Abstract
Expression of Hsp70 is an endogenous mechanism by which living cells adapt to stress and the protection of Hsp70 may interfere with the apoptotic machinery in a variety of ways. Here, we observed the change of Hsp70 expression in rat myocardium under stress and explored the protective effect of Hsp70 on the Fas-mediated pathway to cardiomyocyte apoptosis. The results showed that restraint stress led to cardiac dysfunction and structural damage of the myocardium, as well as activation of the Fas pathway. A similar increase in the Fas expression level, caspase-8/3 activity, and the apoptotic rate of the cardiomyocyte also were found, which indicated that Fas-mediated apoptosis of cardiomyocytes might be one of the mechanisms of cardiomyocyte injury induced by stress. Changes in Hsp70 levels and distribution occurred during the stress process, which correlated with the severity of myocardium injury. Heat preconditioning induced the upregulation of Hsp70 synthesis, which in turn may have mitigated subsequent restraint stress–induced damage, including electrocardiography (ECG) abnormality, myocardium damage, and cell death. Moreover, Hsp70 overexpression induced by heat preconditioning had no effect on Fas expression in the cardiomyocyte, but could inhibit activation of caspase-8/3 induced by the Fas signaling pathway and, as a result, prevent cell apoptosis. These results suggest that Hsp70 is capable of protecting the cardiomyocyte from stress-induced injury by inhibiting Fas-mediated apoptosis, and Hsp70 could be considered a target in future drugs to prevent cardiovascular injury caused by stress.
INTRODUCTION
Stress is defined as an adaptive physiological response to disruption of homeostasis. Moderate stress load can invoke protection, though stress overload can cause injury or contribute to diseases, such as diabetes, gastric ulcer, obesity, cancer, and Parkinson's disease. Data suggest a relationship between stress and the risk of cardiovascular disease, and most experts agree that stress does contribute to heart disease, high blood pressure, high cholesterol, and other cardiac risk factors. A number of studies have shown that the cardiovascular system is the major organ targeted by stress, and that stress is the most important etiologic factor in cardiovascular diseases. Moreover, whereas cardiomyocyte death is considered an important cellular basis for stress-induced cardiovascular injury and disease (Feuerstein and Young 2000), the mechanism of adaptation to stressful events remains unclear.
Heat shock protein 70 (Hsp70) works as a molecular chaperone and plays an important role in the adaptive response. A cellular level stress response has been observed in nearly all organisms, and its characteristic feature is the induction of Hsps (Basu et al 2001). Hsps encompass several families of cytoprotective proteins. The Hsp70 family is highly evolutionarily conserved and both its chaperone function in general and in the cardiovascular system have been studied. Indeed, an inducible isoform of Hsp70 has been shown to protect cells against apoptosis induced by thermal stress, oxidative stress, radiation, and chemical toxins. Hsp70 can interfere directly with the apoptotic machinery in a variety of ways, but the potential mechanisms responsible for the protective effects of Hsp70 on stress-induced injury remain unknown.
Apoptosis plays a pivotal role in the loss of cells not only during physiological phenomena but also in many pathological processes (Majno and Joris 1995). Evidence is accumulating that the apoptotic mechanism is involved in the loss of myocytes in various human heart disorders (Kawano et al 1994; Narula et al 1996; Olivetti et al 1997). Apoptosis may occur by two fundamental pathways: the death receptor pathway and the mitochondrial pathway (Yoon and Gores 2002). The Fas antigen (Fas/APO-1/ CD95) is a cell surface receptor that belongs to the tumor necrosis factor receptor superfamily that triggers apoptosis in sensitive cells when bound to the Fas ligand (FasL) or agonistic anti-Fas antibodies (anti-Fas Ab). The Fas signaling pathway involves a series of intracellular protein-protein interactions that result in a cascade of protease activation, cleavage of cellular substrates, and cell death (Nagata 1997; Ashkenazi and Dixit 1998).
Previous studies suggested that the Fas system plays an important role in the regulation of physiological homeostasis in the immune system. Subsequently, an immunologic mechanism has been confirmed in some cardiovascular disease, leading scientists to pay more attention to the interplay of the Fas pathway with cardiovascular disease. Investigators found a relationship between the overexpression of Fas induced by intense exercise with cardiomyopathy and myocardial infarction and found that the Fas-pathway also participates in cardiomyocyte apoptosis induced by antitumor drugs (Kajstura et al 1996; Nakamura et al 2000; Kalivendi et al 2005). The presence of Hsp70 inhibits Fas-mediated apoptosis in tumor cells, but it is uncertain whether induction of Fas by stress is involved in cardiomyocyte apoptosis. The precise means by which Hsp70 protects the cardiomyocyte against damage caused by stress remains enigmatic.
Against this background, the aims of this study were to observe the change of Hsp70 expression and distribution in the rat myocardium under restraint stress and the mediation of the Fas pathway to cardiomyocyte apoptosis induced by stress. Furthermore, this study explored the effect of Hsp70 on Fas-mediated cardiomyocyte apoptosis and its molecular mechanisms. Our findings suggest that Hsp70 is capable of protecting cardiomyocytes from stress-induced injury through inhibiting Fas-mediated apoptosis. Hsp70, therefore, should be explored as a potential therapy against cardiovascular injury caused by stress.
MATERIALS AND METHODS
Experimental animal model of restraint stress
Using the method of Galea et al (1997), with slight modifications, adult male Wistar rats weighing 180–200 g were divided randomly into 7 groups (6 groups of differing degrees of restraint and 1 control group). The restrained rats were put in a specially built size-manipulable cabin at various designated stress loads. All rats were housed in a pathogen-free environment at room temperature ([RT]; 22–25 °C) and maintained on rat food and water ad libitum before restraint stress. In the acute test, the rats were restrained for 1, 3, and 6 hours and were sacrificed 2 hours after release; in the chronic test the rats were restrained for 6 hours per day from 9:00 AM to 13:00 PM for 2, 3, and 4 weeks and were sacrificed 24 hours after release.
Cardiomyocyte model of stress
Stress can activate the hypothalamic pituitary adrenocortical axis and the sympathetic-adrenomedullary system, and the increased content of glucocorticoid (GC) and catecholamine in plasma are considered as a significant biological basis for evaluating stress load. The major component of GC is corticosterone (CORT) in rodents.
The cardiomyocytes were isolated from neonatal Wistar rats (48–72 hours old) by the trypsin digestion method as previously described (Qian et al 2004). After being cultured for 5 days, the culture bottle of ∼95% confluent cardiomyocytes, 90% of which beat about 100 times per minute, were selected for in vitro experiments. To establish the cell model of stress, the cardiomyocytes were cultured in serum-free minimum essential medium (MEM) for 2 hours before stress and then were incubated with CORT at different concentrations (10−5, 10−6, 10−7) and with 10−6 mol/L CORT for 3, 6, 12, and 24 h, respectively.
The cardiac function was evaluated by electrocardiography recording
Rats were anesthetized with 1% sodium pentobarbital (45 mg/kg) by intraperitoneal injection and kept in a supine position. Limb-lead electrocardiography (ECG) was recorded with an 8-channel physiological recorder (Nihon Kohden Q1-160, Tokyo, Japan); P-R interval, Q-T interval, R wave, and T wave were measured to detect the effect of restraint stress on rat cardiac function.
Myocardium ultrastructure was observed by electron microscopy
The left ventricular muscle (1 mm3) from restraint-stressed rats was fixed for 2 hours in precooled 2.5% phosphate-buffered Glutaral and 1% phosphate-buffered osmium tetroxide in turn, dehydrated in precooled, graded ethanol series, and then soaked in Epon812 embedding medium/100% aceton (1:1) for 30 minutes. The tissues were immersed in pure Epon812 embedding medium overnight, then embedded in embedding board and polymerized at 45°C for 12 hours and at 60°C for 24–48 hours. After sample blocks were treated through reparation, semithin section fixation, ultrathin section, uranium staining, and lead staining, the sections were examined on an electron microscope ([EMS], Hitachi, Tokyo, Japan) to analyze pathological alterations in the myocardium.
Apoptosis rate was detected with flow cytometry
Apoptotic nuclei appeared as a broad hypodiploid DNA peak that was easily distinguished from the narrow peak of nuclei with normal (diploid) DNA content in the red fluorescence channel. Quantification of apoptotic nuclei was assessed by staining. The cardiomyocytes were washed twice with ice-cold phosphate-buffered saline (PBS), and the pellets were resuspended in 0.3 mL PBS containing 5% fetal bovine serum after centrifugation. After fixation with 0.7 mL ethanol absolute at −20°C for at least 24 hours, the cells were centrifuged at 95 × g for 10 minutes and washed once with PBS. The cells were filtered with grid (200 mesh) and incubated with RNaseA (0.2 mg/mL) at 37°C for 30 minutes, then stained with propidium iodide (PI) at 4°C for 30 min away from light. The PI fluorescence was determined by flow cytometry ([FCM], FACS Caliber, BD Biosciences, Sanjoe, CA, USA).
Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide test
The metabolic activity was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay, in which the yellow tetrazolium salt is metabolized by NAD-dependent dehydrogenase (in active mitochondria) to form a dark blue formazan product (Mosmann 1983; Widestrand et al 1999). Briefly, the cardiomyocytes (2–4 × 104 cells/ml) were seeded into 96-well plates. After establishing the stressed cardiomyocyte cultures, the cells were washed and incubated in serum-free medium. Subsequently, the cells were incubated in phosphate buffer containing MTT (0.5 mg/mL, Sigma, St Louis, MO, USA) for 4 hours at 37°C. The blue formazan crystals, formed by viable cells, were dissolved completely by the addition of 100 μL 10% sodium dodecyl sulfate (SDS), followed by overnight incubation. The optical density was measured by an enzyme-linked immunosorbent assay reader (Spectra Rainbow, Tecan, Salzburg, Austria) at 490 nm. Cell viability was expressed as percent of viable control cells.
Caspase-3/8 activity was measured using fluorometry with AC-DEFH-DA and IETD-AFC
The cells (1–2 × 106 cells/sample) were harvested and washed twice in ice-cold PBS and subsequently resuspended in lysis buffer (containing 50 mM Tris-HCl pH 7.4, 1 mM ethylenediamine-tetraacetic acid [EDTA], 50 μM digitonin). After the cells were incubated at 37°C for 10 minutes, the lysates were centrifuged at 15 000 rpm for 3 minutes. The supernatant was incubated with 10 mM AC-DEFH-DA (Sigma) and reaction buffer (containing 50 mM Tris-HCl pH 7.4, 1 mM EDTA , 50 μM digitonin) at 37°C for 10 minutes. The sample was measured by a spectrofluorometer (Hitachi F-4500), using an excitation wavelength of 380 nm and an emission wavelength of 460 nm.
Caspase-8 activity was determined according to the manufacturer's protocol (ApoAlert Caspase Fluorescent Assay Kits, Clontech, USA). Samples were incubated with 50 μL of reaction buffer (50 mM Hepes, 0.2% Chaps, 20% glycerol, 2 mM EDTA, and 10 mM DTT) and 5 μL Caspase-8 substrate (1 mM IETD-AFC) for 1 hour at 37°C, then Caspase-8 activity was determined using excitation at 400 nm in a spectrophotometer.
Hsp70 and Fas expression analyzed with Western blotting
Samples were prepared from myocardium or cardiomyocytes that were homogenized in a buffer that protects the protein from degradation. Equal amount of protein extracts were loaded and separated by SDS–polyacrylamide gel electrophoresis (PAGE) with molecular weight standards and transferred from gels onto nitrocellulose membrane by incubation for 4.5 hours at RT. After staining the membrane with ponceau S stains, the nonspecific binding sites were blocked for 2 hours in 3% bovine serum albumin (BSA) buffer at RT. The blots were probed with either mouse monoclonal antibody to Hsp70 (H 5147, 1:1000 dilution, Sigma, USA) or rabbit polyclonal antibody to mouse/rat Fas (M-20, 1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C, and then incubated with horseradish-peroxidase-conjugated secondary antibody (dilution of 1:1000, Zhongshan, Beijing, China) for 3h at RT. Between each procedure, the sections were extensively rinsed. The immunoreactive sites were visualized using chemiluminescence and exposure to X-ray film. The relative intensities of the bands were analyzed by scanning the film using an image software package (ImageMaster VDS, Amersham Pharmacia Biotech, Uppsala, Sweden).
Hsp70 distribution was observed by immunohistochemistry and immunoelectron microscopy
Immunohistochemistry
The fixation of tissues and the embedding and sectioning of paraffin blocks were performed by a histology or pathology laboratory, where the sections were transferred onto slides and allowed to dry for at least 12 hours at 60°C. Tissue sections were deparaffinized and rehydrated through graded alcohols using standard procedures. Endogenous peroxidase activity was quenched by a 30-minute incubation with 0.3% H2O2 in methanol. Nonspecific binding sites were blocked by incubation with goat serum from the same host species as the secondary antibody. Samples were incubated for 3 hours with a primary antibody and sequentially for 1 hour with secondary and tertiary antibodies (as mentioned in Western blot) at RT. Sections were developed with 3,3′-diaminobenzidine (DAB) as the chromogen substrate, rinsed, and counterstained with hematoxylin. Negative control slides were processed at the same time with omission of the primary antibody. The slide was examined under light microscope after mounting with coverslips.
Immunoelectron microscopy
The fixation and embedding of tissue was performed as mentioned above. Ultrathin sections (50–70 nm) were obtained and collected on nickel grids (200–300 mesh). The sections were treated with 1% H2O2 so as to remove osmium tetroxide and promote penetration of resin, and then incubated with normal goat serum for 60 minutes at RT to saturate aldehyde groups and block nonspecific binding sites. The samples were incubated with primary antibody for 1 hour at RT and overnight at 4°C. The sections were placed in PBS containing 1% BSA for 5 minutes, followed by washing with PBS (3 times, 3 minutes each), and then incubated with gold-conjugated secondary antibody for 1 hour at RT. After rinsing in distilled water (3 times, 3 minutes each), the sections were counterstained with uranyl acetate and lead citrate for 5 minutes, then examined by electron microscopy.
Protein determination
The protein content in lysates of myocardium and cardiomyocyte was determined by means of the modified Folin-phenol method, using BSA as a standard substance.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). Data were analyzed by double-tail Student's t-test. The differences were considered significant at P < 0.05.
RESULTS
Pathological alterations of myocardial function and ultrastructure in stressed rats
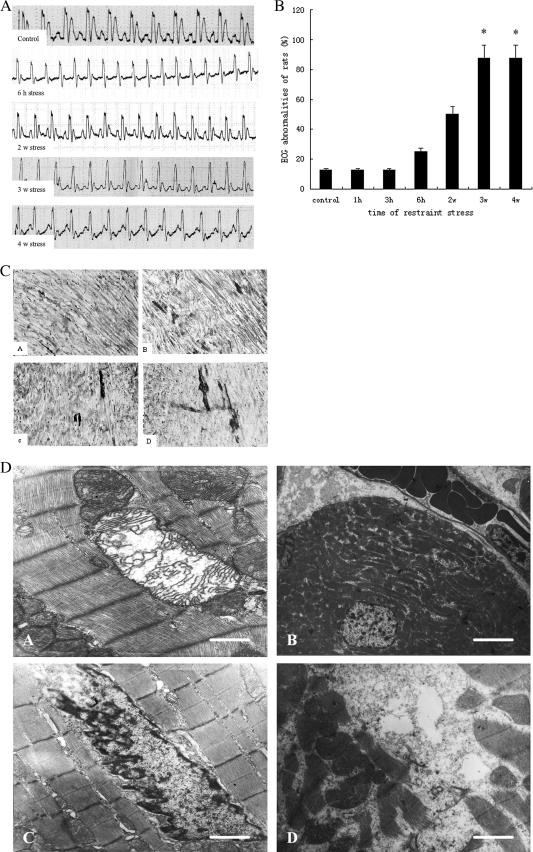
The ECG is one of the clinical tests used for diagnosis of cardiovascular diseases, and specific changes of ECG patterns have emerged as markers for different stages of these diseases (Okin et al 2004). Abnormalities in the ECG were detected in stressed groups such as elevation in the slope of the ST segment (that is the isoelectric period which is important in the diagnosis of ventricular ischemia or hypoxia), and inversion or corona of the T wave (Fig 1A). The results showed that ECG abnormalities began to rise after restraint for 6 hours and that the risk of abnormality increased with the prolongation of the restraint time. In groups restrained for 3 and 4 weeks, ECG abnormalities were significantly higher than those in the control group (P < 0.05; Fig 1B). These results indicate that restraint stress may lead to cardiac dysfunction in rats.
Fig 1.
Pathological alterations of myocardial function and ultrastructure in stressed rat. (A) Changes in electrocardiography of restraint-stressed rats. Rats were anesthetized with 1% sodium pentobarbital (45 mg/kg) by intraperineal injection and kept in a supine position. Limb-lead electrocardiography (ECG) was recorded with an 8-channel physiological recorder. The results showed that abnormalities in the ECG were detected in stressed groups. The elevation in slope or level of ST segment and the two-way of T-wave occurred in groups stress for 2 and 3 weeks, but the elevation in slope of ST segment was much more obvious and the typical inversion or corona of T-wave was found in group 4 weeks after stress. (B) Changes of cardiac function in restraint-stressed rats. The results showed that ECG abnormalities began to rise after restraint for 6 hours, and that the risk of abnormality increased with the prolongation of the restraint time. In groups restrained for 3 and 4 weeks, ECG abnormalities were significantly higher (P < 0.05) than those in the control group. (C) Early pathological change in myocardium of stressed rats by acid fuchsin staining. Acid fuchsin–positive cells were defined as those staining bright red, clearly distinct from the green staining of the control, with the stained areas and intensity representing the severity of injury. Pathologic tissue slices showed that acid fuchsin–positive cells gradually increased as the durations of stress were prolonged. a: control; b: stress for 2 weeks; c: stress for 3 weeks; d: stress for 4 weeks. (D) Changes of myocardial ultrastructure in stressed rats by electron microscopy. The left ventricular muscle (1 mm3) from restraint-stressed rats was ultrathin sectioned and stained after routine treatment and then was examined on the electron microscope. Micrographs showed that changes of myocardial ultrastructure occurred in chronic stress groups. a: mitochondrial swelling and interrupted cristae, the bar in the lower right panel represents 0.9 μm; b: erythrocyte congestion in peripheral vasculature, bar = 5 μm; c: nuclear membrane shrinkage and chromatin margination; d: myofibril breakage, bar = 1.5 μm, which has the same magnification as c
The early pathological change in the heart was observed by acid fuchsin staining. Acid fuchsin–positive cells were defined as those staining bright red, clearly distinct from the green staining of the control, with the stained areas and intensity representing the severity of injury. Pathologic tissue slices showed that acid fuchsin– positive cells gradually increased as the durations of stress were prolonged (Fig 1C). As shown in Figure 1D, chronic stress for 3 and 4 weeks led to severe changes of myocardial ultrastructure, including erythrocyte congestion in peripheral vasculature, mitochondrial swelling and vacuoles, myofibril breakage, and perinuclear space widening. Typical apoptosis characteristics such as nuclear membrane shrinkage and chromatin margination also were found following 3-week restraint stress. The findings indicate that the myocardium injury was more severe with the enhancement of the restraint stress load.
Activation of Fas signal transduction pathway and cell apoptosis induced by restraint stress
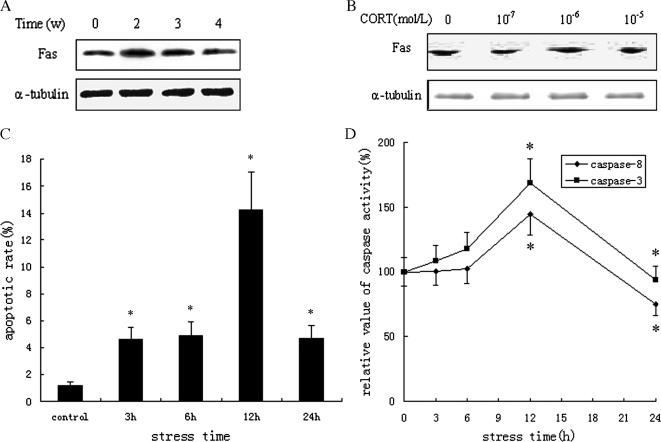
After protein extraction from stressed rat myocardium and cardiomyocytes treated with CORT at various concentrations (10−5, 10−6, or 10−7 mol/L) for 3 hours in vitro Western blotting was used to measure the levels of Fas expression. It was found that the levels of Fas expression were increased markedly in groups of acute stress for 3 and 6 hours (estimated at 4.3-fold and 2.8-fold, respectively, vs control) and in groups of chronic stress for 2 and 3 weeks (estimated at 4.7-fold and 3.8-fold, respectively, vs control; Fig 2A). Consistent with in vitro experimental findings, overexpression of Fas protein was observed in the cardiomyocytes exposed to 10−6 mol/L CORT (Fig 2B), which showed that restraint stress could activate the Fas signal transduction pathway in the heart.
Fig 2.
Activation of Fas signal transduction pathway induced by restraint stress. (A, B) Upregulation of Fas expression in myocardium of stressed rats and cardiomyocytes treated with corticosterone (CORT). The results from Western blotting showed that the levels of Fas expression were increased markedly in groups of chronic stress for 2 and 3 weeks (estimated at 4.7-fold and 3.8-fold vs control), and overexpression of Fas protein was observed in the cardiomyocytes exposed to 10−6 mol/L CORT. Blots were reprobed with α-tubulin as a loading control. (C) Increase of apoptotic rate in cardiomyocytes treated with CORT. The cardiomyocytes were collected and stained with propidium iodide (PI); the PI fluorescence was determined by flow cytometry (FCM). The cell apoptotic rates were 4.6, 4.9, 14.2, and 4.7% when the cardiomyocyte was treated with 10−6 mol/L CORT for 3, 6, 12 and 24 hours, respectively, and all were significantly higher (P < 0.05) than the control (1.2%). (D) Activation of caspase-8/3 in cardiomyocytes treated with CORT. Specific fluorescent substrates, IETD-AFC and AC-DEFH-DA, were used to detect the activity of caspase-8/3 of cardiomyocytes exposed to CORT for 3, 6, 12, and 24 hours in vitro. The results showed similar increased trends in caspase-8 and caspase-3 activity, and activity reached maximum levels following 12 hours stress compared with the control, increasing by 45 and 68%, respectively (P < 0.05)
FCM analysis showed that the cell apoptotic rates were 4.6, 4.9, 14.2, and 4.7% when the cardiomyocyte was treated with 10−6 mol/L CORT for 3, 6, 12, and 24 hours, respectively, and all were significantly higher than the control (1.2%; Fig 2C). The results of the MTT assay showed that, in a time- and dose-dependent manner, the reduction in cell survival rate was linked to the elevation of CORT concentration and the prolongation of restraint time. Our results found that the apoptosis rate was increased notably in the stressed cardiomyocyte and indicated that apoptosis is an important mechanism of cardiomyocyte injury caused by restraint stress.
Most data suggest that caspase-8 is an initiator caspase that is part of the Fas-mediated apoptotic pathway, and caspase-3 is a critical effector caspase of the apoptotic process. Specific fluorescent substrates, IETD-AFC and AC-DEFH-DA, were used to detect the activity of caspase-8 and caspase-3 of cardiomyocytes exposed to CORT for 3, 6, 12, and 24 hours in vitro. The results showed there were similar increased trends in caspase-8 and caspase-3 activity, and that the activity reached maximum levels following 12-hour stress compared with the control, increasing by 45 and 68%, respectively (Fig 2D). Together with the cell apoptotic rate, the findings indicated that cardiomyocyte apoptosis in stressed rats might be executed through a Fas-mediated pathway.
Changes of expression and distribution of Hsp70 in stressed cardiomyocytes
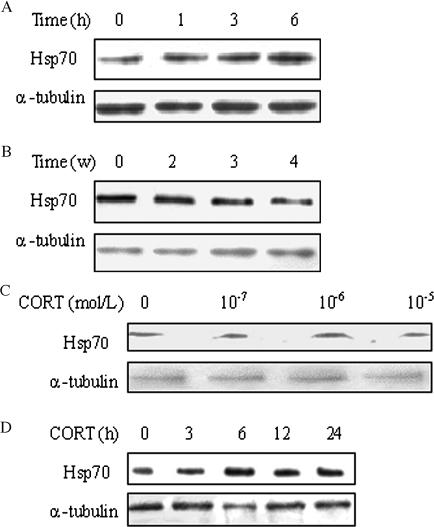
Results from Western blotting showed that restraint stress had different effects on Hsp70 expression in the rat myocardium. Compared with the control, the Hsp70 level was increased by 38% after 6 hours of restraint stress (Fig 3A), but its expression gradually declined with restraint time prolongation in chronic stress groups with about 12, 13, and 48% inhibition of expression, respectively (Fig 3B). Additionally, treating cardiomyocytes with CORT at 10−6 mol/L concentration in vitro significantly increased expression of Hsp70 (Fig 3C). The Hsp70 levels increased approximately to 108, 139, 118, and 109% in cardiomyocytes treated for 3, 6, 12, and 24 hours, respectively (Fig 3D).
Fig 3.
Changes of Hsp70 expression in myocardium and cardiomyocytes under stress. Compared with the control, the results from Western blotting showed (A) the Hsp70 level was increased by 38% after 6 hours of restraint stress in the rat myocardium. (B) The Hsp70 levels gradually declined with restraint time prolongation in chronic stress groups with about 12, 13, and 48% inhibition of expression, respectively. (C) The Hsp70 level significantly increased to 140% in cardiomyocytes treated with corticosterone (CORT) at 10−6 mol/L concentration in vitro. (D) The Hsp70 levels increased approximately to 108, 139, 118, and 109% in cardiomyocytes treated for 3, 6, 12 and 24 hours, respectively. In all Western blots, uniformity of sample loading was confirmed by probing for α-tubulin
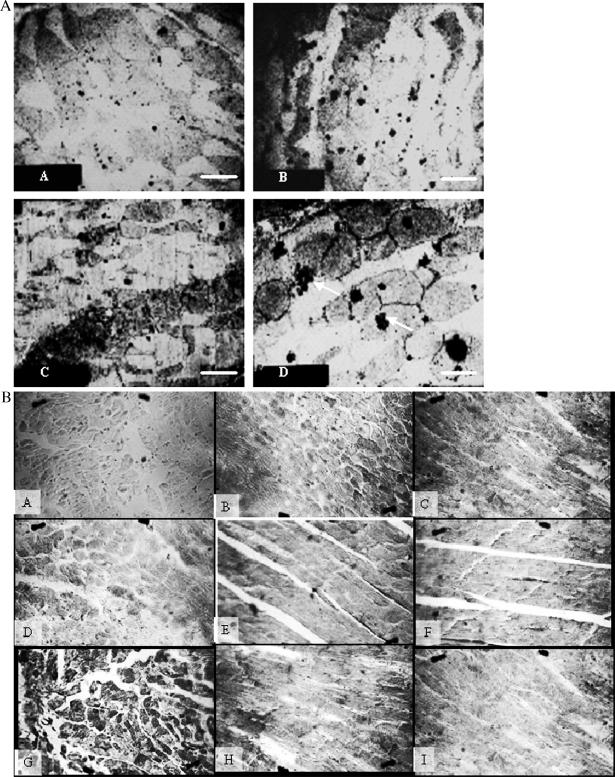
The change of Hsp70 distribution in the myocardium of stressed rats was observed using immunohistochemistry and immunoelectron microscopy (IEM). The results showed that Hsp70 distributes both in cytoplasm and nuclei under normal and stress conditions. A higher content of Hsp70 was observed in both, especially evident in the cytoplasm, and most of the inducible Hsp70 protein distributed near or in the mitochondria (Fig 4). Our findings suggest that the Hsp70 levels and distribution were altered during the restraint stress process.
Fig 4.
Variation of Hsp70 distribution in restraint-stressed rat myocardium. (A) Immunoelectron microscopy showed the localization of Hsp70 in the myocardium of stressed rats and controls. Most of the inducible Hsp70 protein was observed near or in the mitochondria. a: control, bar = 2.1 μm, which has the same magnification as b and c; b: acute stress; c: chronic stress; d: acute stress, bar = 1.5 μm, the gold particles (Hsp70 protein) in clusters were noted (arrows). (B) Immunohistochemistry showed that Hsp70 distributes both in cytoplasm and nuclei under normal conditions and under stress conditions. a: negative control, no positive Hsp70 cell (ie, brown granule in cytoplasm and nuclei) occurred in the myocardium; b: control, there was a little positive Hsp70 cell; c: stress for 6 hours, a higher content of Hsp70 was observed in both, especially evident in the cytoplasm; d–f: stress for 2, 3, and 4 weeks, the content of Hsp70 gradually declined; g–i: stress for 2, 3, and 4 weeks following heat preconditioned for 1 week, respectively, the content of Hsp70 was more than the relevant groups that were not preconditioned
Upregulation of Hsp70 expression by heat preconditioning
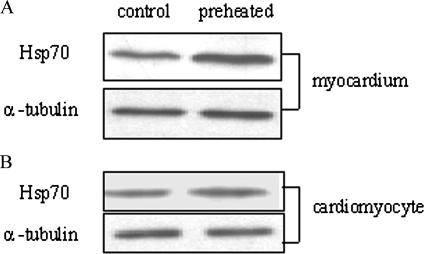
Heat pretreatment was used to raise the endogenous levels of Hsp70. Rats were treated with preheating to activate inducible Hsp70 expression before restraint stress. Briefly, the rat was restricted in a ventilated heating chamber at 35°C with a circulating water bath until its rectal temperature reached 38.5 ± 0.2°C and then was kept for 2 hours (from 9:00 AM to 11:00 AM), as in our previous work. Subsequently the rat was exposed to different degrees of restraint stress after 1-week heat pretreatment. Western blot showed there was a 30% increase in Hsp70 expression in the myocardium compared with the group that was not preconditioned (Fig 5A), and similar findings were confirmed by immunohistochemistry (Fig 4B, g–i).
Fig 5.
Upregulation of Hsp70 expression by heat preconditioning. Heat pretreatment was used to raise the endogenous levels of Hsp70. Rats and cardiomyocytes were treated with preheating to activate inducible Hsp70 expression before stress as in our previous work. Western blot showed (A) a 30% increase in Hsp70 expression in the myocardium compared with the group that was not preconditioned, and similar findings were confirmed by immunohistochemistry (Fig 4B, G–I). (B) Significantly increased expression of Hsp70 in preheated cardiomyocytes, approximately 48% more than control. α-Tubulin was used as the loading control
Primary cardiomyocytes were submerged in a water bath at 41.5°C for 45 minutes, which may result in upregulation of Hsp70 expression, and then were treated with CORT following routine recovery for 6 hours at 37°C. Cells that were not preconditioned were treated identically but were incubated at 37°C. In vitro experiments also showed significantly increased expression of Hsp70 in preheated cardiomyocytes, approximately 48% more than control (Fig 5B).
Hsp70 overexpression mitigated stress-induced cardiomyocyte injury
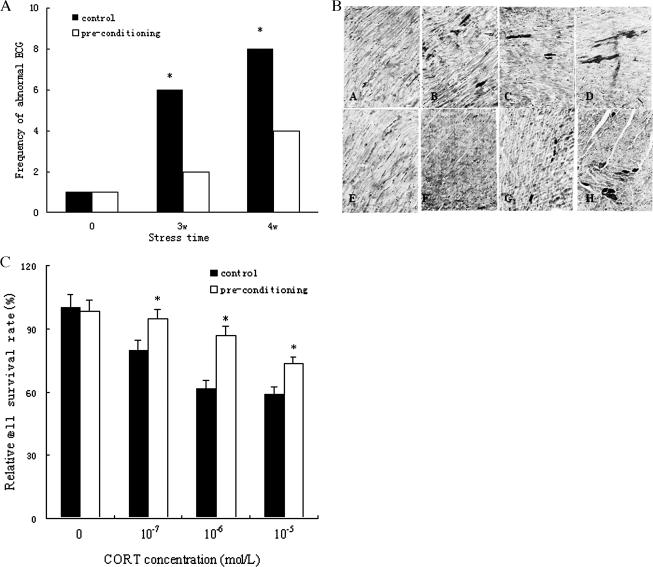
As shown in Figure 6A, the elevated Hsp70 correlated with significantly decreased frequency of abnormal ECGs of rats after 3 and 4 weeks of stress, to 67 and 50% less, respectively, than controls that were not preconditioned. Additionally, the pathological slice viewed with routine light microscopy showed acid fuchsin–positive cells decreased in the myocardium of preheated rats, which had higher levels of Hsp70 compared with the non-preheated group. Also, the specific staining grade became lighter and the stain area became smaller (Fig 6B), indicating that the severity of myocardium injury decreased. Furthermore, upregulation of Hsp70 was accompanied by a significant increase in cell viability in cardiomyocytes treated with CORT for 6 hours, and increased 13, 33, and 16% in response to 10−7, 10−6, and 10−5 mol/L CORT stress, respectively (Fig 6C). The results above indicated that increased levels of Hsp70 correlated with markedly reduced ECG abnormality, myocardium damage, and cell death, which suggested that heat pretreatment could mitigate subsequent restraint stress damage, and this effect may be related to the induction of Hsp70. That is, activation of inducible Hsp70 expression may enable cells to resist various forms of stress and to survive.
Fig 6.
Hsp70 overexpression mitigates stress-induced cardiomyocytes injury. (A) Protection of Hsp70 on cardiac function of stressed rats. The elevated Hsp70 correlated with significantly decreased frequency of abnormal electrocardiographies (ECGs) of rats after 3 and 4 weeks of stress, to 67 and 50%, respectively, less than controls that were not preconditioned. (B) Hsp70 lessens the severity of injury of stressed rat myocardium. a: control; b–d: stress for 2, 3, and 4 weeks, respectively; e: preconditioned control; f–h: stress for 2, 3, and 4 weeks following preconditioned for 1 week, respectively. The pathological slice viewed with routine light microscopy showed acid fuchsin–positive cells decreased in the myocardium of preheated rats, such that the specific staining grade became lighter and the stain area became smaller. (C) Hsp70 augments cell viability in cardiomyocytes treated with corticosterone (CORT). Upregulation of Hsp70 was accompanied by a significant increase in cell viability in cardiomyocytes treated with CORT for 6 hours, and increased 13, 33, and 16% in response to 10−7, 10−6, and 10−5 mol/L CORT stress, respectively. * P < 0.05 vs control
Hsp70 cytoprotection by inhibiting Fas-mediated apoptosis
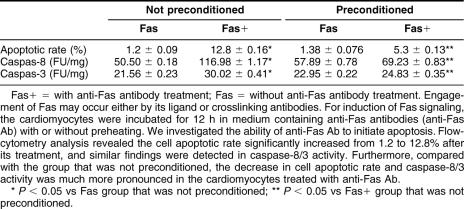
As stated in the Introduction, engagement of Fas may occur either by its ligand or crosslinking antibodies. For induction of Fas signaling, the cardiomyocytes were incubated for 12 hours in medium containing anti-Fas Ab (200 ng/mL, Santa Cruz Biotechnology) with or without preheating. First, we investigated the ability of anti-Fas Ab to initiate apoptosis. FCM analysis revealed the cell apoptotic rate significantly increased from 1.2% to 12.8% after its treatment, and similar findings were detected in caspase-8/3 activity, which indicated that anti-Fas Ab could activate the Fas signaling pathway and induce a significant degree of apoptosis in the cardiomyocyte. Furthermore, compared with the group that was not preconditioned, the decrease in cell apoptotic rate and caspase-8/3 activity was much more pronounced in the cardiomyocytes treated with anti-Fas Ab (Table 1). Our results showed that higher Hsp70 levels might inhibit activation of caspase-8/3 induced by the Fas signaling pathway, and thus prevent cell apoptosis, but have no effect on Fas expression. This suggested that Hsp70 might protect the cardiomyocyte from stress-induced injury by inhibiting Fas-mediated apoptosis.
Table 1.
Inhibition of Hsp70 on Fas-mediated apoptosis in cardiomyocytes
DISCUSSION
Stress is a nonspecific biological response, as well as an adaptive mechanism formed by evolution. It has been proven that the effects of stress on organisms are paradoxical: resisting trauma or inducing trauma. This is because all organisms have limits within which imposed stress is met and dealt with by metabolic adjustments. This can range from near instantaneous compensation for small variations in stressors to prolonged adjustments that deal with major, long-lasting changes in environmental parameters and require substantial metabolic reorganization. Beyond these limits, however, extreme stress would cause injury or even death. It has been found that the cardiovascular system is the major target organism of stress injury, and some scientists even consider that stress is the most important etiologic factor of cardiovascular diseases, such as hypertension, atherosclerosis, and even sudden cardiac failure (Dipak 1999; Schwartz et al 2003). Our findings showed that ECG abnormality in rats began to rise 6 hours after restraint and significantly increased after stress sustained for 3 weeks. Changes of myocardial ultrastructure, however, were observed only in chronic stress groups and there was a dose-dependent relationship between the severity of the injury and the intensity of stress, implying that acute stress only causes functional changes, whereas chronic stress may lead to structural injury of the heart. Additionally, the number of apoptotic cardiomyocytes increased with the deterioration of morphological markers and cardiac function, indicating that apoptosis may be an important mechanism of pathological change and cardiac dysfunction induced by stress.
Hsps are members of multigene families that range in molecular weight from 10 to 150 kDa (eg, the 70-kDa Hsp70) and are present in most cells, serving as molecular chaperones, where they play a role in cell protection from damage due to stressful stimuli. The chaperone function of Hsps in general and in the cardiovascular system has been reported. In addition to being expressed constitutively, the synthesis of these proteins can be induced markedly by a range of cellular insults, the function of which is stabilization and adaptation (Hightower 1991; Welch 1993). The Hsp70 family is highly evolutionarily conserved and the most intensively studied group of Hsps because it is induced often in stressed cells and is thought to represent a basic feature of the ability of cells to cope with adverse conditions. Numerous studies showed that Hsp70 contributes to thermotolerance, as when elevated Hsp70 levels decrease cell sensitivity to heat stress, and protect the cell against toxicity induced by tumor necrosis factor (TNF), ultraviolet ray (UV), oxidative stress, and some chemicals. This ability of Hsp70 to protect cells has been shown recently to be a consequence of inhibition of apoptosis (Mosser and Martin 1992; Malihos et al 1993; Simon et al 1995).
Most of these studies have indicated that the level of Hsp70 induction is correlated positively with the degree of cell thermotolerance, implying that there may be a direct link between the cytoprotective effects of Hsp70 and the expression and distribution of Hsp70. Thus, it has been demonstrated that a transient conditioning ischemia in gerbils leading to ischemic tolerance at 24 hours displays a corresponding increase in Hsp70 in the hippocampal CA1 region and that such an induction will persist for at least 48 hours (Kato et al 1991). Comparable data were reported in a rat global cerebral ischemia model (Nishi et al 1993), a rabbit spinal ischemia model (Sakurai et al 1998), and a dog spinal ischemia model (Matsuyama et al 1997). The former observed that improved postischemic cardiac performance after heat stress is related to the amount of Hsp70 present in the heart at the time the organ is subjected to the subsequent ischemic insult (Yamashita et al 1997). More recently, experiments on transgenic mice, overexpressing Hsp70 in the heart, strongly suggest that Hsp70 is involved casually in the observed cardioprotection (Marber et al 1995; Radford et al 1996). Our results from Western blots showed that restraint stress had a different effect on Hsp70 expression in the rat myocardium. The Hsp70 level increased in acute stress groups and was maximal at 6 hours stress, but Hsp70 expression decreased as restraint time was prolonged in chronic stress and was less than control, suggesting that it may be a consequence of physiological compensation to pathological development in the face of stress overload. ECG abnormality in rats was significantly enhanced and damage to myocardial ultrastructure was much more severe, also in accordance with the inhibition of Hsp70 expression. Previous research proved that the mitochondrion is a major target attacked by stress. The IEM results of the present study demonstrate that most of the inducible Hsp70 protein is distributed near or in the mitochondrion, suggesting that Hsp70 may protect mitochondria from restraint stress injury. Further investigation found that induction of Hsp70 by preheating may play a role in the protection of cardiac function, because levels are correlated not only with markedly reduced myocardium damage, but also with augmented cardiomyocyte viability under stress. The evidence presented above indicates that overexpression of Hsp70 could mitigate stress-induced cardiomyocyte injury and exert its cytoprotective action on the heart.
The Fas pathway is an important pathway of apoptosis that controls cell proliferation and tissue remodeling (Song et al 2000). Triggering of Fas by either agonistic antibodies or FasL results in receptor oligomerization and recruitment of the adaptor protein, Fas-associated death domain (FADD), which along with procaspase-8, forms a death-inducing signaling complex (DISC) (Kischkel et al 1995). The underlying molecular mechanisms are very intricate and downstream effectors are different based on different cell types. Once caspase-8 is activated, the effector caspases, including caspase-3, are activated directly (type-I cells) or indirectly by cytochrome c–mediated activation of caspase-9 (type-II cells) (Salvesen and Dixit 1997). In type II cells, mitochondria are used as amplifiers to initiate the executionary apoptotic caspase cascade. Besides preventing protein aggregation, Hsp70 can interfere directly with the apoptotic machinery in a variety of ways and protect cells from a number of apoptotic stimuli. So far, studies on the antiapoptotic effect of Hsp70 concentrated mostly on preventing processing in the mitochondrion pathway (Chunying et al 2000). However, there have been few studies about the interplay of Fas and Hsp70.
In previous studies, it was suggested that the Fas system plays an important role in the regulation of physiological homeostasis in the immune system. Recent studies have shown that the Fas signal transduction pathway is involved in various types of stress-induced apoptosis, such as how a functional Fas system contributes to cell death in cardiac cells in response to ischemia/reperfusion injury (Jeremias et al 2000). Furthermore, overexpression of Fas has been reported in a variety of conditions: cardiomyopathy induced by rapid pacing in dogs, myocardial infarction in rats (Kajstura et al 1996), or hypoxia in cultured neonatal rat cardiomyocytes (Tanaka et al 1994). Our research also found that levels of Fas were upregulated after restraint stress in vivo and in vitro experiments, and, moreover, that the cell apoptotic rate and caspase-8/3 activity also increased, indicating that Fas-mediated apoptosis may be one of the mechanisms of cardiomyocyte injury induced by stress. It has been shown that the overexpression of Hsp70 protects the cell against toxicity induced by TNFa (Nishimura et al 1997; Ahn et al 1999). In addition, induction of Fas-mediated signaling was followed by a rapid decrease in inducible Hsp70 expression, and activation of Hsp70-suppressed Fas-mediated apoptosis (Schett et al 1999). These data imply that the inducible Hsp70 possibly acts as a negative regulator of Fas-mediated apoptosis in the protection. Our results show cell apoptotic rates and caspase-8/3 activity were decreased significantly in the preconditioned group, which had higher Hsp70 expression compared with the cells that were not preconditioned, whereas there was no difference in Fas expression between those with or without preconditioning. Accordingly, we infer that Hsp70 may block the Fas signaling pathway by inhibiting activation of caspase-8/3 and conferring cytoprotection against stress, but the crucial regulatory molecule through which Hsp70 exerts its antiapoptotic function has not been fully elucidated.
In conclusion, our results support the hypothesis that restraint stress results in cardiac dysfunction and structural injury of the heart, and that Fas-mediated apoptosis is one of the main mechanisms of cardiomyocyte injury induced by stress. Moreover, there is a clear correlation between the change of expression and distribution of Hsp70 under stress and the severity of myocardium injury. Additionally, we show that the upregulation of Hsp70 synthesis by preheating confers protection to the heart against stress. It not only tones up cardiac function, and abates the severity of injury to the myocardium, but also enhances cell viability in cardiomyocytes treated with CORT. This suggests that Hsp70 may protect the cardiomyocyte from stress-induced injury by inhibiting Fas-mediated apoptosis, but the underlying mechanism remains to be investigated further.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China ([NNSFC] 30430590 and 30370586) and the National Basic Research Priorities Program of China (2001CB510206).
REFERENCES
- Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS. Suppression of ceramide-mediated apoptosis by Hsp70. Mol Cells. 1999;9:200–206.1016-8478(1999)009[0200:SOCABH]2.0.CO;2 [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305.0193-4511(1998)281[1305:DRSAM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Basu N, Nakano T, Grau EG, Iwama GK. The effects of cortisol on heat shock protein 70 levels in two fish species. General and Comparative Endocrinology. 2001;124:97–105. doi: 10.1006/gcen.2001.7688.1095-6840(2001)124[0097:TEOCOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chunying L, Jaeseon L, Younggyu K, Jongii K, Jeongsun S. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. JBC. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- Das DK, Engelman RM, and Maulik N 1999 Oxygen free radical signaling in ischemic preconditioning. In: Heart in Stress. ed Das DK. Ann NY Acad Sci. 874:49–65. [DOI] [PubMed] [Google Scholar]
- De-Saint-Jean M, Debbasch C, Rahmani M, Brignole F, Feldmann G, Warnet JM, Baudouin C. Fas- and interferon gamma-induced apoptosis in Chang conjunctival cells: further investigations. Invest Ophthalmol Vis Sci. 2000;41:2531–2543.0146-0404(2000)041[2531:FAIGAI]2.0.CO;2 [PubMed] [Google Scholar]
- Feuerstein GZ, Young PR. Apoptosis in cardiac diseases: stress- and mitogen-activated signaling pathways. Cardiovasc Res. 2000;45:560–569. doi: 10.1016/s0008-6363(99)00372-7.0008-6363(2000)045[0560:AICDSA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, and Tanapat P. et al. 1997 Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neurosci. 81:689–697. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2.0092-8674(1991)066[0191:HSSPCA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, Debatin KM. Involvement of CD95/Apo1/ Fas in cell death after myocardial ischemia. Circulation. 2000;102:915–920. doi: 10.1161/01.cir.102.8.915.0009-7322(2000)102[0915:IOFICD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kajstura J, Cheng W, and Reiss K. et al. 1996 Apoptotic and necrotic myocyte cell death are independent contributing variables of infarct size in rats. Lab Invest. 74:86–107. [PubMed] [Google Scholar]
- Kalivendi SV, Konorev EA, Cunningham S, et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285.0264-6021(2005)389[0527:DANFOA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Liu Y, Araki T, Kogure K. Temporal profile of the effects of pretreatment with brief cerebral ischemia on the neuronal damage following secondary ischemic insult in the gerbil: cumulative damage and protective effects. Brain Res. 1991;553:238–242. doi: 10.1016/0006-8993(91)90831-f.0006-8993(1991)553[0238:TPOTEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kawano H, Okada R, and Kawano Y. et al. 1994 Apoptosis in acute and chronic myocarditis. Jpn Heart J. 35:745–750. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/ CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x.1460-2075(1995)014[5579:CACPFA]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55:621–627. doi: 10.1016/0306-4522(93)90428-i.0306-4522(1993)055[0621:HSPNCF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15.0002-9440(1995)146[0003:AOANAO]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Marber MS, Mestril CH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815.0021-9738(1995)095[1446:OOTRIK]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Chiba Y, Ihaya A, Kimura T, Tanigawa N, Muraoka R. Effect of spinal cord preconditioning on paraplegia during crossclamping of the thoracic aorta. Ann Thorac Surg. 1997;63:1315–1320. doi: 10.1016/s0003-4975(97)00104-5.0003-4975(1997)063[1315:EOSCPO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunology Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Martin LH. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol. 1992;151:561–570. doi: 10.1002/jcp.1041510316.0021-9541(1992)151[0561:ITTAIA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7.0092-8674(1997)088[0355:ABDF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats. Circulation. 2000;102:572–578. doi: 10.1161/01.cir.102.5.572.0009-7322(2000)102[0572:FAIACI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Narula J, Haider N, and Virmani R. et al. 1996 Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 335:1182–1189. [DOI] [PubMed] [Google Scholar]
- Nishi S, Taki W, Uemura Y, Higashi T, Kikuchi H, Kudoh H, Satoh M, Nagata K. Ischemic tolerance due to the induction of Hsp70 in a rat ischemic recirculation model. Brain Res. 1993;615:281–288. doi: 10.1016/0006-8993(93)90039-p.0006-8993(1993)615[0281:ITDTTI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nishimura H, Emoto M, Kimura K, Yoshikai Y. Hsp70 protects macrophages infected with Salmonella choleraesuis against TNF-alpha-induced cell death. Cell Stress Chaperones. 1997;2:50–59. doi: 10.1379/1466-1268(1997)002<0050:hpmiws>2.3.co;2.1466-1268(1997)002[0050:HPMIWS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okin PM, Devereux RB, and Nieminen MS. et al. 2004 Electrocardiography strain pattern and prediction of cardiovascular morbidity and mortality in hypertensive patients. Hypertension. 44:48–54. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Abbi R, and Quaini F. et al. 1997 Apoptosis in the failing human heart. N Engl J Med. 336:1131–1141. [DOI] [PubMed] [Google Scholar]
- Qian L, Song X, Ren H, Gong J, Suqi C. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 2004;9:281–293. doi: 10.1379/CSC-20R.1.1466-1268(2004)009[0281:MMOHSI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford NB, Fina M, and Benjamin IJ. et al. 1996 Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc Natl Acad Sci USA. 93:2339–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M, Hayashi T, Abe K, Aoki M, Sadahiro M, Tabayashi K. Enhancement of heat shock protein expression after transient ischemia in the preconditioned spinal cord of rabbits. J Vasc Surg. 1998;27:720–725. doi: 10.1016/s0741-5214(98)70238-1.0741-5214(1998)027[0720:EOHSPE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4.0092-8674(1997)091[0443:CISBP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schett G, Steiner CW, and Groger M. et al. 1999 Activation of Fas inhibits heat-induced activation of HSF1 and up-regulation of Hsp70. FASEB. 13:833–842. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Gerin W, Davidson KW, Picking TG, Brosschot JF, Thayer JF, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosom Med. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61.0033-3174(2003)065[0022:TACMOC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jaattela M, Schwarz T. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995;95:926–933. doi: 10.1172/JCI117800.0021-9738(1995)095[0926:HSPOAT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Sapi E, and Brown W. et al. 2000 Roles of Fas and Fas ligand during mammary gland remodeling. J Clin Invest. 106:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Ito H, and Adachi S. et al. 1994 Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. 75:426–433. [DOI] [PubMed] [Google Scholar]
- Welch WJ. How cells respond to stress. Sci Am. 1993;268:56–64. doi: 10.1038/scientificamerican0593-56.0036-8733(1993)268[0056:HCRTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Widestrand J, Lundh T, Pettersson H, Lindberg JE. Cytotoxity of four trichothecenes evaluated by three colorimetric bioassays. Mycopathologia. 1999;147:149–155. doi: 10.1023/a:1007127919901.0301-486X(1999)147[0149:COFTEB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yamashita HS, Nishidam IJ, Aokik H, Kuzuya TM. Time course of tolerance to ischemia-reperfusion injury and induction of heat shock protein 72 by heat stress in the rat heart. J Mol Cell Cardiol. 1997;29:1815–1821. doi: 10.1006/jmcc.1997.0416.0022-2828(1997)029[1815:TCOTTI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yoon J-H, Gores GJ. Death receptor-mediated apoptosis and the liver. Journal of Hepatology. 2002;37:400–410. doi: 10.1016/s0168-8278(02)00209-x.0168-8278(2002)037[0400:DRAATL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]