Abstract
We describe an approach to produce an autologous therapeutic antitumor vaccine using hydroxyapatite (HA) for vaccinating cancer patients. The novel approach involved (1) the purification of part of the self-tumor antigens/ adjuvants using column chromatography with HA, (2) the employ of HA as a medium to attract antigen-presenting cells (APCs) to the vaccination site, and (3) the use of HA as a vector to present in vivo the tumor antigens and adjuvants to the patient's APCs. The vaccine was prepared using and combining HA particles, with at least 3 heat shock proteins (gp96 was one of them possibly with chaperoned proteins/peptides as shown in the slot blots) and with proteins from the cell membrane system (including Hsp70, Hsp27, and membrane proteins). The timing of HA degradation was tested in rats; the HA particles administered under the skin attracted macrophages and were degraded into smaller particles, and they were totally phagocytized within 1 week. In patients (n = 20), the vaccine was then administered weekly and showed very low toxicity, causing minor and tolerable local inflammation (erythema, papule, or local pain); only 1 patient who received a larger dose presented hot flashes, and there were no systemic manifestations of toxicity or autoimmune diseases attributed to the vaccine. Our study suggests that this therapeutic vaccine has shown some efficacy producing a positive response in certain patients. Stable disease was noted in 25% of the patients (renal carcinoma, breast carcinoma, and astrocytoma), and a partial response was noted in 15% of the patients (breast carcinoma and astrocytoma). The most encouraging results were seen in patients with recurrent disease; 4 patients in these conditions (20%) are disease free following the vaccine administration. However, we do not want to overstate the clinical efficacy in this small number of patients. The therapeutic vaccine tested in our study is working by activating the T-cell response as was shown in the comparative histological and immunohistochemical study performed in the pre- and postvaccine biopsy taken from a patient with inflammatory breast carcinoma. However, we cannot ruled out that the vaccine could also be producing an antibody(ies)-mediated response. In conclusion, this therapeutic vaccine based on HA ceramic particles and self-antigens can be safely administered and is showing some encouraging clinical results in cancer patients.
INTRODUCTION
Traditional cancer treatments include surgery, radiotherapy, chemotherapy, and in some cases endocrine therapy. Immunotherapy is used in patients with certain cancer types (mainly melanomas as well as bladder and kidney cancer), administrating medicaments that nonspecifically induce an active immune response (eg, BCG, interleukins, interferons) (Steiner et al 1987; Herr 1997; Rosenberg et al 1998, Atkins et al 2004). Another form of immunotherapy, more recently introduced, is the administration of antibodies against specific proteins expressed on the cell surface of specific tumors (eg, trastuzumab against HER-2/neu, rituximab against CD20, bevacizumab against VEGF) (Ménard et al 2003; Yang 2004; see review by Ciocca et al 2006). Finally, the approach to generate cell-mediated immunotherapy by immunization with tumor-cell vaccines is still a research area. One strategy is in the form of adoptive immunotherapy stimulating antigen-specific T lymphocytes (MHC restricted) using tumor infiltrating lymphocytes (TILs) or lymphocytes from the lymph nodes draining the tumor (in both cases a key role is played by the antigen-presenting cells [APCs]) (Huang et al 2006). The difficulty with this strategy is to obtain a relatively large amount of TILs or to expand the lymphocytes from the lymph nodes. Another vaccination procedure is to generate an active immunotherapy based on stimulation of specific T lymphocytes against tumor antigens from the host. This form of immunotherapy is achieved culturing dendritic cells (or another APCs) in contact with the tumor antigen(s) or by presenting the antigens to the APCs in vivo. Among the antigens used for this strategy are (1) inactivated tumor cells or tumor cell lysates, (2) specific antigens and costimulatory molecules, and (3) heat shock proteins (Hsps) as adjuvants mixed with the tumor antigen(s) (Tso et al 2001; Belli et al 2002; Wang et al 2003; Yu et al 2004). Although there are abundant studies on this subject, the use of tumor-cell vaccines is still under evaluation in basic research and in clinical trials (see reviews by Fukao 2002; Fin 2003; Mocellin et al 2004; Nicchitta et al 2004; Rosenberg et al 2004; Mosolits et al 2005).
The introduction of a foreign product (gene or proteins/ peptides) into cells in vivo is often limited to the use of viral vectors, which may present several disadvantages or side effects (Kahn 2000). A number of nonviral vectors have been explored and used (reviewed by Saupe et al 2006). In the present study we have generated an autologous therapeutic vaccine and delivered the vaccine with hydroxyapatite (HA) [Ca10(PO4)6(OH2)]. These ceramics are widely used in human surgery as bone substitutes or as thin layers at the surface of metals allowing to improve bone integration (Frayssinet et al 1992, 1998). The behavior of HA in an organism is well known; these particles are biocompatible and are totally degradable by cells of the monocyte lineage (Frayssinet et al 1994). When HA beads loaded with the lacZ gene were implanted in vivo in rabbit jaws, the first cells to come in contact with the implanted material were circulating cells of the monocyte lineage (Laquerriere et al 2003; Frayssinet et al 2006). During the course of their degradation, ceramic grains are released from the particles and found in phagocytic cells. The ceramic particles are degraded at the grain boundaries and can release submicroscopic grains. The physicochemical characteristics of the grains may activate the phagocytic cells in which they are contained. The synthesis of IL-6 and TNF-α, for example, is dependent on the surface characteristics of the phagocytosed grains (Laquerriere et al 2003). Taking advantage of the HA characteristics, the vaccine prepared in the present study presented the following novel approaches: (1) the purification of tumor adjuvants/antigens using column chromatography with HA, (2) the use of HA as a medium to attract APCs to the injection site, and (3) the use of HA as a vector to present in vivo the tumor antigens and adjuvants to the patient's APCs. Therefore, the same HA ceramic particles were allowed to isolate tumor adjuvants/antigens such as gp96; this mixture was then combined with membrane antigens from the autologous tumor, and the resulting blend of HA and tumor adjuvants/antigens was administrated to the patients. The attraction of APCs to the HA injection site was first tested in the skin of rats, where the time course of HA degradation was also evaluated. In the patients we tested safety and feasibility as the primary endpoints. Additionally, the clinical outcome and immune response of a few patients with advanced cancer disease could also be examined in this pilot study.
MATERIALS AND METHODS
Hydroxyapatite preparation
The HA was obtained by a precipitation method, then sintered before being sieved and/or spray dried: The resulting powder consisted of more than 98% HA (Urodelia, St Lys, France). The amount of CaO and TCP was inferior to 2%, and the powder had a negative surface charge; other powder characteristics have been reported elsewhere (Laquerriere et al 2003). The protein/hydroxyapatite interactions are complex and not fully understood. Amino groups are attracted to crystal phosphates but repelled by crystal calcium ions. It is reversed for carboxyls. Amine binding to crystal phosphate is electrostatic. The binding of carboxyl to crystal calcium ions involves the formation of coordination complexes between Ca and clusters of protein carboxyls. The HA powder (sterile, autoclaved) containing particles of about 80–160 μm was suspended in a sterile phosphate buffer solution (30 mM, pH 6.8).
HA administration to rats
The HA in solution prepared as described previously was injected under the skin of Sprague-Dawley rats. The HA particles showed a tendency to decant; they were maintained in solution by movement of the solution in the flask and in the syringe by hand agitation. The injection site was marked, and biopsies of the injection site were taken at different time periods: 30 minutes, 2 hours, 6 hours, 24 hours, 2 days, 4 days, and 7 days (3 rats/group). In the opposite flank the animals received the vehicle alone (controls). The biopsies were fixed in 10% buffered formalin and processed for paraffin embedding. The tissue sections (5–6 μm) were used for hematoxylin and eosin staining and for immunohistochemistry.
Patients
This vaccine protocol was approved by the Ethics Committee of the Argentine Foundation for Cancer Research, and each patient approved and signed an informed consent. This protocol has been evaluated by the National Administration of Medicaments, Foods and Medical Technology of Argentina (No. 1-47-12542/02-0), and a phase I clinical trial has been approved. Inclusion criteria were advanced cancer patients with solid tumors (confirmed by histopathology) who had finished the traditional standard anticancer treatments, patients who presented progressive disease or recurrent disease, the disease being objectively valuable, the possibility of obtaining fresh tumor to prepare the vaccine (≅1 cm3), and performance status: 0–2 (ECOG/Zubrod). Exclusion criteria were as follows: >80 years, infections, and/or depression of the immune system; life expectancy <12 weeks; and difficulties obtaining the tumor sample or liver or renal failure. Response criteria were evaluated as reported elsewhere (Elledge et al 1997): PD, progressive disease (increase >25% of the tumor mass, new lesions); SD, stable disease (with no decrease in <50% or increase in >25% of the tumor mass); PR, partial response (reduction of at least 50% for 4 weeks, no new lesions); and CR, complete response (confirmed disappearance of the tumor, at least for 4 weeks).
Biopsy collection
We requested from the surgeons at least 1 cm3 of tumor. The biopsy was immediately submitted to the laboratory in a sterile container with ice. Once in the laboratory, in order to confirm presence of tumor tissue, a small piece of the biopsy was processed for histopathological evaluation of the tumor (routine formalin fixation and paraffin embedding). The remaining tissue was frozen and kept at −80°C for vaccine preparation. In previous studies none of the numerous immunological markers examined have been useful in predicting the efficacy of immunotherapies (reviewed by Srivastava 2000). Taking this into consideration, when possible, a second biopsy of the tumor was planned to compare the immunological status/ response in the prevaccine and in the postvaccine tumors. Unfortunately, this was achieved in only 1 patient with inflammatory breast carcinoma (where the postvaccine biopsy was relatively easy to perform) and in another patient with a renal tumor metastatic under the skin.
Vaccine preparation
The tumor tissue and all the materials used to prepare the vaccine were handled under sterile conditions under a laminar flow. The frozen tumor tissue was pulverized in a mortar (kept at −80°C), and the powder was transferred to a Khan tube on ice, adding 750 μL of NaHCO3 (30 mM, pH 7). The tissue was then homogenized and the solution transferred to Eppendorf microcentrifuge tubes (1.5 mL) and centrifuged at 10 000 rpm for 30 min at 4°C. The supernatant (SUP) was saved for later use. The pellet was resuspended in 400 μl of phosphate buffer (30 mM, pH 7) and used for membrane preparation.
Membranes were separated using two Eppendorf microcentrifuge tubes (1.5 mL) each containing a sucrose gradient (400 μl sucrose 40%, 400 μl sucrose 35%, and 400 μl sucrose 30%) (Iyengar et al 1991). Then 200 μl of the resuspended pellet were carefully layered onto the Eppendorf tubes, and the tubes were centrifuged in a microfuge (11 000 rpm for 30 min at 4°C). The material present at the 40% and 35% sucrose interface (which contains mainly plasma membranes, some endoplasmic reticulum membranes, and mitochondria) was recovered with a Pasteur pipette and placed in 4 sterile vaccine glass containers.
The SUP was used for gp96 purification by HA column chromatography with the following steps: (1) Two precipitations with ammonium sulfate (first at 50% and then at 70%) recovering the pellets. The last pellet was resuspended in 1 mL phosphate buffer (20 mM, pH 7). (2) Column preparation (chromatography columns, Poly-prep, Cat. 731-1550, Bio Rad) with HA at 80–160 μm, 2 cm high, equilibrating with 10 volumes of phosphate buffer (20 mM pH7). The resuspended pellet was then added. (3) The column was washed with 3 mL of the following solutions: 50, 100, 200, 300, 400, and 600 mM of NaCl. Fractions of 1 mL each were collected (3/each NaCl concentrations). (4) To test the purification of gp96, slot blots were performed and then SDS-PAGE with silver staining and Western blots. (5) The fractions with higher gp96 content and purification were placed in the 4 glass vaccine containers mixing with the membranes and adding 0.5 mL of HA 45-80 μm in saline solution to each container (the vaccine was then ready to use; the protein concentration ranged from 1,100 to 1,400 μg/mL, and it was kept at −20°C).
Vaccine administration
When enough tumor tissue was received to prepare the vaccine (≥1 cm3), the patients received 0.2 mL (≅240 μg) of the vaccine intradermically in the forearm on day 0 to test the safety of the preparation, and 24 hours later they received the initial dose (usually 0.5 mL) intradermically in the arm. Other doses were also tested when a larger biopsy was obtained. The vaccine was administrated with a tuberculin syringe since the HA particles showed a tendency to decant; the solution was maintained in movement by hand agitation. Dose scheme of treatment: 0.5 mL/dose, every week during 1 month, followed by a month without treatment, and then another month of vaccine treatment. The injection place was alternated in the arms.
Immunological studies
The biopsies were evaluated by immunohistochemistry as described elsewhere (Gago et al 1998), using serial 5-μm-thick sections mounted onto 3-aminopropyltrietoxysilane (Sigma, St. Louis, MO)-coated slides. The following primary antibodies were used: (1) mouse MAb anti-CD20 (used at 1:200 dilution; DAKO Corporation, Carpinteria, CA); (2) mouse MAb anti-CD43 (1:100; DAKO); (3) mouse MAb anti-CD68 (1:100; DAKO); (4) mouse MAb anti-CD57 (1:100; DAKO); (5) mouse MAb anti-CD15 (1:100; DAKO); (6) mouse MAb anti-CD45Ro (1:200; DAKO); and (7) rabbit Ab anti-S100 (1:1500; DAKO). The antigen retrieval protocol with microwave oven was used for tissues incubated with the antibodies CD43 and CD57 to unmask the antigens (30 minutes in citrate buffer 0.01 M, pH 6.0). Tissue sections were incubated with the primary antibodies overnight at 4°C in humidity chambers. A commercial kit to detect mouse and rabbit primary antibodies was used (DAKO EnVision system HRP, DAB). Slides were lightly counterstained with hematoxylin to reveal nuclei and observed with an IM35 microscope (Zeiss, Oberkochen, Germany). The specificity of the antibodies has been evaluated in immunopathology by us. Negative control slides were processed excluding the primary antibody but including all other steps of the procedure. In addition, specificity of the immunoreactions was controlled by using isotype controls, and the proper isotype was obtained from the commercial information provided with the antibodies.
Western blot and slot blots were performed on vaccine samples during different steps of the purification procedure. Total protein samples (40 μg) were subjected to 7.5% SDS-PAGE followed by transfer onto nitrocellulose filters, as previously described (Fanelli et al 1998). The antibodies used were (1) rabbit Ab anti-hybrid Hsp27/ Hsp25 protein (Ciocca et al 2003) used at 1:2000 dilution, (2) mouse MAb anti-Hsp70 (Ciocca et al 2003) used at 1:2000 dilution, (3) mouse MAb anti-gp96 (used at 1 μg/ mL; NeoMarkers, Fremont, CA), (4) rabbit Ab anti-HER-2/neu (used at 1:500; Gago et al 1998), (5) mouse MAb anti-β-catenin (used at 1:500; Zymed Lab, San Francisco, CA), (6) mouse Mab anti-p-cadherin (used at 1:250 dilution; BD Transduction Lab, Lexington, KY), and (7) rabbit Ab anti-survivin (used at 1:1000; Oncogene, La Jolla, CA). For detection of the immunocomplexes, chemiluminescence reagents were used following the manufacturer's instructions (Dupont NEN, Boston, MA).
RESULTS
HA administration to rats
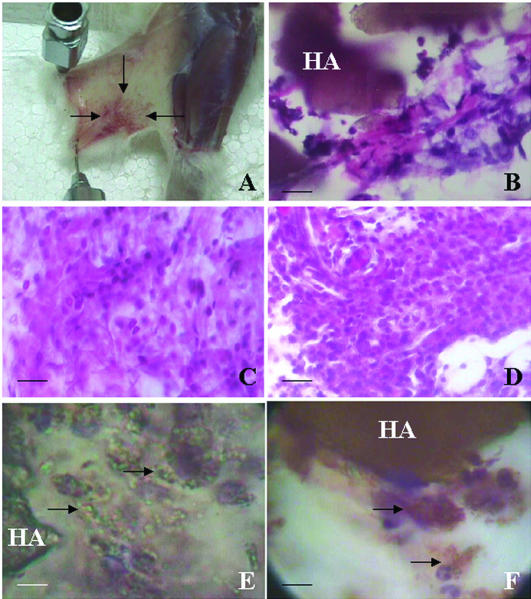
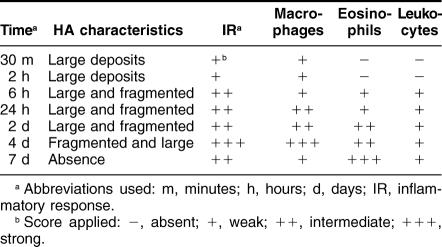
To our knowledge this is the first time that HA was injected in the skin. We performed these injections to know the timing of HA degradation. This information was then used to plan the vaccine administration to the patients. All the animals that received the HA tolerated the ceramic administration. At the local injection site there was erythema due to congestion of the capillaries and a mild inflammatory response (Fig 1A). The animals did not show systemic alterations. At microscopic level, 30 minutes after HA administration a weak inflammatory response was noted at the injection site. This inflammatory response was represented by mononuclear cells with abundant cytoplasm and was more intense at 2 hours surrounding the large HA deposits (Fig 1B). Six hours after HA administration, a fragmentation of the large HA deposits into minor size fragments was noted. Within the inflammatory cells we identified mononuclear cells as well as polymorphonuclear leukocytes and a few eosinophilic leukocytes. At 24 hours a larger inflammatory response was noted, while at 48 hours post-HA administration we noted a greater number of eosinophilic leukocytes, mainly in the areas more distant from the HA deposits (Fig 1C). Four days after HA administration, there were fewer large deposits of HA, and there appeared large clusters of mononuclear cells (Fig 1D). These cells were engulfing the small HA particles (Fig 1E). By immunohistochemistry these cells were not proliferating (absence of PCNA labeling), they were S100 negative, and they were identified as macrophages (CD68+) (Fig 1F). Macrophages (and dendritic cells) belong to the mononuclear phagocyte system and are APCs (Gatti and Pierre 2003). Seven days after HA administration, the HA particles were absent, and eosinophilic leukocytes remained at the injection site. Table 1 summarizes the main changes observed after HA administration.
Fig 1.
Effect of the HA administration in rats. (A) The skin was separated from the muscle of the limb to show the congested injection area (arrows) 7 days after HA administration. (B) Photomicrograph showing large deposits of HA (brown) 2 hours after HA administration. Note the beginning of an inflammatory response around the HA. (C) This photomicrograph shows the increased inflammatory response noted 2 days after HA administration. (D) For days after HA administration, showing the mononuclear cell infiltration. (E) For days after HA administration, a large deposit of HA and degranulation of HA can be seen. The arrows point to macrophages engulfing the small HA granules. (F) Same as above but immunostained to reveal CD68+ macrophages (arrows). Photomicrographs taken from tissue sections stained with hematoxylin and eosin (B–D), stained with hematoxylin alone (E), and after immunohistochemistry (F). Bar = 45 μm (B–D); bar = 28 μm (E–F)
Table 1.
Effects of HA administration under the skin of the rats
Vaccine preparation
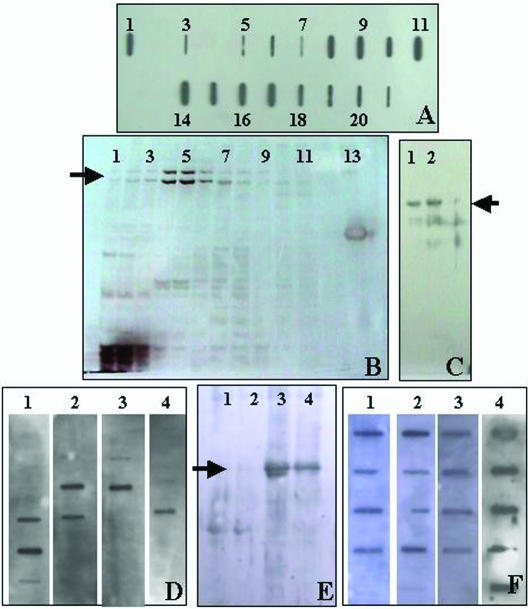
The vaccine was prepared with 2 main approaches, one involving the purification of gp96 with HA chromatography columns. In the first purification step the fractions collected from the HA column were tested with slot blot to identify gp96 (Fig 2A). As can be seen in this figure, the fractions collected with 100, 200, and 300 mM of NaCl were rich in gp96. To further characterized the fractions that contained more gp96, we performed SDS-PAGE. In the silver-stained SDS-PAGE gels, the 200-mM fractions showed abundant gp96 bands and additional lower MW unidentified bands with relatively little protein content (Fig 2B). The isolation of gp96 by HA chromatography columns was confirmed by Western blotting (Fig 2C). We then tried to characterize the proteins/peptides present in the gp96 isolated fractions; β-catenin, P-cadherin, Her-2/neu, and survivin were identified by slot blots in these fractions (Fig 2D). The other purification step was performed in order to isolate proteins from the cell membranes of the tumor cells by sucrose gradient centrifugation. Among the proteins recovered were Hsp70 (Fig 2E) as well as Hsp27, Her-2/neu, β-catenin, and P-cadherin (Fig 2F). So, our vaccine was composed of at least 3 heat shock proteins (gp96 was one of them, possibly with chaperoned proteins/peptides as shown in the slot blots), proteins from the cell membrane system, and HA particles.
Fig 2.
Purification of the vaccine components. (A) Slot blot to reveal gp96 after HA column chromatography. Lane 1: positive control; lane 2: negative control; lane 3: flow-through after column equilibration; lanes 4–6: fractions collected after column washing with 50 mM NaCl; lanes 7–9: fractions collected after column washing with 100 mM NaCl; lanes 10, 11 and 14: fractions collected after column washing with 200 mM NaCl; lanes 15–17: fractions collected after column washing with 300 mM NaCl; lanes 18–20: fractions collected after column washing with 400 mM NaCl; lanes 21, 22: fractions collected after column washing with 500 mM NaCl. (B) SDS-PAGE and silver staining of the fractions collected from the HA chromatography column. Note that the fractions collected with 200 mM of NaCl contained the highest amount of gp96 (arrow), which appeared as a doublet (lanes 4–6). The other lanes were loaded with fractions obtained washing with lower and higher mM of NaCl concentrations (as shown in the slot blot). (C) Western blot showing gp96 as a doublet (arrow) after HA purification. (D) Slot blots of the gp96 purification fractions to show examples of the identified proteins. lane 1: β-catenin; lane 2: Her-2/neu; lane 3: P-cadherin; lane 4: survivin. (E) Western blot to reveal Hsp70 after membrane purification with sucrose gradient centrifugation. Lanes 1 and 2: loaded with 20 and 10 μg of proteins obtained from a patient with a parotid adenocarcinoma; lanes 3 and 4: loaded with 20 and 10 μg of proteins obtained from a patient with a renal clear cell carcinoma. Note that lane 3, loaded with a higher protein concentration, shows 2 Hsp70 bands: the upper corresponds to the constitutive form and the lower corresponds to the inducible form of Hsp70. (F) Slot blots of the purified membranes to show examples of the identified proteins. Lane 1: Her2/neu; lane 2: Hsp27; lane 3: P-cadherin; lane 4: β-catenin
Vaccine administration to patients
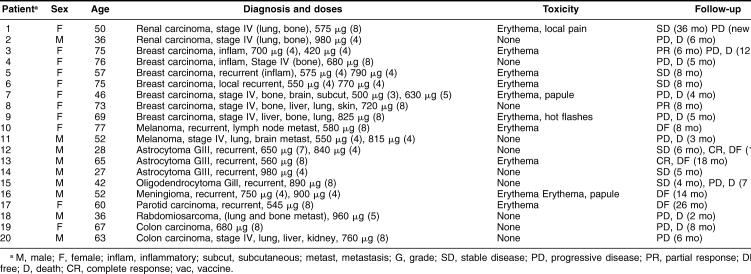
A total of 20 patients entered into this study; the main clinical data of the patients are presented in Table 2. All the patients received a test dose (0.2 mL, ≅240 μg, intradermic, forearm) to observe if the vaccine could produce adverse effects. In all cases the patients tolerated this test dose, with no skin reaction (50%) or with minor discomfort: erythema (40%), erythema with papule (10%), and erythema with tolerable local pain (5%). None of the patients presented systemic reactions attributed to the vaccine, and no autoimmune diseases were present (note that some patients have a relatively long follow-up). Twenty-four hours after the test dose, the patients received the standard vaccine doses, intradermic, once a week during 1 month, followed by a resting period of 1 month, and then another month with vaccine treatment (usually another 4 doses). The intradermic doses administered ranged from 420 to 980 μg/dose (in volumes that ranged from 0.3 to 0.7 mL) (Table 2). These doses were administered in the arms, alternating the place (except in the breast cancer patients, where the arm without lymphadenectomy was used for vaccine administration). It is of interest to mention that the larger vaccine doses (0.7 mL, >800 μg, n = 8) did not produce more local skin reactions, and only 1 patient (12.5%) presented a systemic reaction in the form of hot flashes that disappeared without medication. In all the cases (with the different doses), the erythema and papule disappeared within 3 or 4 days without medication.
Table 2.
Main clinical data of the patients entered into the vaccine study
Two patients with renal cancer, with stage IV disease, entered into this vaccine trial. In one of them the vaccine showed beneficial effects (Table 2). Patient 1 had had a large tumor in 1 kidney (surgically removed), a brain metastasis (surgically removed), and then lung and bone metastases and a metastasis in the contralateral kidney resistant to all standard treatments when she entered into the vaccine trial. After the vaccine, this patient showed a partial response of the bone metastases, with considerable improvement in the calcification of the larger bone lesions (Fig 3) and disappearance of the minor bone metastases. Then she presented a stable disease, as evidenced by the evolution of the lung and bone metastases for a period of 3 years, and thereafter she presented progressive disease at the level of the kidney that was not removed. Actually she is receiving a second vaccine generated from a small biopsy taken from the metastasis in the contralateral kidney. In one of the renal cancer patients (patient 2), pre- and postvaccine biopsies were taken, and in this case there was no immunological response around the tumor cells, and the patient did not respond clinically.
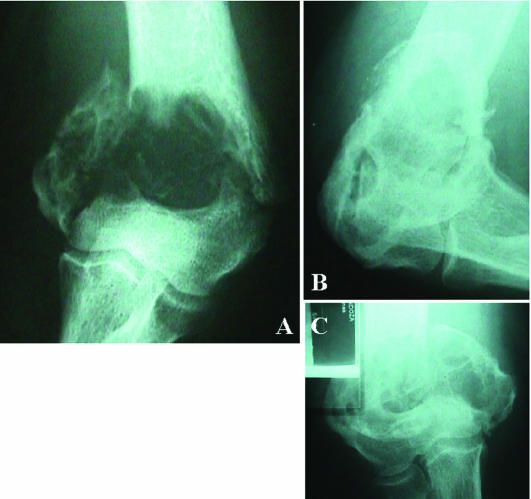
Fig 3.
Patient with renal carcinoma (Table 2, patient 1), effect of the vaccine on a large bone metastasis in the humerus (X-rays). (A) Prevaccine. (B) and (C) 5 months after vaccine administration. One year after the last vaccine administration this large lesion (4.5 cm) showed again progressive disease, but the minor size bone lesions (<1 cm) disappeared or presented stable disease
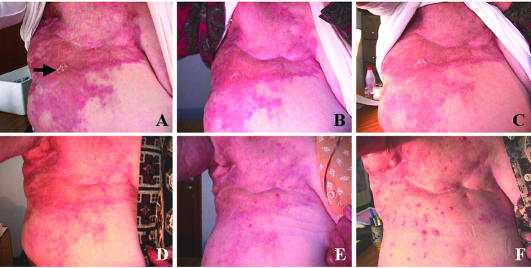
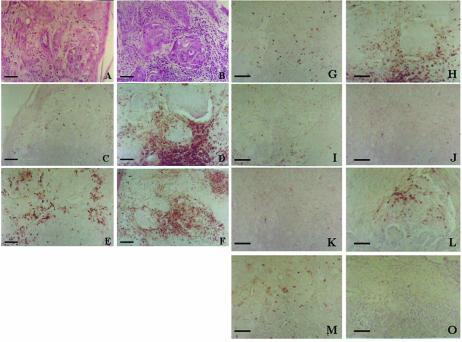
Seven patients with advanced breast cancer, resistant to conventional therapies, entered into this study. Patient 3 had an inflammatory carcinoma. A small biopsy was taken from the affected skin to generate the vaccine. Figure 4 shows the evolution of the skin lesions after the vaccine treatment. The vaccine produced a favorable effect soon after (15 days) the beginning of the first dose. This effect was maximum at 60–90 days where the skin was cleared. The patient could use a brass and begin to sleep resting on the side of the lesion. However, at 90 days a few red spots persisted on certain areas of the skin, and these lesions began to grow as small red papule about 2– 5 mm in diameter. At 180 days postvaccine administration, the papule reached a maximum, the skin between the papule was almost normal in appearance, the patient did not manifest any discomfort, and a second biopsy was taken of one of these lesions. The papule were formed by clusters of tumor cells surrounded by lymphoid cells. Then an immunohistochemical study was performed to compare the prevaccine vs the postvaccine skin lesions (Fig 5). There were more lymphoid cells surrounding the tumor cells in the postvaccine biopsy, with an evident increase in the number of total T cells (CD43+) and mature activated T cells (CD45Ro+) (Fig 5C–F). The postvaccine biopsy also showed an increased in the number of NK cells (CD57+) (Fig 5G,H) and a slight increase in the number of B lymphocytes (CD20+) (Fig 5K–L). There were no significant changes in the number of leukocytes and macrophages. We proposed to the patient a second vaccine generated from these persistent lesions, but she refused. The disease advanced in another areas of the skin (trunk), and 6 months later she died. Two other breast cancer patients with locally recurrent breast cancer showed SD (Table 2, patients 5 and 6), but the patients with distant metastases (with numerous organs involved) did not show a beneficial effect due to the vaccine.
Fig 4.
Patient with inflammatory breast carcinoma (Table 2, patient 3), effect of the vaccine on the skin lesions. (A) Prevaccine. The arrow points to the area where the biopsy was taken to generate the vaccine. (B) 15 days after the beginning of vaccine administration. (C) 30 days after the beginning of vaccine administration. (D) 60 days after the beginning of vaccine administration. (E) 90 days after the beginning of vaccine administration. (F) 180 days after the beginning of vaccine administration
Fig 5.
Immunohistochemical evaluation of the lymphoid cells in the pre- and postvaccine (180 days) biopsies taken from a patient with inflammatory breast carcinoma (Table 2, patient 3; Fig. 4). (A) and (B) Hematoxylin and eosin staining to show the tumor cells infiltrating the skin. (C) and (D) T cells evaluated by CD43. (E) and (F) T cells evaluated by CD45Ro. (G) and (H) NK cells evaluated by CD57. (I) and (J) Macrophages evaluated by CD68. (K) and (L) B cells evaluated by CD20. (M) and (O) Leukocytes evaluated by CD15. Bar = 60 μm (A–F); bar = 100 μm (G–O)
Two patients with melanoma entered into this trial (Table 2, patients 10 and 11). One of them with regional recurrent disease (lymph nodes) is DF, while the other with distant metastases in numerous organs showed PD and then died.
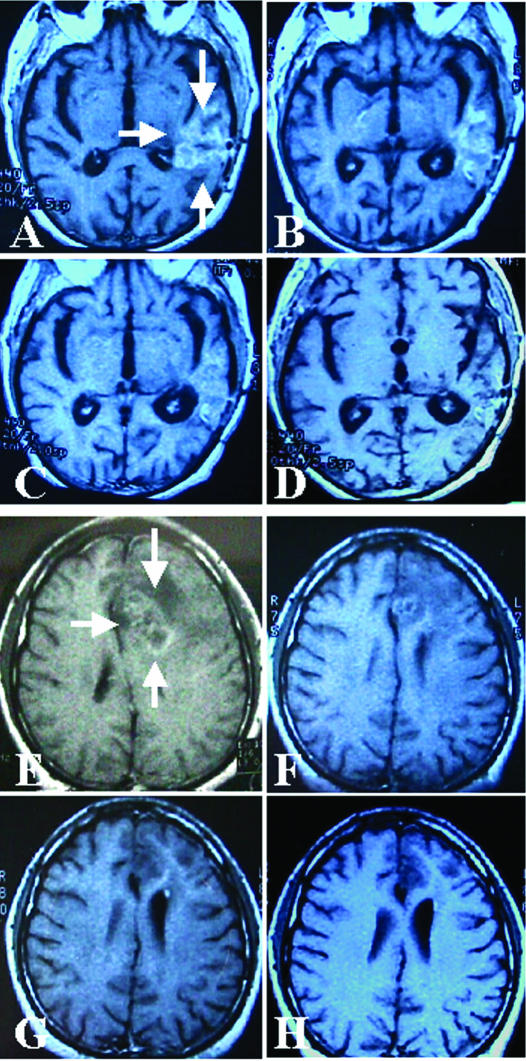
Five patients with central nervous system tumors with recurrent or persistent disease entered into this vaccine trial (Table 2, patients 12–16), 4 with grade III astrocytomas and 1 with a meningioma. All of them have shown a favorable course of the their disease attributed in part to the vaccine. Figure 6A–D shows the evolution of one of the patients with grade III recurrent astrocytoma (Table 2, patient 13). In this patient the disease was diagnosed 13 months before the vaccine treatment. After diagnosis the patient received neoadjuvant radiochemotherapy with no response, followed by surgery (neuronavigator) to reduce the tumor mass and to obtain a biopsy for the vaccine, and then combined radiochemotherapy (carboplatinum and temozolamide) was administered again with no response. One month after the end of this treatment the vaccine administration was started. As can be seen in Figure 6A–D, the vaccine produced an almost complete response. The patient is in very good clinical condition. At this point we cannot rule out that the vaccine could produce this effect, perhaps in association with a long-term effect of the combined radiochemotherapy. However, in another patient (Table 2, patient 12) where the combined radiochemotherapy was stopped 3 months before the vaccine treatment (without response), the effect of the vaccine was also evident in the images of the tumor (Fig 6E–H). In this case the tumor was diagnosed 24 months before the vaccine administration, the patient received neoadjuvant chemotherapy with no response, followed by surgery, and then radiochemotherapy again (with no response). A second surgery was performed 1 month before the vaccine administration to reduce the tumor mass and to obtain material for the vaccine. The patient with recurrent meningioma (Table 2, patient 16) presented the large lesion in contact with the cranial venous sinus. For this reason the meningioma could not be completely removed. The disease recurred 13 months after the first surgery; a second surgery was performed, followed by radiotherapy and then by vaccine administration. At present (14 months) the patient is in CR. In this case the patient entered into the trial mainly to test the toxicity of the vaccine. We are not sure if the patient remains in CR because of the radiotherapy, the vaccine, or the combination of both.
Fig 6.
Effect of the vaccine treatment on 2 grade III recurrent astrocytomas. Patient 13, Table 2 (A–D); patient 12, Table 2 (E–H). MRI with gadolinium for tumor visualization. (A) The arrows show the tumor in this prevaccine image. (B) 30 days after the beginning of the vaccine. (C) 180 days after the beginning of the vaccine. (D) 330 days after the beginning of the vaccine. (E) The arrows show the tumor in this prevaccine image. (F) 30 days after the beginning of the vaccine. (G) 180 days after the beginning of the vaccine. (H) 270 days after the beginning of the vaccine. In both cases note the reduction of the tumor mass, a minor gadolinium uptake in the final MRIs, and the enlargement of the ventricle (retraction like a scar)
Another patient with a recurrent tumor that showed a favorable course of the disease attributed to the vaccine had a parotid carcinoma (Table 2, patient 17). She had had several surgical procedures and radiotherapy. The last surgical removal was performed for tumor reduction and vaccine administration, and at present (26 months) she is SD. In contrast, a patient with a rabdomiosarcoma with metastases in multiple organs showed PD and then died (Table 2, patient 18). Finally, 2 patients with colon carcinoma entered into this trial, and both presented PD.
DISCUSSION
The vaccine tested here was designed to take advantage of data from previous investigations: (1) that HA can be used to purify proteins, (2) that this material is biocompatible, (3) that HA can attract monocytes/macrophages to the implantation area, and (4) that HA can be used as a vehicle to vectorize proteins to APCs (Frayssinet et al 1992, 1994, 1998; Laquerriere et al 2003). We have taken advantage of these HA properties to facilitate antigen preparation and to enhance antigen presentation using multiple antigens or epitopes (Guevara-Patino et al 2006), including certain molecular chaperones (Wang et al 2006). In the present study it was possible to prepare the vaccine using and combining HA particles with at least 3 heat shock proteins (gp96 was one of them, possibly with chaperoned proteins/peptides, as shown in the slot blots) and with proteins from the cell membrane system (including Hsp70, Hsp27, and membrane proteins). Hsps are synthesized by stressed cells. The stressful environment of tumor cells makes these proteins synthesized in large amounts by cancer cells (Ciocca and Calderwood 2005; Calderwood et al 2006). The Hsps are copurified with the proteins/peptides they chaperone; thus, gp96 allows a fingerprint of the tumor cell peptides and is specific of the patient. It means that it needs to be purified from the own patient's tumor in order to be used to enhance the patient's immunity (Srivastava 2000; Belli et al 2002). The immune effect of the gp96 is based on the interaction of the complex gp96/associated peptides with the APCs inducing 2 consequences: (1) stimulation of an innate response and (2) activation of immune events through presentation of Hsps-chaperoned peptides to MHC molecules (Ramirez et al 2005).
In this study we have demonstrated the feasibility and safety of a vaccine composite made of HA and proteins purified from the patient's tumor. In the patients the vaccine toxicity was very low, causing only minor and tolerable local inflammation. Only 1 patient who received a larger dose presented hot flashes. None of the patients needed medications to relieve these symptoms or discontinuation of the vaccine administration. In addition, none of the patients showed systemic manifestation of toxicity attributed to the vaccine or autoimmune diseases. These results support the concept that the vaccine components are relatively innocuous to the body. In previous studies the vaccines prepared from tumors from the same patients (autologous) have shown high tolerability and low toxicity (Disis et al 2003).
Our study suggests that this therapeutic vaccine has shown some efficacy, producing a positive response in certain patients. We do not want to overstate the clinical efficacy in this small number of patients, but it seems important to analyze the clinical response to know about the future of this immunotherapy. SD was noted in 25% of the patients, including those with renal carcinoma (n = 2), breast carcinoma (n = 2), and astrocytoma (n = 1). A PR was noted in 15% of the patients, including those with breast carcinoma (n = 2) and astrocytoma (n = 1). The most encouraging results were seen in patients with recurrent disease. Four patients in these conditions (20%) are DF following the vaccine administration (without counting the meningioma patient). Among them are those with recurrent melanoma (n = 1), astrocytoma (n = 2), and parotid carcinoma (n = 1). These results suggest that the vaccine is working at the doses administered and that 8 vaccine cycles is enough to have effects. The results also suggest that the vaccine could be effective mainly in patients where the tumor is still growing at the primary site and where the tumor mass has been decreased by surgery. It is of interest to point out that astrocytoma patients with grade III tumors are responding. In previous studies astrocytoma patients have also shown a good response to vaccine treatments (Yu et al 2004). Patients with numerous metastases are less responsive, although some benefit was noted in patients with renal carcinomas. Renal cancer is one of the main tumor types responding to vaccine treatments (Avigan 2004). The other is melanoma, which is also responding relatively well to vaccine treatments (Belli et al 2002). In our study we had only 2 melanoma patients. The therapeutic vaccine tested in our study is working by activating the T-cell response, as was shown in the comparative histological and immunohistochemical study performed in the pre- and postvaccine biopsy taken from a patient with inflammatory breast carcinoma. This cancer was highly aggressive and resistant to all conventional therapies. However, we cannot rule out that the vaccine could also be producing an antibody(ies)-mediated response. In conclusion, this therapeutic vaccine based on HA ceramic particles and self-antigens can be safely administered and is showing some encouraging clinical results in cancer patients.
Acknowledgments
The authors thank all individuals who volunteered to participate in this study and their doctors (W. Villalobos, F. Gago, V. Chávez). This work was supported by the Argentine Foundation for Cancer Research.
REFERENCES
- Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004;10:6342s–6346s. doi: 10.1158/1078-0432.CCR-040029.1078-0432(2004)010[6342s:UOTROI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Avigan D. Dendritic cell-tumor vaccines for renal cell carcinoma. Clin Cancer Res. 2004;10:6347s–6352s. doi: 10.1158/1078-0432.CCR-050005.1078-0432(2004)010[6347s:DCVFRC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Belli F, Testori A, and Rivoltini L. et al. 2002 Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 20:4169–4180. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque A, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006.0376-5067(2006)031[0164:HSPICC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1.1466-1268(2005)010[0086:HSPICD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Fanelli MA, and Vargas-Roig LM 2006 Principios Generales de los Tratamientos Biológicos. In: Manual de Oncología Clínica (Principios Biológicos, Diagnóstico, Clínica y Pautas Terapéuticas), ed Huñis A, Alonso D, Gomez D. Universidad Nacional de Quilmes, Buenos Aires, in press. [Google Scholar]
- Ciocca DR, Rozados VR, Cuello-Carrión FD, Gervasoni SI, Matar P, Scharovsky OG. Heat shock proteins 25 and 70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:hahirt>2.0.co;2.1466-1268(2003)008[0026:HSPAIR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis ML, Schiffman K, and Salazar LG. et al. 2003 Antigen-specific cancer vaccines. ASCO, Educational Book, 29–36. [Google Scholar]
- Elledge RM, Green S, and Howes L. et al. 1997 bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest oncology group study. J Clin Oncol. 15:1916–1922. [DOI] [PubMed] [Google Scholar]
- Fanelli MA, Cuello-Carrión FD, Dekker J, Ciocca DR. Serological detection of heat shock protein hsp27 in normal and breast cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:791–795.1055-9965(1998)007[0791:SDOHSP]2.0.CO;2 [PubMed] [Google Scholar]
- Fin OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150.1474-1733(2003)003[0630:CVBTIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Frayssinet P, Cuello-Carrion D, and Ciocca D 2007. Autovaccination against tumors using a mineral/protein composite. In: Heat Shock Proteins in Biology and Medicine. ed Multhoff G, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayssinet P, Fages J, and Bonel G. et al. 1998 Biotechnology, material sciences and bone repair. Eur J Orth Surg Trauma. 8:17–25. [Google Scholar]
- Frayssinet P, Hardy D, Rouquet N, Giammara B, Guilhem A, Hanker J. New observations on middle term hydroxylapatite-coated titanium alloy hip prostheses. Biomaterials. 1992;13:668–674. doi: 10.1016/0142-9612(92)90126-9.0142-9612(1992)013[0668:NOOMTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Frayssinet P, Rouquet N, and Tourenne F. et al. 1994 In vivo degradation of calcium phosphate ceramics. Cells Mater. 4:383–394. [Google Scholar]
- Fukao T. Dendritic-cell-based anticancer vaccination: has it matured? Trends Immunol. 2002;23:231–232. doi: 10.1016/s1471-4906(02)02198-1.1471-4906(2002)023[0231:DAVHIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gago FE, Tello OM, Diblasi AM, Ciocca DR. Integration of estrogen and progesterone receptors with pathological and molecular prognostic factors in breast cancer patients. J Steroid Biochem Mol Biol. 1998;67:431–437. doi: 10.1016/s0960-0760(98)00140-x.0960-0760(1998)067[0431:IOEAPR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gatti E, Pierre P. Understanding the cell biology of antigen presentation: the dendritic cell contribution. Curr Opin Cell Biol. 2003;15:468–473. doi: 10.1016/s0955-0674(03)00069-3.0955-0674(2003)015[0468:UTCBOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Guevara-Patino JA, Engelhorn ME, and Turk MJ. et al. 2006 Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 116:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr HW. Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. Br J Urol. 1997;80:762–765. doi: 10.1046/j.1464-410x.1997.00431.x.0007-1331(1997)080[0762:TPASIP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, Robins PF. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance of the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726.0022-1767(2006)176[7726:MBIOCA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R, Rojas F, Rich KA, and Birnbaumer L 1991 Membrane receptors: criteria and selected methods of study. In: Hormone Action and Molecular Endocrinology, ed Hughes MR, Schrader WT, O'Malley BW. Baylor College of Medicine, Houston, TX, 3–18. [Google Scholar]
- Kahn A. Dix ans de thérapie génique: déceptions et espoirs. Biofutur. 2000;202:16–21.0294-3506(2000)202[0016:DADTGD]2.0.CO;2 [Google Scholar]
- Laquerriere P, Grandjean-Laquerriere A, Jallot E, Balossier G, Frayssinet P, Guenounou M. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials. 2003;24:2739–2747. doi: 10.1016/s0142-9612(03)00089-9.0142-9612(2003)024[2739:IOHPCO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779.0950-9232(2003)022[6570:BATROH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mocellin S, Mandruzzato S, Bronte V, Marincola FM. Cancer vaccines: pessimism in check. Nat Med. 2004;10:1278–1279. doi: 10.1038/nm1204-1278.1078-8956(2004)010[1278:CVPIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mosolits S, Ullenhag G, Mellstedt H. Therapeutic vaccination in patients with gastrointestinal malignancies: a review of immunological and clinical results. Ann Oncol. 2005;16:847–862. doi: 10.1093/annonc/mdi192.0923-7534(2005)016[0847:TVIPWG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nicchitta CV, Carrick DM, Baker-LePain JC. The messenger and the message: gp96 (GRP94)-peptide interactions in cellular immunity. Cell Stress Chaperones. 2004;9:325–331. doi: 10.1379/CSC-62.1.1466-1268(2004)009[0325:TMATMG]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SR, Singh-Jasuja H, Warger T, Braedel-Ruoff S, Hilf N, Wiemann K, Rammensee H-G, Schild H. Glycoprotein 96-activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones. 2005;10:221–229. doi: 10.1379/CSC-117R.1.1466-1268(2005)010[0221:GADCIA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100.1078-8956(2004)010[0909:CIMBCV]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319. doi: 10.1097/00000658-199809000-00004.0003-4932(1998)228[0307:DOCRIP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saupe A, McBurney W, Rades T, Hook S. Immunostimulatory colloidal delivery systems for cancer vaccines. Expert Opin Drug Deliv. 2006;3:345–354. doi: 10.1517/17425247.3.3.345.1742-5247(2006)003[0345:ICDSFC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Srivastava P. Immunotherapy of human cancer: lessons from mice. Nat Immunol. 2000;1:363–366. doi: 10.1038/808795.1529-2908(2000)001[0363:IOHCLF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Steiner A, Wolf C, Pehamberger H. Comparison of the effects of three different treatment regimens of recombinant interferons (r-IFN alpha, r-IFN gamma, and r-IFNalpha + cimetidine) in disseminated malignant melanoma. J Cancer Res Clin Oncol. 1987;113:459–465. doi: 10.1007/BF00390040.0171-5216(1987)113[0459:COTEOT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso CL, Zisman A, and Pantuck A. et al. 2001 Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor. Cancer Res. 61:7925–7933. [PubMed] [Google Scholar]
- Wang XY, Facciponte JG, Subjeck JR. Molecular chaperones and cancer immunotherapy. Handb Exp Pharmacol. 2006;172:305–329. doi: 10.1007/3-540-29717-0_13.0073-0033(2006)172[0305:MCACI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang XY, Kazim L, Repasky EA, Subject JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003;105:226–231. doi: 10.1002/ijc.11058.0020-7136(2003)105[0226:IWTECG]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang JC. Bevacizumab for patients with metastatic renal cancer: an update. Clin Cancer Res. 2004;10:6367s–6370s. doi: 10.1158/1078-0432.CCR-050006.1078-0432(2004)010[6367s:BFPWMR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505.0008-5472(2004)064[4973:VWTLDC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]