Abstract
Cadmium is a heavy metal toxic for living organisms even at low concentrations. It does not have any biological role, and since it is a permanent metal ion, it is accumulated by many organisms. In the present paper we have studied the apoptotic effects of continuous exposure to subacute/sublethal cadmium concentrations on a model system: Paracentrotus lividus embryos. We demonstrated, by atomic absorption spectrometry, that the intracellular amount of metal increased during exposure time. We found, using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay, that long treatments with cadmium triggered a severe DNA fragmentation. We demonstrated, by immunocytochemistry on whole-mount embryos, that treatment with cadmium causes activation of caspase-3 and cleavage of death substrates α-fodrin and lamin A. Incubating the embryos since fertilization with Z-DEVD FMK, a caspase-3 inhibitor, we found, by immunocytochemistry, that cleavage by caspase-3 and cleavage of death substrates were inactivated.
INTRODUCTION
Among the heavy metals, cadmium is considered an important toxicant, and some effects at the cellular level have been recently described. Experimental evidences suggest that the metal is able to cross the plasmalemma as a bivalent metallic ion Cd2+, probably exerting an agonistic role for the specific calcium channels (Rainbow and Black 2005). The metal is highly dangerous not only because it easily penetrates the cells but also because it is eliminated very slowly (Jarup et al 1998). In the majority of cases, it damages chromosomes and inhibits the mechanisms implicated in DNA repair (Schroder et al 1999). It is known that heavy metals interfere on gametogenesis, inhibit embryogenesis, and interrupt development at different stages (Au et al 2001; Fernandez and Beiras 2001). Cadmium exposure can also induce apoptosis in testicular tissue of rat, mouse liver, human T-cells (Xu et al 1996; Habeebu et al 1998; Tsangaris and Tzortzatou-Stathopoulou 1998), and marine sponges (Schroder et al 1999); on the other hand, it has been demonstrated that caspase inhibitors attenuated Cd-induced apoptosis (Galan et al 2000). In response to different stresses, the cells activate the synthesis of heat shock proteins (Hsps), caused by the accumulation of denatured proteins. During the Hsps induction, the cells are refractory to the toxicity of various agents, and the protection of cells is due in part to inhibition of apoptosis (Samali and Cotter 1996). The mechanism by which Hsps may regulate apoptotic pathways is one of the newly emerging interests in the research on apoptosis.
Recently, we found that sea urchin embryos are able to respond with apoptosis to specific external stimuli and that physiological cell death is implicated in development and metamorphosis (Roccheri et al 1997, 2002). Sea urchin embryos represent a good model system for testing the effects of physical and chemical stress, like heavy metals, on development and cell viability (Lesser et al 2003; Kobayashi and Okamura 2004; Vega and Epel 2004; Pellerito et al 2005). Cytotoxic concentrations of this metal induce in Paracentrotus lividus embryos the synthesis of a specific set of highly conserved proteins, the Hsps and/or metallothioneins (MT) (Scudiero et al 1994, 1995; Russo et al 2003; Roccheri et al 2004). We have previously demonstrated that such ability represents a general protective strategy against many stress-inducing agents including heat shock and heavy metals (Roccheri et al 1981, 1988, 1993; Casano et al 1998, 2003; Gianguzza et al 2000; Roccheri et al 2000, 2001). If the concentration of MT and/or Hsps is not enough to block the toxic effect of the metal, cells undergo apoptosis (Samali and Cotter 1996; Hamada et al 1997). The accumulation of damaged proteins acts as signal for induction of stress response and activates the apoptotic program (Latchman 1999).
In the present paper, we have studied the time-dependent accumulation of cadmium in sea urchin embryos and the induction of apoptosis due to exposure to this metal.
MATERIALS AND METHODS
Embryo culture and treatment
Gametes were collected from gonads of the sea urchin P. lividus harvested from the west coast of Sicily. Eggs were fertilized at a concentration of 5000/mL and grown under gentle rotation at 18°C in Millipore-filtered seawater (MFSW) containing antibiotics (50 mg/L streptomycin sulfate and 30 mg/L penicillin). Just after fertilization or at different stages, embryos were continuously cultured for different times (15, 21, and 24 hours) in the presence of 1 mM CdCl2. Development was monitored by optical microscopy. The embryos were recovered from cadmium by repeated washings with MFSW.
Treatment with Z-DEVD-FMK
P. lividus eggs were fertilized in 4-aminobenzoic acid (PABA) to eliminate fertilization membranes and incubated with 250 μM caspase-3 inhibitor (Z-DEVD-FMK). Blastulae (10 hours of development) were then incubated with 1 mM CdCl2 for 24 hours.
Determination of metals
In order to measure cadmium content, 100 mg of embryos were acid digested in 1 mL of 70% HNO3 and 0.5 mL of 30% H2O2 at 150°C in oil bath. The dried residues were reconstituted in 0.2% HNO3 and analyzed for cadmium content by atomic absorption spectrometry (AAS) using a Perkin-Elmer apparatus model 5100 equipped with Zeeman graphite furnace.
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay was performed on whole-mount embryos. Embryos were fixed with a solution of 0.1% formaldehyde in MFSW, washed several times and permeabilized with cold methanol, washed with MFSW and PBST (0.15 M Na2HPO4, 0.04 M NaH2PO4, 0.15 M NaCl, 0.05% Tween 20), and then incubated for 60 minutes at 37°C with 50 μL of a solution containing the following reagents: equilibration buffer, TdT enzyme (10 U), and fluorescent nucleotide mix (5 μM fluorescein 12-dUTP). Negative controls were incubated in the same mixture but without the enzyme; positive controls were pretreated with DNAase (10 μg/mL) before the TdT assay. Nuclei were stained by incubation with propidium iodide (2 μg/ mL). The reaction was interrupted by incubating the embryos with NaCl/sodium-citrate (300 mM/30 mM) for 15 minutes, followed by observations under confocal microscope (Olympus FV 300 with a He-Ne 543-nm laser).
Embryo fixation
Embryos, at different developmental stages, were fixed for 12–16 hours with Bouin fixative (15:5:1 of picric acid solution, 37% formaldehyde, glacial acetic acid, respectively), kept at 4°C in 70% ethanol after serial dehydration.
Immunocytochemistry
Samples of about 1000 embryos were analyzed. After washings in 100% ethanol and in 100% methanol containing 2 mM EDTA, the embryos were rehydrated with decreasing ethanol concentrations, washed 3 times with PBST, and incubated for 12 hours with the following polyclonal antibodies, diluted in PBST in the presence of 3% BSA: anti-caspase-3 (1:100 dilution), anti-pro-caspase-3 (1:50 dilution), anti-cleaved-lamin-A (1:100 dilution), and anti-α-fodrin-cleaved (1:100 dilution). In the negative controls the primary antibodies were omitted. The embryos were washed 3 times with PBST and then incubated for 1 hour with the secondary antibody anti-rabbit IgG alkaline phosphatase (AP) conjugated (1:1000 dilution). The antibody excess was eliminated by washing embryos 3 times with PBST and then with TBS (150 mM NaCl, 10 mM Tris-HCl pH 8). Staining was performed in AP buffer (100 mM NaCl, 100 mM Tris–HCl, pH 9.5, 50 mM MgCl2) containing NBT/BICP. The reaction was blocked with 50% ethanol. Whole embryos resuspended in 80% glycerol in PBST were finally mounted on cover slips, observed under an Axioskope MC80 microscope and photographed with 100 Asa Kodak Gold film.
RESULTS
We have previously demonstrated that subacute/sublethal cadmium concentrations induce morphological abnormalities in the embryos during development and the activation of specific stress proteins, time and dose dependent (Roccheri et al 2004).
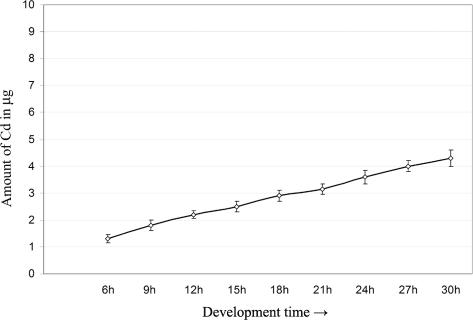
Since our previous results suggested that Cd was partially stored in the embryo cells, we decided to measure the absorption levels of this metal in relation to the treatment period. For this purpose, P. lividus sea urchin embryos, just after fertilization, were continuously cultured in 1 mM CdCl2 for periods of time comprised from 6 to 30 hours. Cadmium content was tested by AAS, measuring the amount of metal in 100 mg of embryos. The analyses demonstrated that the amount of incorporated cadmium highly increased during time (Fig 1).
Fig 1.
Amount of cadmium incorporated during time. The embryos were cultured in 1 mM CdCl2. Cadmium content was tested by atomic absorption spectrometry (AAS), measuring the metal amount in 100 mg of embryos. Graphic was obtained by the mean of 3 experiments; standard deviations are indicated
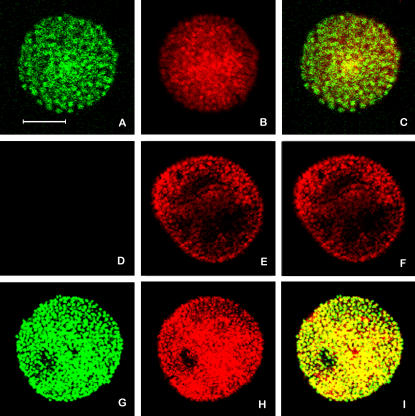
The morphological observations showed that embryos exposed for 15 hours to 1 mM CdCl2 were able to restore the normal development, whereas longer treatments seriously and irreversibly interfered with morphology (Roccheri et al 2004). This suggests that an increasing number of embryo cells was induced to activate the death pathway in response to longer exposure. In order to verify this hypothesis, we performed a TUNEL assay on the embryos treated with 1 mM CdCl2 for 24 hours. Results suggest that the majority of nuclei undergo DNA fragmentation (Fig 2).
Fig 2.
Evaluation of DNA fragmentation by TUNEL assay. The images show equatorial sections observed under confocal laser microscopy. In green DNA fragmentation (A, D, G); in red (propidium iodide) totality of nuclei (B, E, H); merging of green and red (C, F, I). Embryos treated for 24 hours with 1 mM CdCl2 (A–C); control embryos (D–F); control embryos incubated for 10 minutes with DNAase I (G–I). Bar = 40 μM
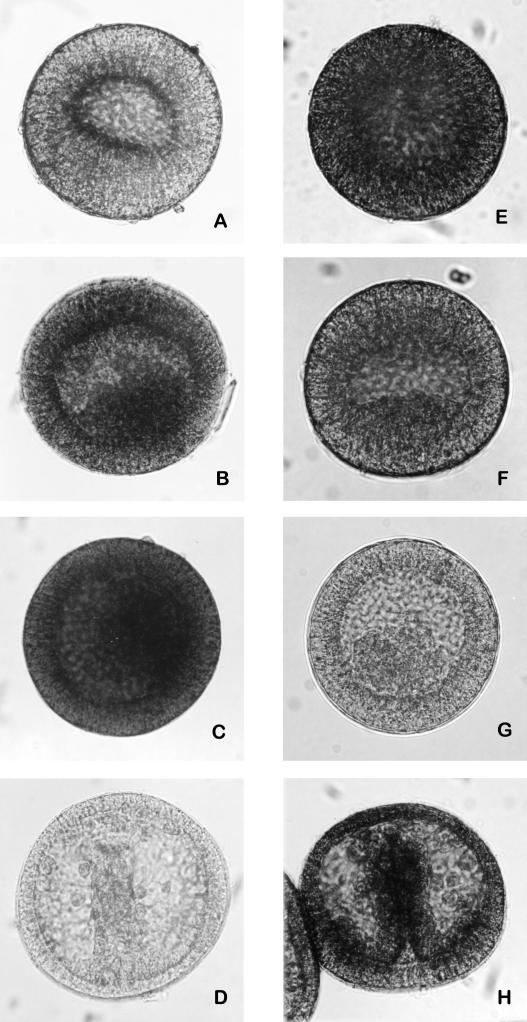
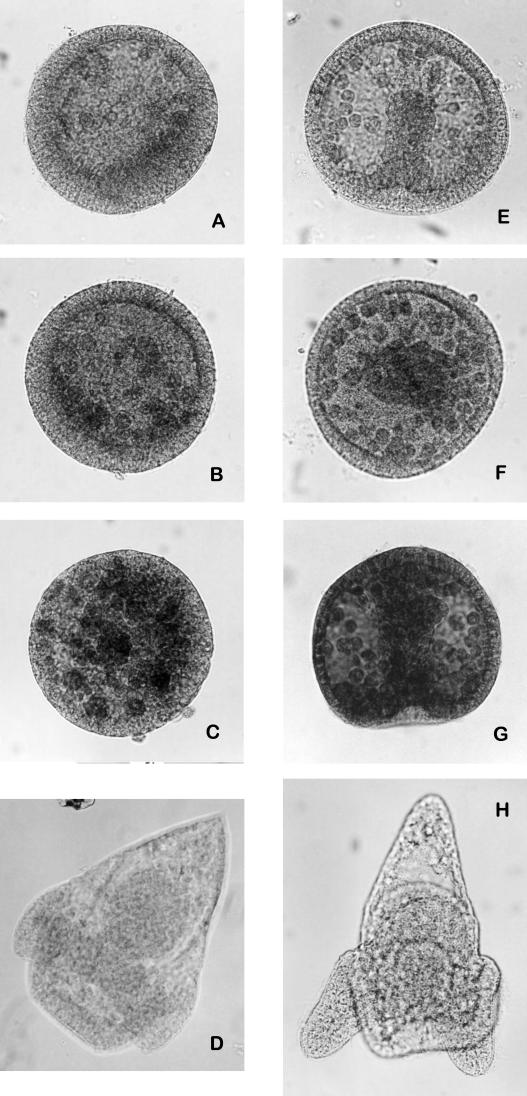
The execution of programmed cell death is usually operated by a proteolytic cascade that involves caspases activation. Immunocytochemical tests performed on whole-mount embryos treated with 1 mM CdCl2 and reacted with anti-caspase-3-cleaved and anti-pro-caspase-3 antibodies showed an increase of caspase-3 cleaved (Fig 3A–D) and a decrease of its zymogene (Fig 3E–H), depending on the length of exposure to the metal. The cleavage of caspase-3 became evident after 21 hours of treatment. On the other hand, embryos untreated or treated for 15 hours showed the expression of pro-caspase-3, while the proenzyme was absent in the embryos treated for longer periods. The remarkable presence of caspase-3 cleaved in embryos treated with 1 mM CdCl2 for 24 hours (Fig 3C) demonstrated that a longer exposure to cadmium led to the increase of apoptotic events.
Fig 3.
Immunolocalization of cleaved-caspase-3 and pro-caspase-3 on whole-mount embryos. Embryos treated with 1 mM CdCl2 since fertilization, respectively, for 15, 21, and 24 hours, incubated with anti-cleaved-caspase-3 antibody (A–C). Embryos treated with 1 mM CdCl2 since fertilization, respectively, for 15, 21, and 24 hours, incubated with anti-pro-caspase-3 antibody (E–G); control embryos (24 hours of development) incubated, respectively, with anti-cleaved-caspase-3 and anti pro-caspase-3 antibodies (D, H)
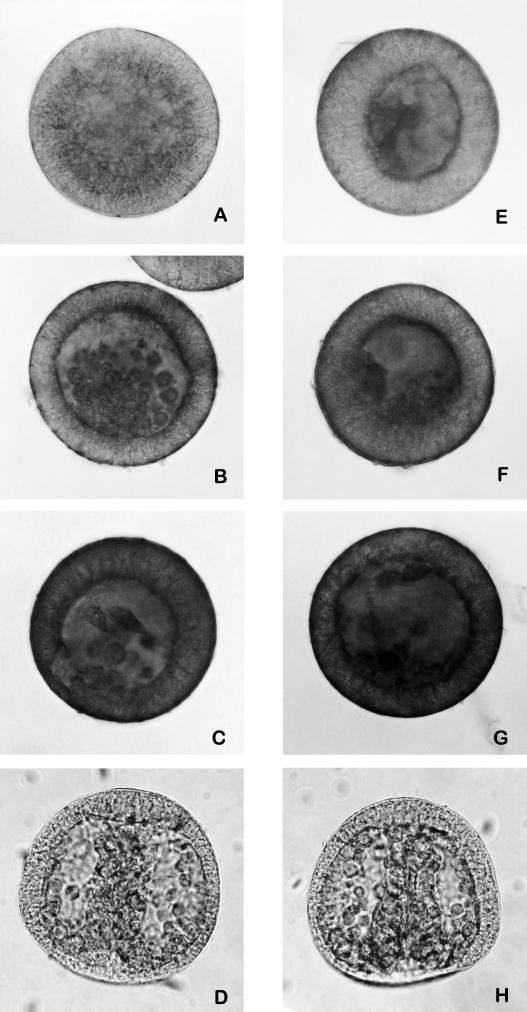
Likewise, the expression of cleaved forms of α-fodrin (an anchorage cytoskeleton protein) and lamin A (nuclear membrane protein), substrates of caspase-3 and caspase-6, respectively, increased during treatment with cadmium (Fig 4).
Fig 4.
Immunolocalization of cleaved-α-fodrin and cleaved-lamin A on whole-mount embryos. Embryos treated with 1 mM CdCl2 since fertilization, respectively, for 15, 21, and 24 hours, incubated with anti-cleaved-α-fodrin (A–C). Embryos treated with CdCl2 1 mM since fertilization, respectively, for 15, 21, and 24 hours, incubated with anti-cleaved-lamin A (E–G). Control embryos (24 hours of development) incubated, respectively, with anti-cleaved-α-fodrin and anti-cleaved-lamin A antibodies (D, H)
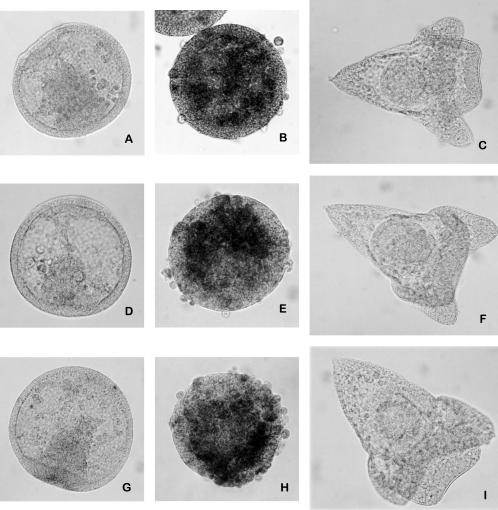
To explore whether the effects previously described may depend on the stage of development, we treated for 15/24 hours blastula and gastrula embryos with 1 mM CdCl2. Immunocytochemical experiments demonstrated that the cleavage of caspase-3, also in this case, increased during the treatment (Fig 5). However, it is noteworthy that the number of cells undergoing apoptosis is lower in the late embryos than in those treated just soon after fertilization (Figs 3 and 5). This difference could be due to the fact that starting from the blastula stage, the embryos are able to synthesize stress proteins (Roccheri et al 1981) with protective and antiapoptotic functions (Samali and Cotter 1996).
Fig 5.
Immunolocalization of cleaved-caspase-3 on whole-mount embryos. Embryos treated with 1 mM CdCl2, respectively, at blastula (A–C) and gastrula (E–G) stages for 15, 21, and 24 hours, incubated with anti-cleaved-caspase-3 antibody. Control embryos (34 and 44 hours of development, respectively) incubated with anti-cleaved-caspase-3 (D, H)
In addition, we incubated embryos soon after fertilization with Z-DEVD-FMK, specific caspase-3 inhibitor (Thornberry and Lazebnik 1998), and at blastula stage we treated with 1 mM CdCl2 for 24 hours in the presence of the inhibitor. Immunocytochemical experiments demonstrated that the inhibitor hampered both the caspase-3 activity and the cleavage of the death substrates α-fodrin and lamin A. However, embryos developed better than those treated with cadmium but were delayed, probably because cadmium induces other cellular damages besides apoptosis (Fig 6).
Fig 6.
Immunolocalization on whole-mount embryos of cleaved-caspase-3 cleaved-α-fodrin and cleaved-lamin A. Embryos treated with Z-DEVD FMK since fertilization and with 1 mM CdCl2 for 24 hours since blastula stage and reacted, respectively, with anti-cleaved-caspase-3, anti-cleaved-α-fodrin, and anti-cleaved-lamin A antibodies (A, D, G). Embryos treated with 1 mM CdCl2 for 24 hours since blastula stage and reacted, respectively, with anti-cleaved-caspase-3, anti-cleaved-α-fodrin, and anti-cleaved-lamin A antibodies (B, E, H). Control embryos (38 hours of development) treated with Z-DEVD FMK since fertilization and reacted, respectively, with anti-cleaved-caspase-3, anti-cleaved-α-fodrin, and anti-cleaved-lamin A antibodies (C, F, I)
DISCUSSION
Morphological observations of P. lividus embryos treated with 1 mM CdCl2 had pointed out the occurrence of aberrations and delay in development. The malformations increased during the time of exposure. On the other hand, the AAS analyses of cadmium absorption demonstrated that the amount of metal incorporated greatly increased during the treatment.
It has been previously demonstrated that cadmium removal from culture medium, after incubation for more than 15 hours (with 1 mM CdCl2), did not allow rescuing of normal embryo development, suggesting that prolonged treatments induced irreversible damage (Roccheri et al 2004).
It is well known that sea urchin embryos use a typical protective strategy against many kinds of stresses (Roccheri et al 1981, 1988, 1993; Casano et al 1998; Gianguzza et al 2000; Roccheri et al 2000, 2001; Casano et al 2003). The fact that the synthesis of stress proteins (Hsps) after 24 hours of cadmium treatment reached plateau (not shown) suggested the existence of a threshold of the cell ability to self-defense. Hence, it may be hypothesized that a prolonged exposure to the metal could induce a toxicity level that would not allow embryos to defend themselves only by Hsps synthesis.
Many organisms can activate mechanisms of cellular programmed death in response to an excessive accumulation of toxic metals like cadmium (Samali and Cotter 1996). On the other hand, in sea urchin embryos apoptotic processes can be induced by stresses or can be physiologically activated in specific developmental stages. If cadmium insult is not too strong, P. lividus embryos restore normal development (Roccheri et al 2004), probably because only a few cells are damaged and removed through apoptosis, allowing the restoring of the physiological morphology. Hence, apoptosis can be considered as part of a defense strategy. In contrast, if cadmium insult were much higher, the cells addressed to death would be more numerous and the damaged DNA molecules so high that the normal development could not be restored. In fact, TUNEL assays demonstrated that DNA fragmentation depended on cadmium concentration. In addition the activation of caspase-3, one of the key molecules of apoptosis, increased in a time-dependent way. In fact, an increase of caspase-3 cleaved form and a decrease of zymogene were shown by immunocytochemistry during cadmium exposure. In the same way the cleavage of caspase-3 substrates, α-fodrin and of lamin A increased. It is well known that α-fodrin is a universally expressed membrane-associated cytoskeleton protein, important both in the maintenance of membrane structures and in the support of cell surface functions. The cleavage of α-fodrin, a target of caspases, leads to membrane malfunctioning and cell shrinkage (Vanags et al 1996). Lamin A, a nuclear membrane structural protein, is essential for the cell cycle control, DNA replication, and chromatin organization. Cleavage of lamin A causes nuclear deregulation and is involved in cell death (Orth et al 1996). The inhibition of the caspase-3 activity resulted in the hamper of α-fodrin and lamin A cleavage and in a reduction of DNA fragmentation.
Moreover, we found that the activity of caspase-3 was higher in embryos exposed to the metal since fertilization than in embryos exposed starting from blastula and gastrula stages for the same periods of time. Such apparent discrepancy can be explained considering that heat shock proteins, synthesized by sea urchin embryos in response to stress only after blastula stage (Roccheri et al 1981), were able to protect, at least partially, the embryos from damages and/or apoptosis.
In conclusion, we demonstrated that cadmium at cytotoxic concentrations, through caspase-3, causes apoptosis that can be repressed by specific inhibitors. In late developmental stages, Hsps interfere with apoptosis. Hence, cadmium interferes with development or by activating cell death pathways through caspase-3 or by other caspases or other mechanisms until now unknown, as suggested by the fact that after inhibition of caspase-3, the embryos showed a severe developmental delay.
In addition, in this paper we demonstrate that sea urchin embryos are able to accumulate cadmium during time; thus we can hypothesize that exposure to even low environmental concentrations could also cause cytotoxic effects. Therefore, sea urchin, an ideal animal model for studying the effects of sea pollution, could represent a good bioindicator for testing environmental cadmium pollution.
Acknowledgments
We thank G. Morici for computing assistance and data elaboration and S. Oddo for language revision. We are deeply indebted to Prof. Silvana Filosa (Univ. of Napoli) for achievement of AAS experiments. This work was supported by grants from MURST (ex 60% and COFIN 2004 prot. 2004057282_003).
REFERENCES
- Au DW, Reunov AA, Wu RS. Reproductive impairment of sea urchin upon chronic exposure to cadmium. Part II: Effects on sperm development. Environ Pollut. 2001;111:11–20. doi: 10.1016/s0269-7491(00)00036-1.0269-7491(2001)111[0011:RIOSUU]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Casano C, Roccheri MC, Maenza L, Migliore S, Gianguzza F. Sea urchin deciliation induces thermoresistance and activates the p38 mitogen-activated protein kinase pathway. Cell Stress Chaperones. 2003;8:70–75. doi: 10.1379/1466-1268(2003)8<70:sudita>2.0.co;2.1466-1268(2003)008[0070:SUDITA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano C, Roccheri MC, Onorato K, Cascino D, Gianguzza F. Deciliation: a stressful event for Paracentrotus lividus embryos. Biochem Biophys Res Commun. 1998;248:628–634. doi: 10.1006/bbrc.1998.9032.0006-291X(1998)248[0628:DASEFP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fernandez N, Beiras R. Combined toxicity of dissolved mercury with copper, lead and cadmium on embryogenesis and early larval growth of the Paracentrotus lividus sea urchin. Ecotoxicology. 2001;10:263–271. doi: 10.1023/a:1016703116830.0963-9292(2001)010[0263:CTODMW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Galan A, Garcia-Bermejo ML, Troyano A, Vilaboa NE, De Blas E, Kazanietz MG, Aller P. Stimulation of p38 mitogen-activated protein kinase is an early regulatory event for the cadmium-induced apoptosis in human promonocytic cells. J Biol Chem. 2000;275:11418–11424. doi: 10.1074/jbc.275.15.11418.0021-9258(2000)275[11418:SOPMPK]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gianguzza F, Ragusa MA, Roccheri MC, Di Liegro I, Rinaldi AM. Isolation and characterization of a Paracentrotus lividus cDNA encoding a stress-inducible chaperonin. Cell Stress Chaperones. 2000;5:87–89. doi: 10.1379/1466-1268(2000)005<0087:iacoap>2.0.co;2.1466-1268(2000)005[0087:IACOAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeebu SS, Liu J, Klaassen CD. Cadmium-induced apoptosis in mouse liver. Toxicol Appl Pharmacol. 1998;149:203–209. doi: 10.1006/taap.1997.8334.0041-008X(1998)149[0203:CAIML]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hamada T, Tanimoto A, Sasaguri Y. Apoptosis induced by cadmium. Apoptosis. 1997;2:359–367. doi: 10.1023/a:1026401506914.1360-8185(1997)002[0359:AIBC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24:1–51.0355-3140(1998)024[0001:HEOCEA]2.0.CO;2 [PubMed] [Google Scholar]
- Kobayashi N, Okamura H. Effects of heavy metals on sea urchin embryo development. 1. Tracing the cause by the effects. Chemosphere. 2004;55:1403–1412. doi: 10.1016/j.chemosphere.2003.11.052.0045-6535(2004)055[1403:EOHMOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Latchman DS 1999 Stress proteins: an overview. In: Stress Proteins, ed. Latchman DS. Springer, New York, 1–7. [Google Scholar]
- Lesser MP, Kruse VA, Barry TM. Exposure to ultraviolet radiation causes apoptosis in developing sea urchin embryos. J Exp Biol. 2003;206:4097–4103. doi: 10.1242/jeb.00621.0022-0949(2003)206[4097:ETURCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem. 1996;271:16443–16446.0021-9258(1996)271[16443:TIPMIA]2.0.CO;2 [PubMed] [Google Scholar]
- Pellerito C, D'Agati P, Fiore T, Mansueto C, Mansueto V, Stocco G, Naji L, Pellerito L. Synthesis, structural investigations on organotin(IV) chlorin-e6 complexes, their effect on sea urchin embryonic development and induced apoptosis. J Inorg Biochem. 2005;99:1294–1305. doi: 10.1016/j.jinorgbio.2005.03.002.0162-0134(2005)099[1294:SSIOOC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rainbow PS, Black WH. Cadmium, zinc and the uptake of calcium by two crabs, Carcinus maenas and Eriocheir sinensis. Aquat Toxicol. 2005;72:45–65. doi: 10.1016/j.aquatox.2004.11.016.0166-445X(2005)072[0045:CZATUO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Agnello M, Bonaventura R, Matranga V. Cadmium induces the expression of specific stress proteins in sea urchin embryos. Biochem Biophys Res Commun. 2004;321:80–87. doi: 10.1016/j.bbrc.2004.06.108.0006-291X(2004)321[0080:CITEOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Barbata G, Cardinale F, Tipa C, Bosco L, Oliva OA, Cascino D, Giudice G. Apoptosis in sea urchin embryos. Biochem Biophys Res Commun. 1997;240:359–366. doi: 10.1006/bbrc.1997.7540.0006-291X(1997)240[0359:AISUE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Cascino D, Giudice G. Two-dimensional electrophoretic analysis of stress proteins in Paracentrotus lividus. J Submicrosc Cytol Pathol. 1993;25:173–179.1122-9497(1993)025[0173:TEAOSP]2.0.CO;2 [PubMed] [Google Scholar]
- Roccheri MC, Di Bernardo MG, Giudice G. Synthesis of “heat shock” proteins in developing sea urchins. Dev Biol. 1981;83:173–177. doi: 10.1016/s0012-1606(81)80020-6.1095-564X(1981)083[0173:SOHSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, La Rosa M, Ferraro MG, Cantone M, Cascino D, Giudice G, Sconzo G. Stress proteins by zinc ions in sea urchin embryos. Cell Differ. 1988;24:209–214. doi: 10.1016/0045-6039(88)90052-8.0045-6039(1988)024[0209:SPBZII]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Onorato K, Tipa C, Casano C. EGTA treatment causes the synthesis of Hsps in sea urchin embryos. Mol Cell Biol Res Commun. 2000;3:306–311. doi: 10.1006/mcbr.2000.0230.1522-4724(2000)003[0306:ETCTSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Patti M, Agnello M, Gianguzza F, Carra E, Rinaldi AM. Localization of mitochondrial Hsp56 chaperonin during sea urchin development. Biochem Biophys Res Commun. 2001;287:1093–1098. doi: 10.1006/bbrc.2001.5503.0006-291X(2001)287[1093:LOMHCD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roccheri MC, Tipa C, Bonaventura R, Matranga V. Physiological and induced apoptosis in sea urchin larvae undergoing metamorphosis. Int J Dev Biol. 2002;46:801–806.0214-6282(2002)046[0801:PAIAIS]2.0.CO;2 [PubMed] [Google Scholar]
- Russo R, Bonaventura R, Zito F, Schroeder HC, Muller I, Muller WEG, Matranga V. Stress to cadmium monitored by metallothionein gene induction in Paracentrotus lividus embryos. Cell Stress Chaperones. 2003;8:232–241. doi: 10.1379/1466-1268(2003)008<0232:stcmbm>2.0.co;2.1466-1268(2003)008[0232:STCMBM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:167–170. doi: 10.1006/excr.1996.0070.0014-4827(1996)223[0167:HSPIRT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schroder HC, Hassanein HM, and Lauenroth S. et al. 1999 Induction of DNA strand breaks and expression of HSP70 and GRP78 homolog by cadmium in the marine sponge. Suberites domuncula. Arch Environ Contam Toxicol. 36:47–55. [DOI] [PubMed] [Google Scholar]
- Scudiero R, Capasso C, De Prisco PP, Capasso A, Filosa S, Parisi E. Metal-binding proteins in eggs of various sea urchin species. Cell Biol Int. 1994;18:47–53. doi: 10.1006/cbir.1994.1006.1065-6995(1994)018[0047:MPIEOV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Scudiero R, Capasso C, Del Vecchio-Blanco F, Savino G, Capasso A, Parente A. Isolation and primary structure determination of a metallothionein from Paracentrotus lividus (Echinodermata, Echinoidea) Comp Biochem Physiol B Biochem Mol Biol. 1995;111:329–336. doi: 10.1016/0305-0491(94)00216-h.1096-4959(1995)111[0329:IAPSDO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312.0193-4511(1998)281[1312:CEW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tsangaris GT, Tzortzatou-Stathopoulou F. Cadmium induces apoptosis differentially on immune system cell lines. Toxicology. 1998;128:143–150. doi: 10.1016/s0300-483x(98)00032-8.0300-483X(1998)128[0143:CIADOI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vanags DM, Porn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075.0021-9258(1996)271[31075:PIIFCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Vega RL, Epel D. Stress-induced apoptosis in sea urchin embryogenesis. Mar Environ Res. 2004;58:799–802. doi: 10.1016/j.marenvres.2004.03.096.0141-1136(2004)058[0799:SAISUE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Xu C, Johnson JE, Singh PK, Jones MM, Yan H, Carter CE. In vivo studies of cadmium-induced apoptosis in testicular tissue of the rat and its modulation by a chelating agent. Toxicology. 1996;107:1–8. doi: 10.1016/0300-483x(95)03195-l.0300-483X(1996)107[0001:IVSOCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]