Abstract
Tyrosinase is a melanocyte-specific enzyme critical for the synthesis of melanin, a process normally restricted to a post-Golgi compartment termed the melanosome. Loss-of-function mutations in tyrosinase are the cause of oculocutaneous albinism, demonstrating the importance of the enzyme in pigmentation. In the present study, we explored the possibility that trafficking of albino tyrosinase from the endoplasmic reticulum (ER) to the Golgi apparatus and beyond is disrupted. Toward this end, we analyzed the common albino mouse mutation Tyr(C85S), the frequent human albino substitution TYR(T373K), and the temperature-sensitive tyrosinase TYR(R402Q)/Tyr(H402A) found in humans and mice, respectively. Intracellular localization was monitored in albino melanocytes carrying the native mutation, as well as in melanocytes ectopically expressing green fluorescent protein-tagged tyrosinase. Enzymatic characterization of complex glycans and immunofluorescence colocalization with organelle-specific resident proteins established that all four mutations produced defective proteins that were retained in the ER. TYR(R402Q)/Tyr(H402A) Golgi processing and transport to melanosomes were promoted at the permissive temperature of 32°C, but not at the nonpermissive 37°C temperature. Furthermore, evidence of protein misfolding was demonstrated by the prolonged association of tyrosinase mutants with calnexin and calreticulin, known ER chaperones that play a key role in the quality-control processes of the secretory pathway. From these results we concluded that albinism, at least in part, is an ER retention disease.
Keywords: calnexin, protein folding, quality control
Mutations in tyrosinase (monophenol, l-dopa:oxygen oxidoreductase, EC 1.14.18.1) are the cause of classic type I oculocutaneous albinism, an autosomal recessive genetic disorder characterized by the absence of melanin in melanocytes (1). The enzyme catalyzes the hydroxylation of tyrosine to dopa, plus the oxidations of dopa to DOPAquinone and 5,6-dihydroxyindole to indole-5,6-quinone, key reactions in melanin biosynthesis (2–4). The amino acid sequences deduced from human and mouse tyrosinase (TYR and Tyr, respectively) cDNAs predict a type I membrane glycoprotein with an N-terminal signal sequence and catalytic copper binding regions with conserved positions of histidine and cysteine residues (5–9). The 60-kDa tyrosinase core polypeptide is modified in the endoplasmic reticulum (ER) by cotranslational addition of multiple N-linked glycans, producing the 70-kDa species (10, 11). Complex sugar modifications in the Golgi apparatus further increases tyrosinase's molecular mass to 80 kDa, the size of the mature wild-type (WT) isoform (10–12).
The early 70-kDa folding intermediate interacts with calnexin and calreticulin (10–12), lectin chaperones known to bind to misfolded proteins possessing monoglucosylated glycans and to help increase folding efficiency (refs. 13 and 14, reviewed in ref. 15). In normal melanocytes the 70-kDa protein eventually is released from this complex and proceeds to the Golgi apparatus en route to the melanosomes, the site of melanin synthesis. In contrast, in amelanotic melanoma cells, WT tyrosinase accumulates as the early 70-kDa species associated with calnexin and subsequently is targeted for degradation by the ubiquitin-dependent proteasome pathway (12).
Tyrosinase from several albino mutant melanocytes also appears as a 70-kDa protein (16–20). In this report, we analyzed the effect on ER processing and intracellular localization of four mutations commonly associated with oculocutaneous albinism. The mutant proteins were studied in their natural form or as tagged proteins in their native environment, the melanocyte. We demonstrate that the albino mutants are underprocessed proteins bound to calnexin and calreticulin that do not progress beyond the ER, indicating that albinism belongs to the growing group of ER retention diseases.
Materials and Methods
WT and Mutant Melanocytes.
Cultured human melanocytes from newborn foreskins (12) were used as WT controls, and melanocytes carrying the common T373K substitution (19), termed TYR(T373K), cultured from an individual with oculocutaneous albinism, were the source for mutant human protein. Immortalized mouse melanocytes established from black B10BR mouse (21), served as WT controls, and albino melan c melanocytes (22) homozygous for the c-locus (Tyr) mutation C85S (6, 23), termed Tyr(C85S), and himalayan homozygous for the temperature-sensitive (ts) H402A mutation (16, 24), termed Tyr(H402A), were the source for albino mouse enzyme. The light and dark himalayan melanocytes originated from the same homozygous mutant mice were of similar passage, except that for as yet undefined variability displayed different levels of pigmentation and possessed different forms of tyrosinase (16, 24).
Construction of Plasmids and Transfection.
Plasmids encoding TYR linked to enhanced green fluorescent protein (EGFP) were constructed by ligation of the N terminus of GFP (from pEGFP-N, CLONTECH) to the C-terminus tyrosinase cDNAs (obtained from R. Spritz, University of Colorado Health Services Center, Denver), encoding human WT or mutant proteins carrying the R402Q (a ts mutation similar to mouse himalayan; ref. 25), or T373K mutation. The resulting fusion proteins in the pCMV/TYR-GFP plasmids, proceeding from the N to the C terminus were tyrosinase, a linker sequence of 14 amino acid residues, and GFP. In addition, we replaced the cytomegalovirus (CMV) promoter in the pCMV/TYR-GFP and pEGFP-N plasmids with the 2,500 bp of Tyr promoter (26), obtained from S. Shibahara (Tohoku University School of Medicine, Sendai, Japan). These plasmids were designated pTyrP/TYR-GFP and pTyrP/GFP. All vectors included the neomycin gene conferring resistance to geneticin (G418). DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate)-encapsulated plasmid DNAs (20 μg/each) were transfected into WT (B10BR) or albino Tyr(C85S) (melan c) mouse melanocytes following the manufacturer's instructions (Boehringer Mannheim). Melanocytes were subjected to selection in 1–1.5 mg/ml G418 starting 4–5 days after transfection. Ectopically expressed fusion proteins were analyzed within 3 weeks of transfection, using pTyrP/GFP as a control.
Western Blotting and Coimmunoprecipitation.
Melanocytes were lysed in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) buffer (2% CHAPS in 50 mM Hepes and 200 mM NaCl, pH 7.5) containing a mixture of protease inhibitors (Complete EDTA-free, Boehringer Mannheim). Western blotting was performed with whole-cell lysates (WCLs, 40 μg protein/lane), immunoprecipitated products (250 μg/precipitation), or bead-bound wheat germ agglutinin (Sigma) affinity-purified glycoproteins.
Tyrosinase was detected with anti-human T311 mouse mAb (27), polyclonal rabbit PEP7 (28), or goat M-19 (Santa Cruz Biotechnology) antibodies raised against the C-terminal peptide of Tyr. Anti-calnexin rabbit antiserum SPA-860 (StressGen Biotechnologies, Victoria, Canada), and anti-calreticulin rabbit polyclonal antibodies SPA-600 (StressGen), or PA3–900 (ABR, Affinity Bioreagents, Golden, CO) were used in coimmunoprecipitation assays as described (12). Antigen-antibody complexes were detected by enhanced chemiluminescence (NEN).
Carbohydrate Cleavage.
Cell lysates (300–400 μg protein) in 100 μl CHAPS lysis buffer were incubated with 50 μl of bead-bound wheat germ agglutinin for 2 h in the cold, under constant rotation. After washing twice with CHAPS buffer and once with PBS, bead-bound glycoproteins were digested with endoglycosidase H (endo H), according to the manufacturer's instruction (Boehringer Mannheim). Reaction products were subjected to Western blotting with anti-tyrosinase antibodies.
Radioactive Pulse/Chase.
Mouse melanocytes were starved for 2 h in Met/Cys-free RPMI 1640 medium, incubated with [35S]Met/Cys (0.88 mCi/ml, EasyTag, NEN) for 30 min, harvested immediately, or further incubated with Met/Cys-supplemented medium for 2 h. Melanocytes were collected and lysed in CHAPS buffer, and cell extracts were immunoprecipitated with anti-tyrosinase PEP7, anti-calnexin, or anti-calreticulin polyclonal antibodies as described (12). To detect calnexin/calreticulin-associated tyrosinase, the respective immunoprecipitates were dissociated by incubation with 1% SDS buffer, diluted 1:5 with 1% Triton X-100, and subjected to a second immunoprecipitation with anti-tyrosinase antibodies. Densities of radioactive bands were determined with a Molecular Dynamics PhosphorImager.
Immunofluorescence Microscopy.
Cells were fixed in 4% formaldehyde/PBS, permeabilized with 0.1% Triton X-100/PBS, incubated with polyclonal antibodies (diluted in 0.1% BSA/PBS) against calnexin (StressGen) to localize the ER, α-1,2-mannosidase II (from M. Farquhar, University of California, San Diego, and K. Moremen, University of Georgia, Athens) to localize the Golgi apparatus, rabbit PEP7, or goat M-19 polyclonal antibodies to detect endogenous tyrosinase, and then with rhodamine anti-rabbit conjugates (Molecular Probes) or fluorescein anti-goat conjugates (Santa Cruz Biotechnology) diluted in 0.1% BSA/PBS. Indirect immunofluorescence was visualized with an inverted Bio-Rad MRC-600 Laser Confocal Microscope System. Images were processed with Bio-Rad Confocal Assistant software.
Results
Albino Tyrosinase Mutants Accumulate as Immature ER Glycoforms.
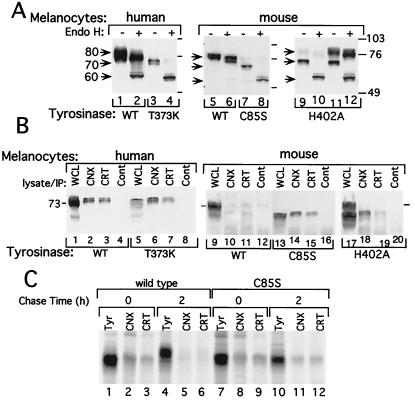
Digestion with endo H was used to determine the state of posttranslational modification because endo H cleaves tyrosinase with early high mannose forms characteristic of ER species, but not forms containing complexed carbohydrate that are attached in the Golgi (12). In agreement with previous results (12), steady-state WT TYR displayed a broad band spanning 70–80 kDa composed of immature and mature species, whereas WT Tyr appeared as a narrower band in the high molecular mass range, presumably representing the mature form (Fig. 1A, lanes 1 and 5, bands marked 80). In contrast, the undigested TYR(T373K), Tyr(C85S), and the amelanotic himalayan Tyr(H402A) migrated as the 70-kDa protein that was less abundant compared with its WT counterpart (Fig. 1A, lanes 3, 7, and 9; bands marked 70). The 70-kDa species of WT TYR and albino melanocytes corresponded to an endo H-sensitive glycoform, digested to a 60-kDa protein (Fig. 1A, lanes 2, 4, 8, and 10), the expected size of the deglycosylated protein (12). Dark himalayan melanocytes possessed the mature endo H-resistant 80-kDa form, in addition to the 70-kDa sensitive form (Fig. 1A, lanes 11 and 12). Interestingly, unlike WT TYR, Tyr was resistant to endo H digestion (Fig. 1A, compare lane 5 to 6), showing that under steady-state conditions, the majority of the enzyme is in the Golgi processed form. TYR(T373K) appeared as a doublet protein migrating at a slightly lower position than the fastest form of TYR (Fig. 1A, compare lane 1 to 3 and lane 2 to 4). This finding is consistent with the preservation of the inefficient glycosylation site Asn-Gly-Thr-Pro (amino acids 290–293) in WT TYR (A. Ujvari, R.A., S. Drabic, E.C., Y.S., R.H., and D.N.H., unpublished work), and loss of a consensus site caused by the substitution of Thr with Lys at position 373 in Asn-Glu-Thr-Met (amino acids 371–374).
Figure 1.
Tyrosinase of albino melanocytes displays characteristics of ER-retained proteins. (A) The 70-kDa tyrosinase proteins are sensitive to endo H digestion. Western blots with anti-tyrosinase antibodies (T311 mAb, lanes 1–4; PEP7 rabbit polyclonal, lanes 5–12) showing nondigested (−) or endo H-digested (+) tyrosinase from WT human or mouse melanocytes, human albino (T373K), mouse melan c (C85S), or himalayan (H402A) melanocytes. The light and dark mouse himalayan melanocytes were collected from different flasks, all grown at 37°C; the difference in pigmentation was caused by yet undefined variability. Arrows indicate sizes of tyrosinase proteins in kDa: 80, mature; 70, immature; and 60, endo H digested. Molecular mass markers are designated in kDa by numbered bars on the left. (B) Immature tyrosinase associates with the ER chaperone calnexin and calreticulin. Western blots with anti-tyrosinase (T311 mAb, lanes 1–8; goat polyclonal, lanes 9–20) of WCL (40 μg protein/lane) from normal human or mouse melanocytes (WT), human albino (T373K), melan c (C85S), or himalayan (H402A), as indicated. Alternatively, cell lysates (250 μg protein/precipitation) were precipitated with rabbit polyclonal antibodies against calnexin (CNX), calreticulin (CRT), or rabbit IgG as a control (Cont). Immunoprecipitates then were subjected to Western blotting. The migration of tyrosinase in all of the coimmunoprecipitated reaction products is artificially retarded because of protein overloading contributed by the presence of high amounts of antibodies. (C) Association of newly synthesized tyrosinase with calnexin and calreticulin in WT and albino (C85S) melanocytes. Melanocytes were pulsed with [35S]-Met/Cys for 30 min and then harvested immediately (lanes 1–3 and 7–9) or further incubated in media (without radioactive Cys/Met) for 2 h (lanes 4–6 and 10–12). Tyrosinase was precipitated directly with anti-tyrosinase antibodies (Tyr) or as an associated protein with anti-calnexin (CNX) or anti-calreticulin (CRT) antibodies. The latter were performed by sequential precipitation first with antibodies against the respective chaperone and, after dissociation of antibody-antigen complex, with a second immunoprecipitation with anti-tyrosinase antibodies. After resolution by SDS/PAGE, the levels of tyrosinase were quantified with a PhosphorImager.
Persistent Chaperone Binding to Albino Mutants.
Calnexin and calreticulin are homologous ER lectin chaperones that assist in folding and retention of proteins in the ER until they are properly folded or assembled (15). Indeed, previous coimmunoprecipitation assays showed that the two remained bound to misfolded WT tyrosinase produced in vivo (12) and in a cell-free system (A. Ujvari, R.A., S. Drabic, E.C., Y.S., R.H., and D.N.H., unpublished work). Similar assays revealed that calnexin and calreticulin associated with the 70-kDa WT TYR glycoform, but not with the fully processed 80-kDa species (Fig. 1B, lanes 2 and 3). There was inefficient binding to WT Tyr (Fig. 1B, lanes 10 and 11), in agreement with the lack of early glycoforms in steady-state conditions, as previously demonstrated by its resistance to endo H digestion (Fig. 1A, lane 6). In all mutant albino melanocytes, a large proportion of the 70-kDa tyrosinase species remained bound to the two chaperones (Fig. 1B, lanes 6, 7, 14, 15, 18, and 19).
A similar pattern of chaperone association was observed with newly synthesized tyrosinase. After a 30-min pulse with [35S]-Met/Cys, the 70-kDa glycoform of WT and albino Tyr(C85S) (Fig. 1C, lanes 1 and 7), was bound to calnexin and calreticulin (Fig. 1C, lanes 2, 3, 8, and 9). The efficiency of binding to calnexin (14% of total Tyr) was similar to that for calreticulin (9%) for both cell types. After a 2-h chase with unlabeled amino acids, WT Tyr migrated as an 80-kDa species that was no longer bound to the lectin chaperones, whereas Tyr(C85S) persisted as the 70-kDa species bound to calnexin and calreticulin, indicating that Tyr(C85S) remained misfolded in the ER for at least 2 h after synthesis.
Albino Tyrosinase Is Localized to the ER.
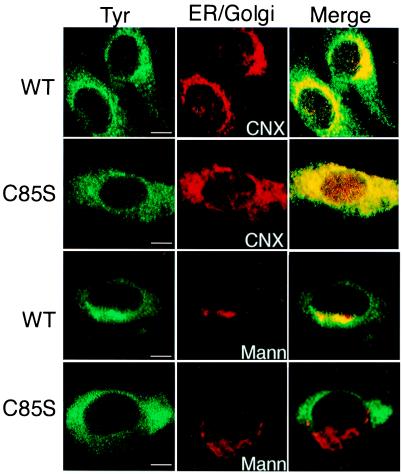
Subcellular localization with indirect confocal immunomicroscopy confirmed our biochemical analyses. In these images WT Tyr was displayed in a perinuclear reticular pattern, coincident with calnexin staining, representative of the ER. In addition, WT Tyr also was localized to bright spots outside the ER perinuclear region (Fig. 2, WT, compare Tyr and CNX, to Merge). In contrast, Tyr(C85S) was found exclusively in the reticular ER, colocalizing with calnexin (Fig. 2, C85S, compare Tyr and CNX, to Merge).
Figure 2.
Albino (C85S) mouse tyrosinase is retained in the ER. Immunofluorescence confocal microscopy of WT and albino (C85S) mouse melanocytes. (Left) The green fluorescein-stained panels represent tyrosinase detected with goat polyclonal anti-mouse tyrosinase antibodies. (Middle) The red rhodamine-stained panels show resident ER and Golgi complex markers, calnexin, and α-1,2-mannosidase II, respectively. (Right) Merged images. Tyrosinase colocalization with each protein is indicated in yellow in the merge images. (The bars represent 1 μm.)
Staining for α-1,2-mannosidase II showed the expected Golgi complex localization in a punctate pattern on one side of the nucleus in WT and albino mouse melanocytes (Fig. 2, Mann). However, whereas WT TYR colocalized with the Golgi apparatus marker, Tyr(C85S) did not (Fig. 2, Merge). We therefore concluded that steady-state WT Tyr was distributed to the ER and the Golgi complex, whereas Tyr(C85S) was retained in the ER.
Trafficking of TYR-GFP Chimeras in Mouse Melanocytes.
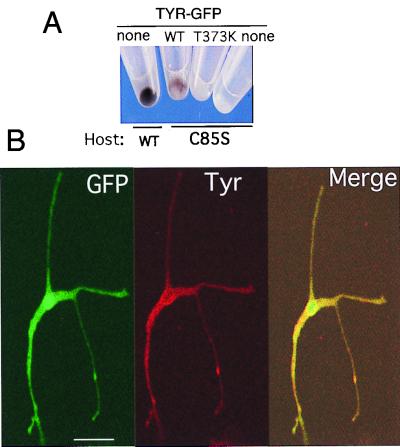
To monitor the trafficking of TYR in melanocytes, we transiently expressed the tyrosinase-GFP chimeras, WT TYR-GFP, TYR(T373K)-GFP, and TYR(R402Q)-GFP, in mouse melanocytes. Functional integrity was confirmed by the conversion of the amelanotic melan c melanocytes to pigment producing cells in response to WT TYR-GFP, but not TYR(T373K)-GFP expression (Fig. 3A), and by the colocalization of the green fluorescence of WT TYR-GFP with endogenous WT Tyr (Fig. 3B).
Figure 3.
Ectopic WT TYR-GFP chimeras display functions characteristic to tyrosinase. (A) Photograph of cell pellets showing pigmented WT mouse melanocytes (Host, WT), pigmented mutant melan c (Host, C85S) transfected with WT TYR-GFP (WT), or unpigmented melan c transfected with TYR(T373K)-GFP (T373K), compared with unpigmented nontransfected melan c melanocytes (none). There were approximately 30% and 80% fluorescing cells in the WT TYR-GFP and TYR(T373K)-GFP transfected melan c cultures. (B) Micrographs of WT mouse melanocytes transiently transfected with WT TYR-GFP. (Left) Green fluorescence from the fusion protein (GFP). (Middle) Red fluorescence of rhodamine-stained image detecting endogenous Tyr with PEP7 antibodies (Tyr). (Right) Merged image of the two. (The bar represents 10 μm.)
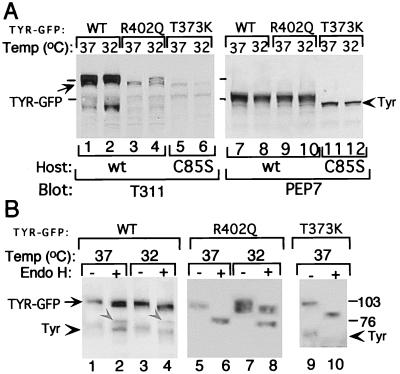
Western blotting also confirmed the synthesis of the predicted size chimeric molecules with the expected phenotype. The TYR-GFP chimeras were distinguished from endogenous WT Tyr by their larger size, composed of the 27-kDa GFP and 70- to 80-kDa tyrosinase, and their preferential immunoreactivity with anti-TYR mAb T311, but not with the mouse-specific PEP7 (Fig. 4A, compare lanes 1–6 to 7–12). Also observed were faster migrating T311 immunoreactive bands comprised of faintly reactive endogenous Tyr and degradation products of chimeric molecules.
Figure 4.
Processing of ectopic TYR-GFP chimeras is similar to native proteins. (A) Western blots of TYR-GFP transfectants (40 μg protein/lane) grown continuously at 37°C or after a 14 h shift to 32°C. Host melanocytes were WT (Host WT, lanes 1–4 and 7–10) or albino (Host, C85S, lanes 5, 6, 11, and 12). The same membrane was first blotted with T311 mAb (lanes 1–6), and then, without stripping, with PEP7 (lanes 7–12). Melanocytes were harvested 1 week after transfection with WT TYR-GFP (WT), TYR-GFP(T373K) (T373K), or TYR(R402Q)-GFP (R402Q). Arrow indicates chimeric proteins of 100–110 kDa, and arrowhead points at endogenous Tyr. Bars indicate molecular mass protein markers. (B) Western blots with T311 mAb of undigested (−) and endo H-digested (+) proteins. Glycoproteins from extracts (300–400 μg/transfectant) were precipitated with wheat germ agglutinin-bound beads for 2 h and then incubated without (−) or with (+) endo H overnight. Arrow indicates the position of undigested chimeric proteins, gray arrowheads (in lanes 2 and 4) point to endo H-digested WT TYR-GFP proteins, and arrowheads mark endogenous Tyr. Host mouse melanocytes are as in A.
Endo H digestion further extended the similarities shared by the chimeric proteins and native tyrosinase species. Regardless of temperature, WT TYR-GFP appeared as a broad endo H-resistant protein band of 100–110 kDa (Fig. 4A, 1–2), with only a small portion cleaved to the 80-kDa chimeric form (Fig. 4B, lanes 2 and 4; marked with gray arrowheads). Likewise, TYR(R402Q)-GFP appeared as ≈100-kDa doublet at 37°C (Fig. 4A, lane 3) that was completely sensitive to endo H digestion (Fig. 4B, compare lane 5 to 6). Shifting the transfected melanocytes to the permissive temperature of 32°C for 14 h enabled the production of tyrosinase chimeric protein of higher molecular mass (Fig. 4A, lane 4) that was endo H resistant (Fig. 4B, compare lane 7 to 8), confirming temperature sensitivity conferred by the mutation. TYR(T373K)-GFP, as expected, was produced as the early high mannose (Fig. 4A, compare lane 5 to 6), endo H-sensitive glycoform (Fig. 4B, compare lane 9 to 10), regardless of temperature.
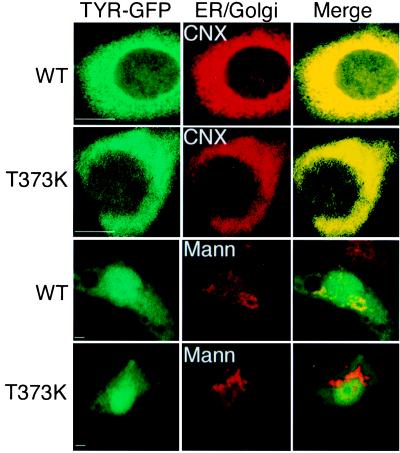
Immunofluorescence confocal microscopy provided direct evidence that unlike WT tyrosinase, the mutant forms remained in the ER. WT TYR-GFP expressed in mouse melanocytes colocalized with the ER resident chaperone calnexin and the Golgi complex resident protein α-1,2-mannosidase II (Fig. 5, WT). In contrast, TYR(T373K) colocalized with calnexin, but not with α-1,2-mannosidase II, demonstrating that TYR(T373K) was exclusively in the ER and did not reach the Golgi apparatus (Fig. 5, T373K).
Figure 5.
The albino T373K TYR mutation causes retention in the ER. Micrographs showing green fluorescence from the chimeric proteins WT TYR-GFP (WT) and TYR(T373K)-GFP (T373K) (Left), transiently expressed in WT mouse melanocytes, calnexin (CNX), and α-1,2-mannosidase II (Mann) stained with rhodamine (Middle), and merge of the two respective images (Right). (The bars represent 1 μm.)
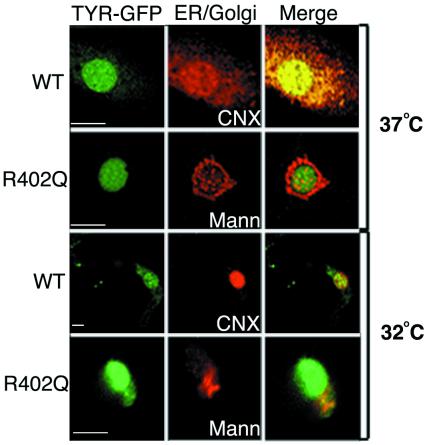
Similar analysis showed that TYR(R402Q)-GFP was restricted to the ER at the nonpermissive temperature of 37°C, but progressed to the Golgi complex at the permissive temperature of 32°C (Fig. 6). At 37°C, TYR(R402Q)-GFP was localized solely to the calnexin-stained ER, being excluded from the α-1,2-mannosidase II-stained Golgi complex. In contrast, at 32°C TYR(R402Q)-GFP was distributed to the ER and Golgi apparatus, as demonstrated by the colocalization with both markers, calnexin and α-1,2-mannosidase II. Furthermore, when visualized at lower magnification, TYR(R402Q)-GFP also was observed in punctate spots at distal sites in the extending dendrites likely representing melanosomes. Therefore, the R402Q substitution causes temperature-dependent retention in the ER.
Figure 6.
Exit of albino R402Q TYR from the ER is temperature dependent. TYR(R402Q)-GFP was transiently expressed in WT mouse melanocytes grown continuously at 37°C or shifted to the permissive temperature of 32°C for 14 h, as indicated. The micrographs show green fluorescence emitted from the chimeric protein TYR(R402Q)-GFP (Left), red fluorescence detecting calnexin (CNX) or α-1,2-mannosidase II (Mann) stained with rhodamine (Middle), and merged images (Right). (The bars represent 2 μm.)
Discussion
Pigmentation is a multistep process critically dependent on the functional integrity of tyrosinase, the rate-limiting enzyme in melanin synthesis. Here we show that the quality-control system of the secretory pathway monitors the integrity of tyrosinase maturation. Mutant proteins, representatives of the albino phenotype, are retained in the ER bound to calnexin and calreticulin and are not released to the targeted organelle, the melanosome. Our observations place albinism with an increasing number of disease states associated with retention of malfolded protein in the ER that include cystic fibrosis and emphysema (reviewed in refs. 29–32).
Tyrosinase is a highly conserved protein possessing 40% identity with two other melanocyte-specific proteins termed tyrosinase-related protein 1 and 2, i.e., TRP1/b-locus/gp75 (33) and TRP2/DOPAchrome tautomerase (for review see refs. 20, 34, and 35). Currently, approximately 100 different mutations in tyrosinase have been reported in individuals with oculocutaneous albinism, clustering in five regions, which include two copper binding sites (CuA and CuB), domains flanking CuB, and the C domain at the N terminus (1, 35), some directly affecting copper binding critical for enzymatic activity (36).
The C85S mutation (6, 23), and its human homologue C89R (37), reside in a critical domain (Cys-Gly-Asn-Cys) conserved in tyrosinases of different species and in TRP1 and TRP2. The brown mouse harbors a similar mutation, C86Y (38), that produces an unprocessed TRP1 (39). We showed that Tyr(C85S) was an unprocessed and misfolded glycoform that remained in the ER, efficiently bound to calnexin and calreticulin. It is likely that the close proximity of C85 to the N terminus cotranslationally produces an essential disulfide bond, critical for maintaining the integrity of the vectorial folding process in the ER (40).
N-linked glycans are likely to be required for the maturation and stability of tyrosinase because glycosylation inhibitors abolish pigmentation in mouse melanoma cells (41). Human and mouse tyrosinase possess seven and six consensus glycosylation sites, respectively. The TYR ER glycoforms appear as a 70-kDa doublet, because of inefficient glycosylation of the consensus site at Asn-290 (not present in the mouse sequence) caused by a proximal Pro (Asn-Gly-Thr-Pro) (A. Ujvari, R.A., S. Drabic, E.C., Y.S., R.H., and D.N.H., unpublished work). TYR(T373K) lacks the conservative glycosylation site at Asn-371, producing a lower molecular weight doublet representing a protein with five or six glycans that is retained in the ER, presumably caused by misfolding. In addition, the T to K mutation introduces an additional positive charge in close proximity to the CuB region. This protein, once again, was bound to calnexin and calreticulin, indicating that additional glycosylation sites participate in the attachment of these two lectin chaperones to tyrosinase.
TYR(R402Q)/Tyr(H402A) display a ts phenotype where the enzyme is functional when produced at 32°C but not at 37°C (16, 42). Here we demonstrate that the basis for this thermosensitivity is the retention of the defective protein in the ER. At the permissive temperature of 32°C, the protein was able to reach vesicles in the dendrites representative of melanosomes. In contrast, at the elevated temperature of 37°C, the protein maintained high mannose endo H-sensitive glycans and completely colocalized with the ER marker calnexin. TYR(R402Q)/Tyr(H402A) behaved like the much-studied CFTR(ΔF508) mutation that is responsible for the large majority of cases of cystic fibrosis (reviewed in ref. 29), and the model trafficking thermosensitive protein vesicular stomatitis virus G protein (tsO45 strain) (43).
Mutations in proteins involved in the transport of tyrosinase to melanosomes, such the adaptor protein AP-3, also can cause albinism. AP-3 binds to a synthetic tail peptide of tyrosinase in vitro (44), and mutations in the β3A subunit of AP-3 were identified in two albino siblings with Hermansky–Pudlak syndrome (45) and the pearl mouse (46, 47), whereas the mocha mouse carries a mutation in the δ subunit of AP-3 (48). All three conditions showed altered trafficking of lysosomal proteins, leading to defective platelets and lysosomal abnormalities. Presumably, the transport of WT tyrosinase to the melanosomes also is affected by these mutations.
Although certain chaperones and folding factors are found abundantly in the secretory pathway in all cell types, interacting promiscuously with a large number of substrates (primary quality control, such as calnexin and calreticulin), other chaperones interact with selected proteins and are found only in specific cell types (secondary quality control) (refs. 13 and 14, reviewed in refs. 15, 49, and 50). The secondary quality-control system is likely to play a critical role in melanocytes where tyrosinase can comprise up to 0.4% of the total cellular protein (10). This notion is supported by studies of tyrosinase processing and maturation in human melanoma cells that have lost at least part of their differentiated phenotype (12) and by experiments using heterologous cellular systems, such as COS7 cells (51). In both cases tyrosinase remains mostly as the immature 70-kDa glycoform that is bound to calnexin. At least two melanocyte-specific proteins, TRP1 and the pink-eyed dilution, contribute to tyrosinase's stability (52, 53), which also might be affected in melanoma cells. The observations that WT tyrosinase behaves like mutant proteins in nonheterologous or dediffentated cell types (12, 51) underscore the importance of exploring maturation, quality control, and degradation in normal host cells that contain all of the components required to lead tyrosinase to the melanosomes, its final destination in melanocytes.
Acknowledgments
We thank Dr. R. Spritz (Human Medical Genetics Program, University of Colorado Health Sciences Center, Denver) for the human tyrosinase cDNA clones, Drs. S. Pomerantz (Rehovot, Israel), L. Old (Ludwig Institute for Cancer Research, Memorial Sloan-Kettering Cancer Center, New York), and V. Hearing (Laboratory of Cell Biology, National Institutes of Health, Bethesda, MD) for anti-tyrosinase antibodies, Drs. M. Farquhar (Departments of Cellular and Molecular Medicine and Pathology, University of California, San Diego) and K. Moremen (Department of Biochemistry and Molecular Biology, University of Georgia, Athens) for the anti-α-1,2-mannosidase II antibodies, and Dr. Shigeki Shibahara, Department of Molecular Biology and Applied Physiology, Tohoku University School of Medicine, Sendai, Japan, for the tyrosinase promoter. This work was supported by National Institutes of Health Grants AR39848 to R.H. and AR41942 (Yale Skin Diseases Research Center; R. E. Tigelaar, Program Investigator), and grants from The Medical Foundation, Edward Mallinckrodt, Jr. Foundation, and National Institutes of Health Grant CA79864 to D.N.H.
Abbreviations
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- endo H
endoglycosidase H
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- TRP
tyrosinase-related protein
- ts
temperature sensitive
- TYR
human tyrosinase
- Tyr
mouse tyrosinase
- WCL
whole-cell lysate
- WT
wild type
References
- 1.Oetting W S, King R A. Hum Mutat. 1999;13:99–115. doi: 10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Lerner A B, Fitzpatrick T B, Calkins E, Summerson W H. J Biol Chem. 1949;178:185–195. [Google Scholar]
- 3.Körner A, Pawelek J. Science. 1982;217:1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi R K, Hearing V J, Urabe K, Aroca P, Spritz R A. J Biol Chem. 1992;267:23707–23712. [PubMed] [Google Scholar]
- 5.Kwon B S, Haq A K, Pomerantz S H, Halaban R. Proc Natl Acad Sci USA. 1987;84:7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon B S, Haq A K, Wakulchik M, Kestler D, Barton D E, Francke U, Lamoreux M L, Whitney J B D, Halaban R. J Invest Dermatol Symp Proc. 1989;93:589–594. doi: 10.1111/1523-1747.ep12319693. [DOI] [PubMed] [Google Scholar]
- 7.Muller G, Ruppert S, Schmid E, Schutz G. EMBO J. 1988;7:2723–2730. doi: 10.1002/j.1460-2075.1988.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto H, Takeuchi S, Kudo T, Sato C, Takeuchi T. Jpn J Genet. 1989;64:121–135. doi: 10.1266/jjg.64.121. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard B, Fuller B B, Vijayasaradhi S, Houghton A N. J Exp Med. 1989;169:2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halaban R, Pomerantz S H, Marshall S, Lambert D T, Lerner A B. J Cell Biol. 1983;97:480–488. doi: 10.1083/jcb.97.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halaban R, Pomerantz S H, Marshall S, Lerner A B. Arch Biochem Biophys. 1984;230:383–387. doi: 10.1016/0003-9861(84)90121-8. [DOI] [PubMed] [Google Scholar]
- 12.Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, Setaluri V, Hebert D N. Proc Natl Acad Sci USA. 1997;94:6210–6215. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert D N, Simons J F, Peterson J R, Helenius A. Cold Spring Harbor Symp Quant Biol. 1995;60:405–415. doi: 10.1101/sqb.1995.060.01.045. [DOI] [PubMed] [Google Scholar]
- 14.Hebert D N, Foellmer B, Helenius A. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- 15.Ellgaard L, Molinari M, Helenius A. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 16.Halaban R, Moellmann G, Tamura A, Kwon B S, Kuklinska E, Pomerantz S H, Lerner A B. Proc Natl Acad Sci USA. 1988;85:7241–7245. doi: 10.1073/pnas.85.19.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon B S, Wakulchik M, Haq A K, Halaban R, Kestler D. Biochem Biophys Res Commun. 1988;153:1301–1309. doi: 10.1016/s0006-291x(88)81370-6. [DOI] [PubMed] [Google Scholar]
- 18.Chintamaneni C D, Halaban R, Kobayashi Y, Witkop C J, Jr, Kwon B S. Proc Natl Acad Sci USA. 1991;88:5272–5276. doi: 10.1073/pnas.88.12.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park K C, Chintamaneni C D, Halaban R, Witkop C, Jr, Kwon B S. Am J Hum Genet. 1993;52:406–413. [PMC free article] [PubMed] [Google Scholar]
- 20.Halaban R, Moellmann G. J Invest Dermatol. 1993;100,Suppl.:176s–185s. [PubMed] [Google Scholar]
- 21.Tamura A, Halaban R, Moellmann G, Cowan J M, Lerner M R, Lerner A B. In Vitro Cell Dev Biol. 1987;23:519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- 22.Bennett D C, Cooper P J, Dexter T J, Devlin L M, Heasman J, Nester B. Development (Cambridge, UK) 1989;105:379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- 23.Jackson I J, Bennett D C. Proc Natl Acad Sci USA. 1990;87:7010–7014. doi: 10.1073/pnas.87.18.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon B S, Halaban R, Chintamaneni C. Biochem Biophys Res Commun. 1989;161:252–260. doi: 10.1016/0006-291x(89)91588-x. [DOI] [PubMed] [Google Scholar]
- 25.Giebel L B, Tripathi R K, King R A, Spritz R A. J Clin Invest. 1991;87:1119–1122. doi: 10.1172/JCI115075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata K, Muraosa Y, Tomita Y, Tagami H, Shibahara S. J Biol Chem. 1992;267:20584–20588. [PubMed] [Google Scholar]
- 27.Chen Y T, Stockert E, Tsang S, Coplan K A, Old L J. Proc Natl Acad Sci USA. 1995;92:8125–8129. doi: 10.1073/pnas.92.18.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez M, Maloy W L, Hearing V J. J Biol Chem. 1989;264:3397–3403. [PubMed] [Google Scholar]
- 29.Kopito R R. Physiol Rev. 1999;79:S167–S173. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- 30.Callea F, Brisigotti M, Fabbretti G, Bonino F, Desmet V J. Liver. 1992;12:357–362. doi: 10.1111/j.1600-0676.1992.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 31.Sifers R N. Nat Struct Biol. 1995;2:355–357. doi: 10.1038/nsb0595-355. [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsov G, Nigam S K. N Engl J Med. 1998;339:1688–1695. doi: 10.1056/NEJM199812033392307. [DOI] [PubMed] [Google Scholar]
- 33.Jackson I J. Proc Natl Acad Sci USA. 1988;85:4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsukamoto K, Jackson I J, Urabe K, Montague P M, Hearing V J. EMBO J. 1992;11:519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oetting W S. Anatomy of Pigment Cell Genes Acting at the Subcellular Level. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 36.Spritz R A, Ho L, Furumura M, Hearing V J., Jr J Invest Dermatol. 1997;109:207–212. doi: 10.1111/1523-1747.ep12319351. [DOI] [PubMed] [Google Scholar]
- 37.Spritz R A, Strunk K M, Hsieh C L, Sekhon G S, Francke U. Am J Hum Genet. 1991;48:318–324. [PMC free article] [PubMed] [Google Scholar]
- 38.Zdarsky E, Favor J, Jackson I J. Genetics. 1990;126:443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halaban R, Moellmann G. Proc Natl Acad Sci USA. 1990;87:4809–4813. doi: 10.1073/pnas.87.12.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Helenius J, Braakman I, Helenius A. Proc Natl Acad Sci USA. 1995;92:6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imokawa G, Mishima Y. Cancer Res. 1982;42:1994–2002. [PubMed] [Google Scholar]
- 42.Tripathi R K, Strunk K M, Giebel L B, Weleber R G, Spritz R A. Am J Med Genet. 1992;43:865–871. doi: 10.1002/ajmg.1320430523. [DOI] [PubMed] [Google Scholar]
- 43.Presley J F, Cole N B, Schroer T A, Hirschberg K, Zaal K J, Lippincott-Schwartz J. Nature (London) 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 44.Honing S, Sandoval I V, von Figura K. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dell'Angelica E C, Shotelersuk V, Aguilar R C, Gahl W A, Bonifacino J S. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 46.Feng L, Seymour A B, Jiang S, To A, Peden A A, Novak E K, Zhen L, Rusiniak M E, Eicher E M, Robinson M S, et al. Hum Mol Genet. 1999;8:323–330. doi: 10.1093/hmg/8.2.323. [DOI] [PubMed] [Google Scholar]
- 47.Zhen L, Jiang S, Feng L, Bright N A, Peden A A, Seymour A B, Novak E K, Elliott R, Gorin M B, Robinson M S, Swank R T. Blood. 1999;94:146–155. [PubMed] [Google Scholar]
- 48.Kantheti P, Qiao X, Diaz M E, Peden A A, Meyer G E, Carskadon S L, Kapfhamer D, Sufalko D, Robinson M S, Noebels J L, Burmeister M. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 49.Vassilakos A, Cohen-Doyle M F, Peterson P A, Jackson M R, Williams D B. EMBO J. 1996;15:1495–1506. [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon K S, Helenius A. J Biol Chem. 1999;274:7537–7544. doi: 10.1074/jbc.274.11.7537. [DOI] [PubMed] [Google Scholar]
- 51.Toyofuku K, Wada I, Hirosaki K, Park J S, Hori Y, Jimbow K. J Biochem. 1999;125:82–89. doi: 10.1093/oxfordjournals.jbchem.a022272. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi T, Imokawa G, Bennett D C, Hearing V J. J Biol Chem. 1998;273:31801–31805. doi: 10.1074/jbc.273.48.31801. [DOI] [PubMed] [Google Scholar]
- 53.Orlow S J, Brilliant M H. Exp Eye Res. 1999;68:147–154. doi: 10.1006/exer.1998.0599. [DOI] [PubMed] [Google Scholar]