similar in the hypertensive (155 +/- 15 g) and the normotensive (164 +/- 13 g) subjects. In the hypertensives, both whole body (28 +/- 3 vs 44 +/- 3 mumol/kg per min, P < 0.01) and femoral (64 +/- 11 vs 94 +/- 8 mumol/kg muscle per min, P < 0.05) glucose uptake rates were decreased compared to the controls. In contrast, heart glucose uptake was 33% increased in the hypertensives (939 +/- 51 vs 707 +/- 46 mumol/kg muscle per min, P < 0.005), and correlated with systolic blood pressure (r = 0.66, P < 0.001) and the minute work index (r = 0.48, P < 0.05). We conclude that insulin-stimulated glucose uptake is decreased in skeletal muscle but increased in proportion to cardiac work in essential hypertension. The increase in heart glucose uptake in mild essential hypertensives with a normal left ventricular mass may reflect increased oxygen consumption and represent an early signal which precedes the development of left ventricular hypertrophy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P., Li P., Malhotra A., Zhang X., Herman M. V., Capasso J. M. Effects of hypertension and coronary constriction on cardiac function, morphology, and contractile proteins in rats. Am J Physiol. 1993 Aug;265(2 Pt 2):H713–H724. doi: 10.1152/ajpheart.1993.265.2.H713. [DOI] [PubMed] [Google Scholar]
- Camici P., Marraccini P., Marzilli M., Lorenzoni R., Buzzigoli G., Puntoni R., Boni C., Bellina C. R., Klassen G. A., L'Abbate A. Coronary hemodynamics and myocardial metabolism during and after pacing stress in normal humans. Am J Physiol. 1989 Sep;257(3 Pt 1):E309–E317. doi: 10.1152/ajpendo.1989.257.3.E309. [DOI] [PubMed] [Google Scholar]
- Choi Y., Hawkins R. A., Huang S. C., Gambhir S. S., Brunken R. C., Phelps M. E., Schelbert H. R. Parametric images of myocardial metabolic rate of glucose generated from dynamic cardiac PET and 2-[18F]fluoro-2-deoxy-d-glucose studies. J Nucl Med. 1991 Apr;32(4):733–738. [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Devereux R. B., Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977 Apr;55(4):613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Buzzigoli G., Bonadonna R., Giorico M. A., Oleggini M., Graziadei L., Pedrinelli R., Brandi L., Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987 Aug 6;317(6):350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- Frohlich E. D. Left ventricular hypertrophy, cardiac diseases and hypertension: recent experiences. J Am Coll Cardiol. 1989 Dec;14(7):1587–1594. doi: 10.1016/0735-1097(89)90001-6. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saubert C. W., 4th, Piehl K., Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972 Sep;33(3):312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Grover-McKay M., Schwaiger M., Krivokapich J., Perloff J. K., Phelps M. E., Schelbert H. R. Regional myocardial blood flow and metabolism at rest in mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1989 Feb;13(2):317–324. doi: 10.1016/0735-1097(89)90505-6. [DOI] [PubMed] [Google Scholar]
- Hamacher K., Coenen H. H., Stöcklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986 Feb;27(2):235–238. [PubMed] [Google Scholar]
- Huang S. C., Phelps M. E., Hoffman E. J., Sideris K., Selin C. J., Kuhl D. E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980 Jan;238(1):E69–E82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhlin-Dannfelt A., Frisk-Holmberg M., Karlsson J., Tesch P. Central and peripheral circulation in relation to muscle-fibre composition in normo- and hyper-tensive man. Clin Sci (Lond) 1979 Apr;56(4):335–340. doi: 10.1042/cs0560335. [DOI] [PubMed] [Google Scholar]
- Kagaya Y., Kanno Y., Takeyama D., Ishide N., Maruyama Y., Takahashi T., Ido T., Takishima T. Effects of long-term pressure overload on regional myocardial glucose and free fatty acid uptake in rats. A quantitative autoradiographic study. Circulation. 1990 Apr;81(4):1353–1361. doi: 10.1161/01.cir.81.4.1353. [DOI] [PubMed] [Google Scholar]
- Katz A., Nyomba B. L., Bogardus C. No accumulation of glucose in human skeletal muscle during euglycemic hyperinsulinemia. Am J Physiol. 1988 Dec;255(6 Pt 1):E942–E945. doi: 10.1152/ajpendo.1988.255.6.E942. [DOI] [PubMed] [Google Scholar]
- Kissling G., Rupp H., Malloy L., Jacob R. Alterations in cardiac oxygen consumption under chronic pressure overload. Significance of the isoenzyme pattern of myosin. Basic Res Cardiol. 1982 May-Jun;77(3):255–269. doi: 10.1007/BF01908041. [DOI] [PubMed] [Google Scholar]
- Knuuti M. J., Nuutila P., Ruotsalainen U., Saraste M., Härkönen R., Ahonen A., Teräs M., Haaparanta M., Wegelius U., Haapanen A. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med. 1992 Jul;33(7):1255–1262. [PubMed] [Google Scholar]
- Koivisto V. A., Yki-Järvinen H., DeFronzo R. A. Physical training and insulin sensitivity. Diabetes Metab Rev. 1986;1(4):445–481. doi: 10.1002/dmr.5610010407. [DOI] [PubMed] [Google Scholar]
- Krivokapich J., Huang S. C., Selin C. E., Phelps M. E. Fluorodeoxyglucose rate constants, lumped constant, and glucose metabolic rate in rabbit heart. Am J Physiol. 1987 Apr;252(4 Pt 2):H777–H787. doi: 10.1152/ajpheart.1987.252.4.H777. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- Lassers B. W., Kaijser L., Carlson L. A. Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3 H-palmitate. Eur J Clin Invest. 1972 Aug;2(5):348–358. doi: 10.1111/j.1365-2362.1972.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988 Aug;4(5):517–540. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Young A. A., Culter C. L., Ivy J. L., Abbott W. G., Zawadzki J. K., Yki-Järvinen H., Christin L., Secomb T. W., Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987 Aug;80(2):415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- Manchester J., Kong X., Nerbonne J., Lowry O. H., Lawrence J. C., Jr Glucose transport and phosphorylation in single cardiac myocytes: rate-limiting steps in glucose metabolism. Am J Physiol. 1994 Mar;266(3 Pt 1):E326–E333. doi: 10.1152/ajpendo.1994.266.3.E326. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Modan M., Halkin H., Almog S., Lusky A., Eshkol A., Shefi M., Shitrit A., Fuchs Z. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985 Mar;75(3):809–817. doi: 10.1172/JCI111776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossberg K. A., Rowe R. W., Tewson T. J., Taegtmeyer H. Rabbit hindlimb glucose uptake assessed with positron-emitting fluorodeoxyglucose. J Appl Physiol (1985) 1989 Oct;67(4):1569–1577. doi: 10.1152/jappl.1989.67.4.1569. [DOI] [PubMed] [Google Scholar]
- Natali A., Santoro D., Palombo C., Cerri M., Ghione S., Ferrannini E. Impaired insulin action on skeletal muscle metabolism in essential hypertension. Hypertension. 1991 Feb;17(2):170–178. doi: 10.1161/01.hyp.17.2.170. [DOI] [PubMed] [Google Scholar]
- Nuutila P., Knuuti J., Ruotsalainen U., Koivisto V. A., Eronen E., Teräs M., Bergman J., Haaparanta M., Voipio-Pulkki L. M., Viikari J. Insulin resistance is localized to skeletal but not heart muscle in type 1 diabetes. Am J Physiol. 1993 May;264(5 Pt 1):E756–E762. doi: 10.1152/ajpendo.1993.264.5.E756. [DOI] [PubMed] [Google Scholar]
- Nuutila P., Knuuti M. J., Raitakari M., Ruotsalainen U., Teräs M., Voipio-Pulkki L. M., Haaparanta M., Solin O., Wegelius U., Yki-Järvinen H. Effect of antilipolysis on heart and skeletal muscle glucose uptake in overnight fasted humans. Am J Physiol. 1994 Dec;267(6 Pt 1):E941–E946. doi: 10.1152/ajpendo.1994.267.6.E941. [DOI] [PubMed] [Google Scholar]
- Nuutila P., Koivisto V. A., Knuuti J., Ruotsalainen U., Teräs M., Haaparanta M., Bergman J., Solin O., Voipio-Pulkki L. M., Wegelius U. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest. 1992 Jun;89(6):1767–1774. doi: 10.1172/JCI115780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985 Dec;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pearlman E. S., Weber K. T., Janicki J. S., Pietra G. G., Fishman A. P. Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab Invest. 1982 Feb;46(2):158–164. [PubMed] [Google Scholar]
- Pollare T., Lithell H., Berne C. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism. 1990 Feb;39(2):167–174. doi: 10.1016/0026-0495(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Ratib O., Phelps M. E., Huang S. C., Henze E., Selin C. E., Schelbert H. R. Positron tomography with deoxyglucose for estimating local myocardial glucose metabolism. J Nucl Med. 1982 Jul;23(7):577–586. [PubMed] [Google Scholar]
- Schelbert H. R., Henze E., Schon H. R., Keen R., Hansen H., Selin C., Huang S. C., Barrio J. R., Phelps M. E. C-11 palmitate for the noninvasive evaluation of regional myocardial fatty acid metabolism with positron computed tomography. III. In vivo demonstration of the effects of substrate availability on myocardial metabolism. Am Heart J. 1983 Mar;105(3):492–504. doi: 10.1016/0002-8703(83)90368-x. [DOI] [PubMed] [Google Scholar]
- Spinks T. J., Guzzardi R., Bellina C. R. Performance characteristics of a whole-body positron tomograph. J Nucl Med. 1988 Nov;29(11):1833–1841. [PubMed] [Google Scholar]
- Yki-Järvinen H., Consoli A., Nurjhan N., Young A. A., Gerich J. E. Mechanism for underestimation of isotopically determined glucose disposal. Diabetes. 1989 Jun;38(6):744–751. doi: 10.2337/diab.38.6.744. [DOI] [PubMed] [Google Scholar]
- Yonekura Y., Brill A. B., Som P., Yamamoto K., Srivastava S. C., Iwai J., Elmaleh D. R., Livni E., Strauss H. W., Goodman M. M. Regional myocardial substrate uptake in hypertensive rats: a quantitative autoradiographic measurement. Science. 1985 Mar 22;227(4693):1494–1496. doi: 10.1126/science.3975623. [DOI] [PubMed] [Google Scholar]
- Zaninetti D., Greco-Perotto R., Jeanrenaud B. Heart glucose transport and transporters in rat heart: regulation by insulin, workload and glucose. Diabetologia. 1988 Feb;31(2):108–113. doi: 10.1007/BF00395557. [DOI] [PubMed] [Google Scholar]