Abstract
Neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis have been termed “protein misfolding disorders.” These diseases differ widely in frequency and impact different classes of neurons. Heat shock proteins provide a line of defense against misfolded, aggregation-prone proteins and are among the most potent suppressors of neurodegeneration in animal models. Analysis of constitutively expressed heat shock proteins revealed variable levels of Hsc70 and Hsp27 in different classes of neurons in the adult rat brain. The differing levels of these constitutively expressed heat shock proteins in neuronal cell populations correlated with the relative frequencies of the previously mentioned neurodegenerative diseases.
INTRODUCTION
Neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis (ALS), are characterized by the accumulation of intracellular and extracellular protein aggregates and selective neuronal loss in the central nervous system. Increasing evidence supports the view that these neurodegenerative diseases have common molecular mechanisms associated with protein misfolding and aggregation; hence, they have been termed “protein misfolding disorders” (Muchowski and Wacker 2005). These diseases impact different classes of neurons. In Alzheimer's disease, neuronal loss is prominent in the entorhinal cortex and hippocampus and is accompanied by memory loss and dementia (Martin 1999). Parkinson's disease is characterized by a loss of dopaminergic neurons in the substantia nigra and is accompanied by muscle rigidity, brandykinesia, and resting tremor (Schapira and Olanow 2004). In ALS, motor neurons of the spinal cord and motor cortex are selectively lost, resulting in progressive muscle wasting and weakness and culminating in paralysis and respiratory failure (Bruijn et al 2004).
Neurons are particularly vulnerable to the detrimental effects of misfolded and /or aggregated proteins because they are postmitotic and cannot dilute potentially toxic species through cell division; hence, misfolded proteins accumulate in neurons during aging (Muchowski and Wacker 2005). Alzheimer's disease, Parkinson's disease, and ALS impact different classes of neurons and differ widely in frequency (Martin 1999; Bruijn et al 2004; Schapira and Olanow 2004). This suggests that all types of neurons may not be equally susceptible to protein misfolding disorders that are the hallmarks of neurodegenerative diseases.
Heat shock proteins (Hsps) are the major molecular chaperones whose function is to mediate the proper folding of other proteins and to ensure that these proteins maintain their native conformations during conditions of stress (Becker and Craig 1994; Morimoto et al 1997). In addition, Hsps are required for protein trafficking to target organelles and to facilitate the transfer of misfolded proteins to the proteasome for degradation (Becker and Craig 1994). Hsps can be induced by various stresses such as heat shock, ischemia, hypoxia, heavy metals, and amino acid analogs. Some Hsps are expressed constitutively in unstressed cells (Morimoto et al 1997). Mammalian Hsps have been classified into families on the basis of their molecular weight including Hsp110, Hsp90, Hsp70, Hsp60, Hsp40, and Hsp27. The Hsp70 family is comprised of constitutively expressed Hsc70 and stress-inducible Hsp70. Because of the functions of Hsps in protein quality control, recent studies have investigated the role of these proteins in neurodegenerative diseases and found that Hsps provide a line of defense against misfolded or aggregation-prone proteins and are among the most potent suppressors of neurodegeneration in animal models (Sherman and Goldberg 2001; Muchowski and Wacker 2005).
Previously we have shown that constitutively expressed Hsc70 is enriched in the mammalian nervous system (Manzerra et al 1997). Here, we examine levels of constitutively expressed Hsps in different classes of neurons that are affected in Alzheimer's disease, Parkinson's disease, and ALS. We find that motor neurons of the spinal cord show significantly higher levels of Hsc70 and Hsp27 than dopaminergic neurons of the substantia nigra, which in turn have higher Hsc70 expression than neurons of the entorhinal cortex and hippocampus. The overall level of constitutively expressed Hsps in different classes of neurons correlates with the frequency of Alzheimer's disease, Parkinson's disease, and ALS.
MATERIALS AND METHODS
Treatment of animals
All procedures using animals were approved by the Animal Care Committee of the University of Toronto and were in accordance with the guidelines established by the Canadian Council on Animal Care. The body temperature of adult male Wistar rats (42 days old) was raised 3.5°C above normal (38°C) by using a dry incubator set at 45°C. Body temperature of the animal was monitored with a rectal thermal probe (Physitemp Instruments, Clifton, NJ, USA) and maintained at the elevated temperature for 1 hour. After heat shock, the rats were placed at room temperature to recover for 24 hours.
Immunohistochemistry
Rats were anesthetized with sodium pentobarbital and perfused intracardially with cold 0.1 M phosphate-buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde in PBS. Brain and spinal cord tissues were removed and fixed overnight in 4% paraformaldehyde in PBS at 4°C. Tissue was then equilibrated with 20% sucrose in PBS, mounted in OCT embedding compound (Miles Inc., Elkhart, IN, USA), and frozen at −70°C until use. Cryostat sections (10 μm) were collected on gelatin-coated microscope slides and air dried for 3 h. Following rehydration in PBS-T buffer (0.1 M PBS, pH7.4, 0.2% Triton X-100, 0.1% BSA), tissue sections were blocked in 10% goat serum in PBS-T for 1 hour. Sections were incubated overnight with primary antibodies diluted in PBS-T: 1:500 mouse anti-NeuN (MAB377, Chemicon, Temecula, CA, USA) or 1:300 mouse anti-tyrosine hydroxylase (MAB5278, Chemicon) in combination with one of the following Hsps antibodies: rabbit 1:500 anti-Hsp110 (SPA-1103, StressGen Biotechnologies, Victoria, British Columbia, Canada), 1:500 mouse anti-Hsp90 (SPA-830, StressGen Biotechnologies), 1:1000 rat-anti-Hsc70 (SPA-815, StressGen Biotechnologies), 1:1000 rabbit anti-Hsp70 (SPA-812, StressGen Biotechnologies), 1:1000 rabbit anti-Hsp60 (SPA-805, StressGen Biotechnologies), 1:1000 rabbit anti-Hsp40 (SPA-400, StressGen Biotechnologies), or 1:1000 rabbit Hsp25 (SPA-801, StressGen Biotechnologies). After washing in PBS-T, sections were incubated for 2 hours with secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted in PBS-T consisting of Cy5-labeled 1:100 anti-mouse IgG and 1:200 Cy3-labeled anti-rabbit IgG or Rodamine-red-X labeled anti-rat IgG diluted in PBS-T for 2 hours. For nucleic acid staining, slides were then incubated with 2 μM Yo-pro-1 (Invitrogen, Carlsbad, CA, USA) in PBS-T for 30 minutes. Following washes in PBS-T, sections were mounted with antifading fluorescent mounting medium (DAKO, Glostrup, Denmark).
Image analysis
Quantitative immunohistochemistry with confocal microscopy and image analysis software has been used widely in biomedical research to compare fluorescent intensities representing proteins levels in selected image areas (Kretz et al 2004; Buttini et al 2005). Slides were analyzed using a LSM510 confocal microscope (Carl Zeiss Inc., Germany). Detection configurations were adjusted to obtain images with a pixel intensity within a linear range and were kept constant for different regions of the brain that were examined on each microscope slide. Image analysis was carried out with Zeiss LSM510 software (version 3.2 with SP2). NeuN, a neuronal specific marker (Mullen et al 1992), or tyrosine hydroxylase (TH) , a dopaminergic neuron marker, was used to outline the cell body of individual neurons, and fluorescent intensity of Hsps was measured within those areas as average intensity per pixel. Fifty neurons for each neuronal population were randomly selected for fluorescent intensity quantification. The immunohistochemical results are from 3 animal trials. Omission of either the primary or the secondary antibody resulted in a total loss of fluorescent signal.
Western blotting
Regions of the rat nervous system were harvested and kept at −70°C until use. Tissues were homogenized in ice-cold 0.32 M sucrose with a protease inhibitor cocktail containing 5 μg/mL of antipain, aprotinin, and leupetin (BioShop Canada Inc., Burlington, Ontario, Canada). Protein concentration of the homogenates was determined with a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein samples were solubilized by boiling in 2 × SDS loading buffer (100 mM Tris, pH 7.4, 4% SDS, 200 mM DTT, 20% glycerol, 0.2% bromophenol blue), separated by SDS-PAGE with 5% stacking gel and 10% separating gel, and transferred to a nitrocellulose membrane. After blocking for 1 hour with 5% fat-free milk powder in TBST (10 mM Tris, 250 mM NaCl, 0.5% Tween-20, pH 7.4), blots were incubated overnight at room temperature with primary antibodies diluted 1:5000 for Hsp110 (SPA-1103, StressGen Biotechnologies), 1:3000 for Hsp90 (SPA-830, StressGen Biotechnologies), 1:30 000 for Hsc70 (SPA-815, StressGen Biotechnologies), 1:5000 for Hsp70 (SPA-810, StressGen Biotechnologies), 1:5000 for Hsp60 (SPA-805, StressGen Biotechnologies), 1:5000 for Hsp40 (SPA-400, StressGen Biotechnologies) and 1:10 000 for Hsp25 (SPA-801, StressGen Biotechnologies). Following washes, blots were incubated with HRP-conjugated secondary antibodies (Sigma, St. Louis, MO, USA) for 2 hours at room temperature. Immunoactivity was visualized with ECL Western blot detection reagents (Amersham, Piscataway, NJ, USA). Data representative of 3 experimental repeats are shown.
Statistical analysis
The average fluorescent intensity of Hsps in entorhinal cortical and hippocampal neurons (n = 50) was standardized as 100%, and the fluorescent intensity of other populations of neurons (n = 50) was calculated accordingly. The results are expressed as the mean ± SD, and the statistical significance was assessed by 1-way analysis of variance.
RESULTS
Frequencies of neurodegenerative diseases
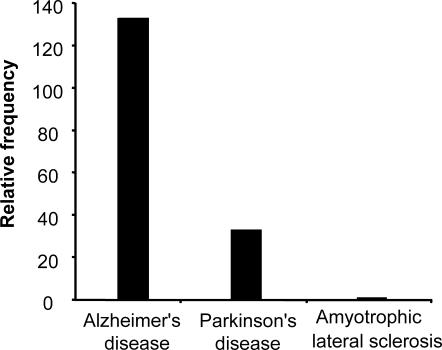
Neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and ALS have been termed as “protein misfolding disorders.” Hsps have been shown to provide a line of defense against misfolded proteins and are among the most potent suppressors of neurodegeneration in animal models (Muchowski and Wacker 2005). Alzheimer's disease, Parkinson's disease, and ALS affect approximately 4 million (Martin 1999), 1 million (Schapira and Olanow 2004), and 30 000 people (Bruijn et al 2004), respectively, in the United States, whose population was 281 421 906 in 2000 (US Census Bureau). Therefore, the occurrence rate per 100 000 is 1421 for Alzheimer's, 355 for Parkinson's, and 10.7 for ALS. Hence, Alzheimer's disease is 4-fold more frequent than Parkinson's disease and 133-fold more frequent than ALS, while Parkinson's is 33-fold more frequent than ALS (Fig 1).
Fig 1.
Relative frequencies of Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis (ALS). Alzheimer's disease is 4-fold more frequent than Parkinson's disease and 133-fold more frequent than ALS, while Parkinson's disease exhibits a frequency that is 33-fold greater than ALS. Calculations are based on the prevalence of these neurodegenerative diseases in the US population
Constitutive expression of Hsc70 and Hsp27 in different classes of neurons
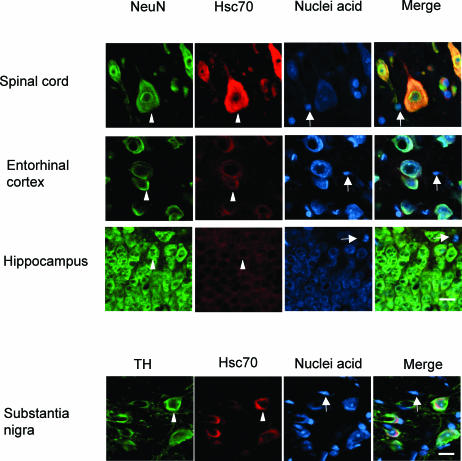
Our results demonstrate that different populations of neurons in the mammalian brain show widely differing levels of the constitutively expressed Hsc70 and Hsp27. As indicated in Figure 2a by the arrowheads, motor neurons in the rat spinal cord show high levels of Hsc70 compared to dopaminergic neurons of the substantia nigra, while neurons in the entorhinal cortex and hippocampus demonstrate even lower levels of Hsc70. Glial cells in these brain regions (indicated by the arrows) do not show a detectable signal for Hsc70. Neurons were identified by the neuron-specific marker NeuN or tyrosine hydroylase (TH) for dopaminergic neurons in the substantia nigra.
Fig 2.
Constitutive expression of heat shock proteins in different classes of neurons. (a) Constitutive Hsc70 expression pattern. As indicated by the arrowheads, motor neurons in the spinal cord, labeled with the neuron-specific marker NeuN, exhibited a high level of constitutively expressed Hsc70. Dopaminergic neurons of the substantia nigra, labeled with tyrosine hydroxylase (TH), showed moderate Hsc70 expression, whereas neurons in the entorhinal cortex and hippocampus exhibited low levels of Hsc70. Glial cells in these regions (indicated by the arrows) did not show a detectable signal for expression of constitutive Hsc70
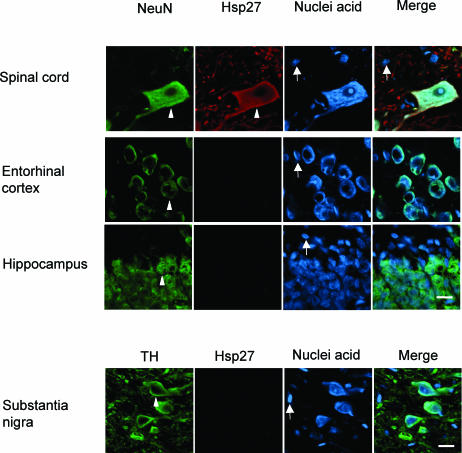
Hsp27 is constitutively expressed at high levels in motor neurons of the spinal cord but is not detectable in neurons of the entorhinal cortex, hippocampus, and substania nigra (Fig 2b, indicated by arrowheads). Glial cells (indicated by arrows in Fig 2b) do not show a detectable signal for constitutive expression of Hsp27.
Fig 2.
Continued. (b) Constitutive Hsp27 expression patterns. As indicated by the arrowheads, motor neurons of the spinal cord demonstrated a high level of constitutive Hsp27 expression. An Hsp27 signal was not detectable in dopaminergic neurons of the substantia nigra and neurons of the entorhinal cortex and hippocampus. Glial cells (indicated by arrows) did not show detectable constitutive Hsp27 in any of these regions. Bar = 16 μm
Quantitative analysis of constitutively expressed Hsps in neurons
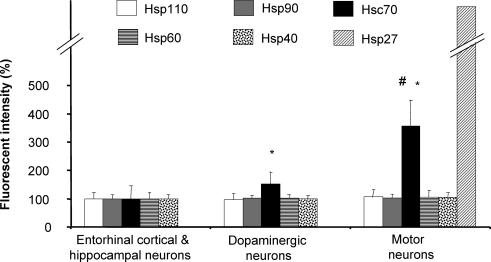
A quantitative analysis confirmed that Hsc70 is present at significantly higher levels in motor neurons of the spinal cord compared to dopaminergic neurons of the substania nigra, which in turn express higher levels of Hsc70 than neurons of the enthorhinal cortex and hippocampus (Fig 3). Other constitutively expressed Hsps, such as Hsp110, Hsp90, Hsp60, and Hsp40, do not demonstrate significant differences in levels in the various classes of neurons.
Fig 3.
Quantitative analysis of constitutively expressed heat shock proteins in neuronal populations. Motor neurons, that are impacted in ALS, have a higher defense capacity against protein-misfolding disorders because of their elevated levels of Hsc70 and Hsp27 compared to dopaminergic neurons of the substantia nigra that are affected during Parkinson's disease, which in turn have higher levels of these constitutively expressed Hsps than entorhinal cortical and hippocampal neurons that are impacted in Alzheimer's disease. Fluorescence intensity is shown relative to a 100% value for entorhinal cortical and hippocampal neurons. Mean and standard deviation are shown. (n = 50; *, P < 0.05, compared to entorhinal cortical and hippocampal neurons; #, P < 0.05, compared to dopaminergic neurons)
Effect of thermal stress on neural expression of Hsps
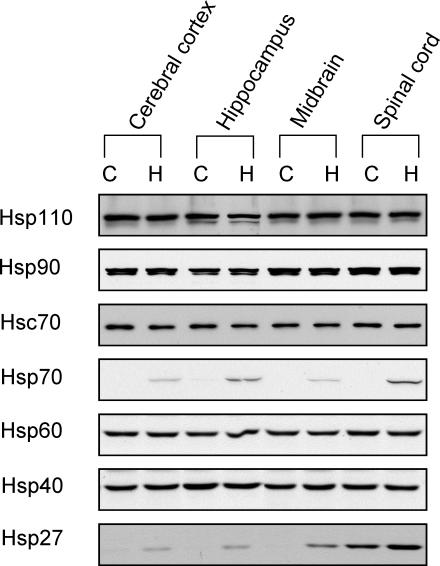
To confirm that Hsc70 is a constitutively expressed Hsp that is not stress inducible, the effect of elevation of body temperature was examined. A Western blot analysis revealed that thermal stress did not induce changes in levels of the constitutively expressed Hsp110, Hsp90, Hsc70, Hsp60, or Hsp40 in various regions of the rat nervous system (Fig 4). However, thermal stress induced expression of stress-inducible Hsp70 and Hsp27.
Fig 4.
Western blot analysis of the effects of heat stress on the expression of heat shock proteins in regions of rat nervous system. Hsp110, Hsp90, Hsc70, Hsp60, and Hsp40 were constitutively expressed in the cerebral cortex, hippocampus, midbrain, and spinal cord. Thermal stress did not alter levels of expression of these constitutive Hsps; however, it did induce expression of Hsp70 and Hsp27. C, control; H, heat shock (24-hour recovery)
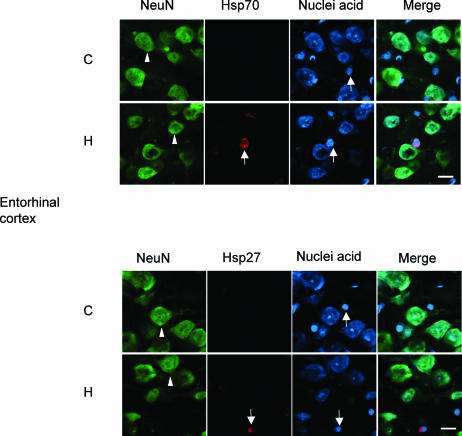
Cellular expression pattern of stress-inducible Hsp70 and Hsp27
An immunocytochemical investigation was undertaken to explore if elevation of body temperature affected the level of Hsps in the neuronal cell populations that were under investigation. Immunocytochemistry revealed that thermal stress induced Hsp70 and Hsp27 in glial cells (arrows) and not in neurons (arrowheads) in all neural regions that were examined. Representative data are shown in Figure 5 for the entorhinal cortex. A similar pattern of glial induction in response to thermal stress and lack of induction in neuronal cells was evident in the hippocampus, substantia nigra, and spinal cord (data not shown). Our previous reports have fully documented this glial response to hyperthermic stress (reviewed by Brown and Sharp 1999).
Fig 5.
Cellular expression pattern of stress-inducible Hsp70 and Hsp27 in the entorhinal cortex. Thermal stress induced expression of stress-inducible Hsp70 (upper panel) and Hsp27 (lower panel) in glial cells (indicated by arrows) but not in neuronal cells (indicated by arrowheads) in the entorhinal cortex. C, control; H, heat shock (24-hour recovery). Bar = 16 μm
DISCUSSION
Evidence is accumulating to suggest that neurodegenerative diseases are caused by a combination of events that impair normal neuronal function. The intracellular and extracellular accumulation of protein aggregates is the hallmark of these diseases. Although the specific proteins that aggregate in particular neurodegenerative disorders are unrelated in size and primary amino acid sequence, the characteristic lesions of each disease typically contain fibrillar, amyloid-like structures with common biochemical features (Dobson 2003). While it is controversial whether these protein aggregates are pathogenic, inert, or even protective, many studies indicate that intermediates formed during the aggregation process, termed diffusible oligomers or protofibrils, are potent neurotoxins that may trigger neuronal dysfunction by initiating a cascade of events that culminates in neuronal degeneration (Ross and Poirier 2004; Muchowski and Wacker 2005). Toxic diffusible oligomers from different neurodegenerative diseases appear to share conformational similarities. It has been demonstrated that a single monoclonal antibody that recognizes a common conformational epitope displayed by several disease-associated protein oligomers, including Aβ of Alzheimer's disease, α-synuclein of Parkinson's disease, and Huntingtin of Huntington's disease, can block the toxicity of these oligomers when applied to cultured cells (Kayed et al 2003). These commonalities suggest that a conserved mechanism of pathogenesis may connect phenotypically diverse neurodegenerative diseases (Ross and Poirier 2004; Muchowski and Wacker 2005). The accumulation of misfolded proteins, protofibril formation, and ubiquitin-proteosome system dysfunction represent unifying events in these neurodegenerative diseases (Bossy-Wetzel et al 2004).
Hsps acting as molecular chaperones have essential roles in many cellular processes associated with protein quality control, including protein folding, targeting, transport, and degradation (Becker and Craig 1994; Morimoto et al 1997). This has led to the hypothesis that Hsps may provide a line of defense against protein misfolding in neurodegenerative diseases. Recent evidence from in vitro and in vivo studies indicates that Hsps are potent suppressors of neurodegeneration and are therefore promising therapeutic targets for neurodegenerative disorders (Sherman and Goldberg 2001; Muchowski and Wacker 2005).
In Alzheimer models, overexpression of Hsp70 rescues neurons from Aβ42 mediated toxicity (Magrane et al 2004). Hsp27 decreases the level of hyperphosphorylated Tau, another Alzheimer-related disease protein, and suppresses Tau-mediated cell death (Shimura et al 2004). Knockdown of Hsp70 and Hsp90 by RNAi increases the accumulation of insoluble, aggregated Tau and impairs the association of Tau with microtubules (Dou et al 2003).
Expression of human Hsp70 suppresses α-synuclein-mediated dopaminergic neuron toxicity in a Drosophila model of Parkinson's disease (Auluck et al 2002). Hsp70 overexpression protects cultured cells from α-synuclein-dependent toxicity and influences the aggregation process of α-synuclein in a transgenic mouse model (Klucken et al 2004). Overexpression of Hsp27 has a potent protective effect against α-synuclein-induced cell death in mammalian neuronal cells (Zourlidou et al 2004).
In ALS models, intranuclear co-microinjection of the expression vectors for Hsp70 and mutant SOD1, one of the familial ALS disease proteins, into primary cultured motor neurons reduces the toxicity of mutant SOD1 and enhances motor neuron survival (Bruening et al 1999). Hsp27 and Hsp70, overexpressed in combination, have a potent protective effect against SOD1-mutant-induced cell death in mammalian neuronal cells (Patel et al 2005). Arimoclomol, a small molecule that acts as a co-inducer of the heat shock response, increases Hsp70 expression in spinal cord motor neurons and improves behavioral phenotype, prevents neuronal loss, and extends survival rate in transgenic ALS mice (Kieran et al 2004).
Many of these studies have focused on the effects of engineering elevations of inducible Hsp70 on neuronal protection. Hsc70, the constitutively expressed protein of the Hsp70 family, shares about 90% amino acid homology with Hsp70 and has almost identical molecular structure and indistinguishable biochemical functions with Hsp70 (Welch 1992). Interfering with endogenous Hsc70 accelerates dopaminergic neuronal loss in an α-synuclein transgenic Drosophila model for Parkinson's disease, suggesting that constitutively expressed Hsc70 protects dopaminergic neurons against α-synuclein toxicity by delaying the onset of degeneration (Auluck et al 2002). The previously mentioned studies emphasize the defense capacity of Hsps against neurodegeneration and suggest that the overall levels of Hsps in neurons, including constitutively expressed members, may be intimately related to the age of onset, kinetics of progression, and severity of pathological and behavioral phenotypes in experimental models of neurodegenerative diseases (Muchowski 2002).
Neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and ALS exhibit common molecular mechanisms associated with protein misfolding, yet they differ widely in frequency and impact different populations of neurons. Hsps provide a line of defense against misfolded, aggregation-prone proteins and are among the most potent suppressors on neurodegeneration in animal models (Sherman and Goldberg 2001; Muchowski and Wacker 2005). We suggest that differing levels of constitutively expressed Hsc70 and Hsp27 in different neuronal cell populations confers a variable buffering capacity against protein misfolding disorders that correlates with the relative frequencies of these neurodegenerative disorders. The defense capacity against protein misfolding disorders is higher in motor neurons in the spinal cord because of their elevated levels of constitutively expressed Hsc70 and Hsp27, compared to neurons of the substantia nigra, which in turn have a higher defense capacity than neurons of the entorhinal cortex and hippocampus. Levels of these constitutively expressed Hsps may correlate with the low frequency of ALS that impacts motor neurons, whereas a higher disease frequency is apparent for Parkinson's disease that affects neurons of the substania nigra and an even higher frequency for Alzheimer's disease that impacts neurons of the entorhinal cortex and hippocampus. Following stress, such as temperature elevation, the induction of stress-inducible Hsps is selective, occurring primarily in glial cells rather than in neurons. Neuron may rely on their constitutive levels of Hsc70 and Hsp27 as a “preprotection” mechanism for defense against protein misfolding triggered by stressful stimuli or that associated with neurodegenerative diseases. Variable levels of these constitutive Hsps in different neuronal cells types may modulate their relative susceptibility to protein misfolding disorders. Our present investigation has been carried out in the rat brain. It would be beneficial to extend the analysis to relevant neuronal cell populations in the human brain.
Acknowledgments
This study was supported by grants from NSERC Canada to I. Brown who also holds a Canada Research Chair. We thank Raymond Or for technical advice on confocal microscopy and Starlee Lively, Ramona Cheung, and Ari Chow for helpful suggestions.
REFERENCES
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389.0193-4511(2002)295[0865:CSOATI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2.0014-2956(1994)219[0011:HPAMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, and Lipton SA 2004 Molecular pathways to neurodegeneration. Nat Med. 10(Suppl). S2–S9. [DOI] [PubMed] [Google Scholar]
- Brown IR, Sharp FR 1999 The cellular stress response in brain. In: Handbook of Experimental Pharmacology: Stress Proteins, ed Latchman DS. Springer-Verlag, New York, 136:243–263. [Google Scholar]
- Bruening W, Roy J, Giasson B, Figlewicz DA, Mushynski WE, Durham HD. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J Neurochem. 1999;72:693–699. doi: 10.1046/j.1471-4159.1999.0720693.x.0022-3042(1999)072[0693:UOPCPV]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244.0147-006X(2004)027[0723:UTMIIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Buttini M, Masliah E, and Barbour R. et al. 2005 Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease. J Neurosci. 25:9096–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261.1476-4687(2003)426[0884:PFAM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, and Tanemura K. et al. 2003 Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A. 100:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469.0193-4511(2003)300[0486:CSOSAO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kieran D, Kalmar B, Dick JR, Riddoch-Contreras J, Burnstock G, Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021.1078-8956(2004)010[0402:TWAACO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200.0021-9258(2004)279[25497:HRAAAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, and Wehrenberg U. et al. 2004 Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 24:5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004.0270-6474(2004)024[1700:HSPPIT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170:130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P.0021-9541(1997)170[0130:TDIHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507.1533-4406(1999)340[1970:MBOTND]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997;32:17–29.0071-1365(1997)032[0017:THRRAF]2.0.CO;2 [PubMed] [Google Scholar]
- Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. doi: 10.1016/s0896-6273(02)00761-4.0896-6273(2002)035[0009:PMAFAN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587.1471-0048(2005)006[0011:MONBMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201.1011-6370(1992)116[0201:NANSNP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Patel YJ, Payne Smith MD, de Belleroche J, Latchman DS. Hsp27 and Hsp70 administered in combination have a potent protective effect against FALS-associated SOD1-mutant-induced cell death in mammalian neuronal cells. Mol Brain Res. 2005;134:256–274. doi: 10.1016/j.molbrainres.2004.10.028.0169-328X(2005)134[0256:HAHAIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ross CA, Poirier MA 2004 Protein aggregation and neurodegenerative disease. Nat Med. 10(Suppl). S10–S17. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. J Am Med Assoc. 2004;291:358–364. doi: 10.1001/jama.291.3.358.0098-7484(2004)291[0358:NIPDMM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5.0896-6273(2001)029[0015:CDAUPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279:17957–17962. doi: 10.1074/jbc.M400351200.0021-9258(2004)279[17957:BOTTHS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063.0031-9333(1992)072[1063:MSRCPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zourlidou A, Payne Smith MD, Latchman DS. HSP27 but not HSP70 has a potent protective effect against alpha-synuclein-induced cell death in mammalian neuronal cells. J Neurochem. 2004;88:1439–1448. doi: 10.1046/j.1471-4159.2003.02273.x.0022-3042(2004)088[1439:HBNHHA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]