Abstract
A transgenic mouse line (Tg.AC) carrying an activated v-Ha-ras oncogene fused to the embryonic ζ-globin promoter develops an array of spontaneous epithelial and mesenchymal neoplasms. In this report we describe the morphological, immunophenotypic, and molecular features of a unique hematopoietic neoplasm in these mice. The cardinal lesion of this disease is marked hepatomegaly due to leukemic proliferation and infiltration. In the peripheral blood, there is a marked increase in the number of metarubricytes and other less differentiated erythroid progenitor cells. Leukemic cells stain positively with an erythroid-associated nuclear transcription factor (GATA-1). Using a reverse transcription polymerase chain reaction assay, co-expression of GATA-1 and endogenous ζ-globin genes is detected in hematopoietic tissues of nonleukemic transgenic and nontransgenic mice. ras transgene expression is, however, detected only in normal bone marrow and leukemic tissues of transgenic mice, and 5′ mapping experiments using S1 protection analysis of total RNA from leukemic tissue indicates that transcription of the transgene mRNA is initiated from the natural ζ-globin promoter start site, supporting the belief that the ζ-globin promoter directs v-Ha-ras expression in erythroid progenitor cells, ultimately leading to leukemic transformation.
For many years the mouse has served as a model for mammalian hematopoiesis, and malignant neoplasms of the murine hemolymphatic system have been extensively studied and used as models for corresponding human diseases. 1 Of the nonlymphoid neoplasms, erythroleukemia, myeloid leukemia, histiocytic sarcomas, and mast cell tumors are recognized diseases in mice that may occur spontaneously or can be induced by viruses and other experimental methodologies. 2 Of these, erythroleukemia is rare, with the majority of cases reported in mice associated with the experimental inoculation of certain retroviruses, most notably the Friend leukemia virus. 3 It is so rare that only a single case of spontaneous erythroleukemia in a mouse has been reported. 4
The advent of transgenic technology has allowed the introduction of specific oncogenes into mice and targeted tumorigenesis in various tissues, including the hemolymphatic system. 5,6 Transgenic models of lymphoid neoplasia have been developed, but transgenic lines expressing specific disorders of nonlymphoid hematopoietic cells have not been reported.
The Tg.AC transgenic mouse line, carrying a v-Ha-ras oncogene linked to the embryonic ζ-globin promoter, was developed by Leder and colleagues to study the development of the embryonic hematopoietic system. 7 Interestingly, the Tg.AC strain develops an array of spontaneous epithelial and mesenchymal tumors 8,9 and has been used in mechanistic studies of chemically induced skin carcinogenesis due to its sensitivity to induction of skin papillomas. 10,11 However, hematopoietic abnormalities have not been reported other than descriptions of hepatosplenomegaly associated with infiltrations of lymphoid or myelomonocytic cells and typically found in animals bearing skin tumors. 7,8
In our laboratory, cases of hepatosplenomegaly in Tg.AC mice have been observed due to a distinct neoplastic disease of erythroid cells associated with expression of the transgene. We propose that the transcription factors required for the constitutive expression of the endogenous ζ-globin promoter in adult erythroid stem cells are functioning to stimulate expression of the ζ-globin promoted ras transgene and that accumulation of the oncogenic ras protein results in aberrant proliferation of erythropoietic cells. Therefore, in this report we present an initial morphological characterization of this disease as well as an investigation of the roles of the ras transgene and the erythroid-specific transcription factor, GATA-1, in its development.
Materials and Methods
Mice
The animals described in this report were identified from a colony of homozygous Tg.AC transgenic mice and parental strain FVB/N mice obtained from Taconic Farms (Germantown, NY) at approximately 4 to 5 weeks of age, held for up to 10 weeks, and then placed on skin tumor induction protocols as described elsewhere. 10,11 Standard treatment was twice-weekly applications of test chemical or acetone vehicle to the shaved dorsal skin. Two affected animals in this data set, as well as all randomly selected unaffected Tg.AC and FVB/N mice used as controls, were not on any chemical treatment protocol and had no chemical exposure history. Mice were multiply housed (five per cage), kept on a 12-hour light/dark cycle, and fed either NIH-07 pellet ration or Purina Pico Chow 5058 ad libitum.
Pathological Evaluations
Observation of marked abdominal distention due to hepatosplenomegaly provided antemortem identification of affected mice. Blood collections for hematological analyses were made from the retro-orbital venous sinus under anesthesia with 70% CO2/30% O2. Mice were killed by CO2 asphyxiation, and body and organ weights were recorded. Tissues were collected, fixed in 10% neutral buffered formalin, paraffin embedded, sectioned at 6 μm, and stained with hematoxylin and eosin for light microscopic evaluation. Tissues routinely collected at necropsy were liver, spleen, kidney, heart, lung, femur, sternum, and all gross lesions.
Automated complete blood counts were made with a Technicon H*1 hematology analyzer (Miles, Tarrytown, NY) outfitted with specialized rodent software. Hematological measurements included the following parameters: white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hgb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelet count. Nucleated cell differentials were made visually from blood smears stained with Wright-Giemsa solution, and reticulocyte counts were obtained from smears stained with New Methylene Blue (EK Industries, Joliet, IL).
Immunohistochemical Analysis of GATA-1
Four-micron serial sections of formalin-fixed, paraffin-embedded tissues were deparaffinized and rehydrated to 1× Automation buffer (Biomeda Corp., Foster City, CA). Endogenous peroxides were blocked in 3% aqueous H2O2 for tissue sections and 0.3% for air-dried cytocentrifuge preparations. Tissues were subjected to antigen retrieval in the microwave (twice for 5 minutes each at 700 W in 0.1 mol/L citrate buffer, pH 6.0). 12,13 After cooling to room temperature, all slides were blocked with 1% normal goat serum (Vector Laboratories, Burlingame, CA) and then incubated with rat anti-mouse GATA-1 (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:100 dilution for 1 hour at room temperature. Detection was accomplished with StrAviGen supersensitive detection kit (BioGenex Laboratories, San Ramon, CA), followed by incubation with diaminobenzidine as the substrate and counterstaining with Harris or Mayer’s hematoxylin (Sigma Chemical Co.).
Preparation of Total RNA from Leukemic and Nonleukemic Tissue
Liver, spleen, and bone marrow were collected and flash frozen in liquid nitrogen. Total RNA was extracted from frozen tissue using TriReagent (Molecular Research Center, Cincinnati, OH) and following the manufacturer’s protocol. Organs were homogenized in TriReagent using a Brinkmann polytron (Brinkmann Instrument Co., Westbury, NY), extracted once with chloroform, and then precipitated with isopropanol. The final RNA pellet was washed once in 75% ethanol, resuspended in sterile 0.1% diethylpyrocarbonate-treated water, and then heated for 15 minutes at 55°C to facilitate dissolution of the pellet. RNA was quantified by spectrophotometric measurements at A260.
Reverse Transcription PCR
Reverse transcription (RT)-PCR was performed as described previously. 9,13 RNA-specific primers were directed to either the SV40 polyadenylation/splice region of the transgene, 9,13 GATA-1, 14 mouse β2-microglobulin, 13 or endogenous mouse ζ-globin. Primer sequences for ζ-globin were selected from genomic DNA of mouse ζ-globin 15 with the sense primer located in exon 1 from bases 435 to 462 and the antisense primer in exon 2 spanning bases 1509 to 1536. Sequences for the ζ-globin primers are as follows: sense 5′-GCC AGT CTT GAG TGC ACT CAA CTC CAG C-3′ and antisense 5′-CAC TAG AGA GGT TGT CGA TGC TCT TAA C-3′. All primers were synthesized by BioServe Biotechnologies (Laurel, MD). RT-PCR products were 214-bp RNA-derived product and a 279-bp DNA-derived product for the v-Ha-ras transgene, 578 bp for GATA-1, 300 bp for ζ-globin, and 212 bp for mouse β2-microglobulin. Amplification of the cDNA was carried out for 30 cycles using annealing temperatures of 50°C for transgene and mouse β2-microglobulin primers, 55°C for GATA-1 primers, and 60°C for ζ-globin primers. Denaturing, annealing, and extension were all done for 30 seconds. RT-PCR products were electrophoresed on 2% agarose gels prepared with 1× TAE (0.04 mol/L Tris acetate, 0.001 mol/L EDTA) and containing 0.5 μg/ml ethidium bromide.
5′ Transcript Mapping via S1 Nuclease Protection Assay
32P-labeled DNA used for the S1 nuclease protection assay was prepared from a pGEM plasmid containing the mouse ζ-globin promoter (gift from A. Leder, Harvard Medical School). A 4-kb BamH1 fragment was excised, gel purified, and end-labeled with [γ32P]ATP using polynucleotide kinase (Promega, Madison, WI). The labeled fragment was then digested with EcoR1, generating a 1000-bp 32P-end-labeled fragment, which served as the S1 probe, and 0.1 pmol of probe was hybridized to 25 μg of total RNA overnight at 42°C in hybridization buffer (40 mmol/L PIPES, 1 mmol/L EDTA, 0.4 mmol/L NaCl, and 80% formamide). After incubation, the hybridized samples were digested to completion with S1 nuclease (Promega) containing salmon testes DNA (Sigma Chemical Co., St. Louis, MO) as carrier for 1 hour at 37°C, and then the reaction was terminated with the addition of 1 mol/L ammonium acetate, 0.5 mol/L EDTA, and 50 mg/ml tRNA (Sigma). The S1-protected samples were ethanol precipitated, resuspended in formamide loading buffer, and electrophoresed on an 8% polyacrylamide sequencing gel containing 7.7 mol/L urea.
Results
Pathological Findings
Six affected mice with complete histopathological and hematological evaluations were selected to constitute the data set of this report. These cases occurred over a 16-month time span. Because the animal colony is used for various studies and not maintained for tumor incidence statistics, the exact incidence of erythroleukemia in Tg.AC mice has not been documented, but it is estimated to be <5%. Erythroleukemia incidence in control heterozygous females used in chemical testing experiments is approximately 1% (William Eastin, personal communication). Case histories and necropsy data of the six diseased mice as well as age- and sex-matched controls are presented in Table 1 ▶ . Although the six cases evaluated in this study were females, a true gender predisposition could not be determined due to a preponderance of female Tg.AC mice in the animal colony due to study design. The age of onset of hepatomegaly and necropsy ranged from 4 to 9 months. Affected mice were on skin tumor studies and had been treated topically with various agents, including TPA, benzene, and acetone or untreated. To date, no association has been made between occurrence of erythroleukemia and any particular chemical treatment.
Table 1.
Selected Case Histories and Necropsy Data
| Case | Age (months) | Skin Treatment | Body weight (g) | Liver weight (g) | Spleen weight (g) | Gross lesions | |
|---|---|---|---|---|---|---|---|
| Leukemic Tg.AC | 1 | 9 | TPA | 26.6 | 6.2 | ND | Enlarged renal and submandibular nodes |
| 2 | 8 | TPA | 33.2 | 7.6 | ND | None | |
| 3 | 4 | None | 24.0 | 6.8 | ND | None | |
| 4 | 7 | Acetone | 29.5 | 9.2 | 0.83 | Enlarged hepatic node and ovary | |
| 5 | 6 | Benzene | 30.0 | 8.5 | 1.34 | Enlarged axillary, renal, and lumbar nodes | |
| 6 | 6 | None | 31.2 | 9.5 | 0.56 | None | |
| Control Tg.AC | 1 | 6 | None | 23.2 | 1.0 | 0.12 | None |
| 2 | 6 | None | 28.7 | 1.3 | 0.11 | None | |
| 3 | 6 | None | 39.2 | 1.5 | 0.14 | None | |
| Control FVB/N | 1 | 6 | None | 28.3 | 1.4 | 0.13 | None |
| 2 | 6 | None | 26.4 | 1.2 | 0.12 | None | |
| 3 | 6 | None | 26.6 | 1.0 | 0.08 | None |
ND, splenomegaly noted grossly but spleen weight not recorded.
Abdominal distention due to marked hepatomegaly was the distinguishing feature common to all cases. At necropsy, enlarged livers were mottled and friable and were increased in weight severalfold relative to that in control mice (Table 1) ▶ . Splenomegaly of variable severity was also a consistent gross finding. The only other gross observation noted with regularity was one or more enlarged lymph nodes, usually the submandibular and/or abdominal nodes (Table 1) ▶ .
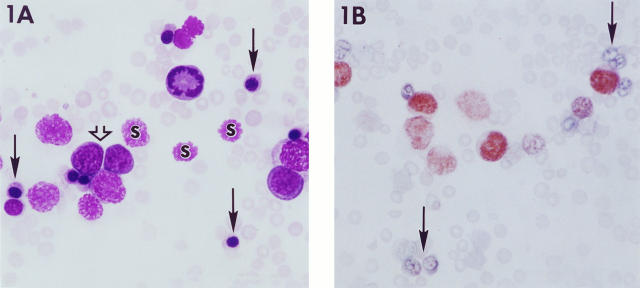
Abnormalities of the peripheral blood were found in all cases evaluated (Table 2) ▶ . Automated counts of total nucleated cells in the peripheral blood were markedly elevated. Examination of blood smears revealed numerous metarubricytes (average of 40% of total nucleated cells), erythroblasts, and smudge cells (Figure 1A) ▶ . In addition to the large numbers of metarubricytes, anisocytosis and polychromasia of the non-nucleated red cells were also apparent, and reticulocyte counts were elevated. Other abnormalities of red blood cell parameters were decreased RBC, Hgb, and Hct and increased MCV, indicating an anemia. Numerous mitotic blasts were present in the peripheral blood of some cases (Figure 1A) ▶ . WBCs corrected for nucleated erythroid and smudge cells ranged from 0.4 × 10 3 to 18 × 103/ml. A small subpopulation in the peripheral blood consisted of cells with horseshoe-shaped nuclei and light blue-gray cytoplasm but without granules, interpreted to be possibly of the myelomonocytic lineage.
Table 2.
Hematology of Tg.AC Leukemic and Control Mice
| TNCs (1000/μl) | Metarubri- cytes (%)* | RBC (mil/μl) | HGB (g/dl) | Hct (%) | MCV (fl) | MCH (pg) | MCHC (g/dl) | Reticulo- cytes (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Leukemic (n = 6 cases) | 243 ± 140 | 39.2 ± 15.1 | 4.8 ± 1.0 | 7.1 ± 1.9 | 24.7 ± 2.7 | 51.2 ± 5.3 | 14.4 ± 2.6 | 28.4 ± 5.4 | 9.2 ± 4.6 |
| Control Tg.AC (n = 3 mice) | 6.4 ± 1.2 | 0 | 10.6 ± 0.9 | 14.6 ± 0.5 | 46.7 ± 2.3 | 44.3 ± 1.5 | 13.8 ± 0.8 | 31.3 ± 0.9 | 2.0 ± 0.2 |
| Control FVB/N (n = 3 mice) | 8.9 ± 4.4 | 0 | 9.3 ± 0.4 | 13.7 ± 0.5 | 43.0 ± 3.0 | 46.7 ± 1.5 | 14.8 ± 0.2 | 31.8 ± 1.4 | 1.8 ± 0.3 |
Results are presented as mean ± SD. TNCs, total nucleated cells.
*Percentage of TNCs.
Figure 1.
Peripheral blood and liver from Tg.AC mice with erythroleukemia. A: Numerous metarubricytes (closed arrows) and smudge cells (s) are intermingled with erythroblasts (open arrow), including a single cell in mitosis. B: Blood smear immunostained for GATA-1 demonstrates nuclear localization in several erythroblasts. Note that the metarubricytes are unstained (arrow). Magnification, ×2500.
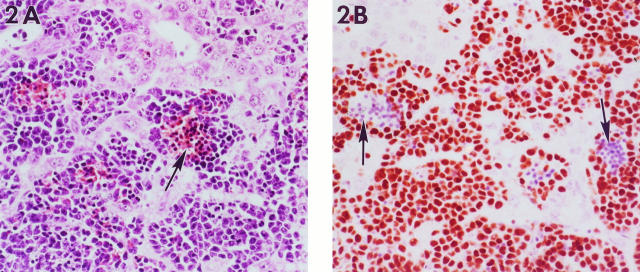
Hepatomegaly corresponded microscopically to diffuse filling of hepatic sinusoids with leukemic cells that effaced the hepatic architecture (Figure 2A) ▶ . The predominant cell type was poorly differentiated with a round to folded nucleus containing coarsely clumped chromatin and a moderate amount of basophilic cytoplasm. Erythroid differentiation was evidenced by intermingled metarubricytes and erythrocytes, often forming sinusoidal blood lakes with more undifferentiated large cells around the periphery. Many of the infiltrating cells stained positive for the cell proliferation marker Proliferating Cell Nuclear Antigen (data not shown). Mitoses were abundant, as well as focal areas of necrosis of both infiltrating and parenchymal cells.
Figure 2.
Liver from a mouse with erythroleukemia. A: The hepatic sinusoids are infiltrated by immature erythroid cells. In some areas, these cells surround blood pools that contain more mature nucleated red blood cells (arrow). B: Immunostaining for GATA-1 demonstrates nuclear immunoreactivity in most infiltrating cells except for more mature nucleated erthryocytes in the center of the sinusoidal pools (arrows). Magnification, ×276.
Changes in the bone marrow and spleen were variable. In some cases, the marrow cavity and splenic red pulp were effaced by neoplastic cells similar to those in the liver sinusoids. In most cases, however, there was no clear neoplastic involvement of these tissues, and the hematopoietic cells at these sites exhibited a variable myeloid/erythroid ratio. In cases of increased granulocytopoiesis, eosinophils were a prominent component in both the marrow and spleen.
In cases with gross involvement of nodes or other tissues (Table 1) ▶ , microscopic examination revealed leukemic infiltration by sheets of poorly differentiated cells with focal areas of erythroid differentiation. An increased number of nucleated cells within blood vessels was apparent in multiple tissues.
Expression of GATA-1 in Erythroleukemic Tissues and Cytological Preparations
GATA-1 has been shown to be a key regulator of erythroid differentiation, may be a central regulator of erythroid-specific gene expression, and has also been shown to modulate all globin gene expression. 16,17 Targeted disruption of mouse GATA-1 as well as differentiation assays show that an absence of GATA-1 results in an arrest of erythroid precursors at the proerythroblast stage. 18-20 Given the important role of GATA-1 in erythropoiesis, peripheral blood smears and paraffin-embedded liver sections were immunostained for protein expression. Intense reactivity was present within neoplastic cells in the peripheral blood (Figure 1B) ▶ and infiltrating the hepatic sinusoids (Figure 2B) ▶ . At both sites, GATA-1 immunolocalization was limited to the nuclei of the more poorly differentiated cells.
The ras Transgene, GATA-1, and Endogenous ζ-Globin Are Co-Expressed in Leukemic Tissues
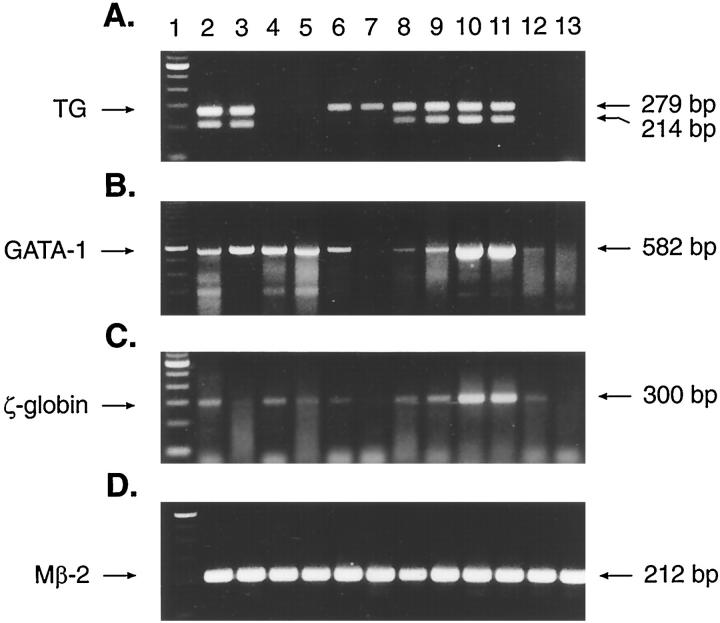
Liver, spleen, and bone marrow from normal (n = 3) and leukemic (n = 5) animals were examined by RT-PCR for the expression of the ras transgene. Results of representative cases are shown in Figure 3A ▶ . Primers directed to the SV40 polyadenylation/splice region of the transgene construct encompass a 65-bp intervening sequence that gives rise to a 279-bp product derived from contaminating DNA and to a 214-bp product derived from processed messenger RNA. As can be seen in Figure 3A ▶ , the 279-bp product was detected in all Tg.AC tissues (lanes 2, 3, and 6 to 11). However, the RNA-specific 214-bp product was found only in normal Tg.AC bone marrow (lanes 2 and 3), leukemic spleen (lanes 8 and 10), and leukemic liver (lanes 9 and 11). Although the normal Tg.AC spleen sample of this study (lane 6) did not show evidence of transgene expression, other studies have demonstrated expression in approximately 50% of normal Tg.AC spleens 8 (and unpublished observations). Background strain FVB/N tissues showed no evidence of either the 279- or 214-bp transgene product (lanes 4, 5, 12, and 13).
Figure 3.
Expression of the v-Ha-ras transgene, GATA-1, and ζ-globin in normal and leukemic tissues from Tg.AC and FVB/N mice using RT-PCR. Total RNA from leukemic and nonleukemic tissues was assayed by RT-PCR using primers specific for the v-Ha-ras transgene (A), GATA-1 (B), ζ-globin (C), and β-2-microglobulin (Mβ2; D). Lane 1, 100-bp molecular weight marker (Gibco BRL); lanes 2 and 3, normal Tg.AC bone marrow; lanes 4 and 5, normal FVB/N bone marrow; and lane 6, normal Tg.AC spleen; lane 7, normal Tg.AC liver; lanes 8 and 10, leukemic spleen; lanes 9 and 11, leukemic liver; lane 12, normal FVB/N spleen; lane 13, normal FVB/N liver.
In addition to GATA-1, we were also interested in determining whether the natural (endogenous) ζ-globin gene was expressed in leukemic tissue in view of the fact that the ras transgene construct contains the ζ-globin promoter sequence. Expression of the endogenous embryonic ζ-globin gene would indicate the presence of transcription factors common to both. RT-PCR results of GATA-1 and endogenous ζ-globin are shown in Figure 3, B and C ▶ , respectively. Nonleukemic bone marrow and spleen from Tg.AC (lanes 2, 3, and 6) and parental strain FVB/N (lanes 4, 5, and 12) mice co-expressed GATA-1 and endogenous ζ-globin. In leukemic liver and spleen from Tg.AC mice (lanes 8 to 11), both GATA-1 and endogenous ζ-globin are expressed in addition to the transgene. Normal liver from both Tg.AC (lane 7) and FVB/N (lane 13) mice show no evidence of transgene (Figure 3A) ▶ , GATA-1 (Figure 3B) ▶ , or ζ-globin (Figure 3C) ▶ .
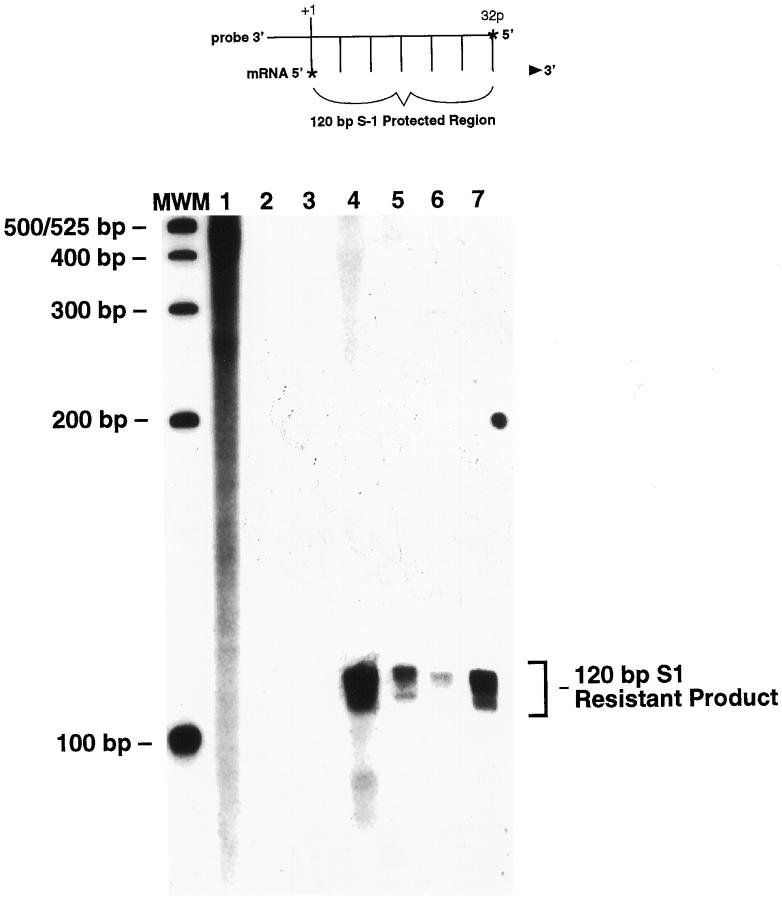
5′ Mapping Assay Demonstrates That the Transgene ζ-Globin Promoter Is Utilized in Erythroleukemia in the Tg.AC Mouse
Expression of the transgene in cutaneous tumors has been shown to be dependent on the ζ-globin promoter of the ras transgene (R.E. Cannon, R.S. Faircloth, J.W. Spalding, C.S. Trempus, K.M. Virgil, M.C. Humble, G.D. Lacks, J.-L. Klein, R.W. Tennant, submitted for publication). To determine whether the transgene initiates transcription from its ζ-globin promoter in erythroleukemia, RNA from leukemic spleen and liver was analyzed by S1 protection experiments. The assay, illustrated at the top of Figure 4 ▶ , predicts that, if transcription initiates from the known natural start site of the mouse ζ-globin promoter of the transgene, a 120-bp 32P-labeled fragment will be protected from S1 nuclease digestion. Figure 4 ▶ shows, as predicted, that a 120-bp S1-resistant product was produced from cell lines derived from squamous cell carcinomas taken from Tg.AC mice (lanes 4 and 5) but not in normal, non-tumor-bearing FVB/N skin (lane 2) or Tg.AC skin (lane 2). An S1-resistant product of identical size was also found in RNA from the spleen (lane 6) and liver (lane 7) of an animal diagnosed with erythroleukemia. The leukemic tissues and cell lines were also found to be expressing the ras transgene by RT-PCR (data not shown). These data indicate that transgene expression in erythroleukemias utilizes the ζ-globin promoter sequences for the start of transcription. The start of transcription identified in these experiments is identical to that observed in cell lines derived from cutaneous, transgene-expressing malignancies taken from Tg.AC mice (Figure 4) ▶ . This suggests that factors necessary for the expression of the transgene are common to cells within both the cutaneous reservoir and progenitor blood cells of erythrocytic origin.
Figure 4.
S1 nuclease demonstration of the transgene ζ-globin promoter as the site of the start of transcription. The probe used in the protection assay was prepared from the endogenous ζ-globin promoter. Shown at the top is a diagram of the S1 protection assay, where the 32P-labeled probe hybridizes to the complementary region of the transgene mRNA. After digestion to remove unhybrized probe, a 120-bp protected product should be observed, as shown in the bottom panel. Lane MWM, molecular weight marker; lane 1, unhybridized 32P-labeled probe; lanes 2 and 3, RNA from normal, non-tumor-bearing FVB/N and Tg.AC skin, respectively; lanes 4 and 5, RNA from epidermal carcinoma cell lines derived from malignancies removed from Tg.AC mice; lanes 6 and 7, RNA from erythroleukemic spleen and liver, respectively.
Discussion
In comparison with lymphoproliferative disease, neoplasms of myeloid cells are relatively rare in mice, and complex classification criteria such as those used in the French American British (FAB) classification of human myeloid leukemias have not been routinely applied to mice. The diagnosis of erythroleukemia in the Tg.AC mice of this study was based on neoplastic effacement of the liver and other tissues by cells exhibiting primarily erythroid differentiation, in addition to the leukemic profile of the peripheral blood, including both atypical blast forms, mitotic blasts, and metarubricytosis. The erythroid lineage of the leukemic cells is further supported by the positive immunostaining of a significant proportion of the infiltrating cells in the liver for the Ter119 erythroid surface marker as detected by flow cytometry (data not shown) and an even more extensive immunostaining for GATA-1, an erythroid lineage-associated DNA-binding nuclear protein and transcription factor. 17,21,22 The more differentiated metarubricytes seen within leukemic solid tissues as well as in the peripheral blood are interpreted to be the progeny of neoplastic progenitor cells in the liver or elsewhere. Alternatively, benign regenerative erythropoiesis due to concurrent anemia could account for some of the increase in metarubricytes, polychromasia, and reticulocytosis in leukemic mice. Although cells of the erythroid lineage predominate in this disorder of Tg.AC mice, a small number of myelomonocytic cells were detected in blood smears as well as in suspensions of liver-infiltrating cells by flow cytometry. This is consistent with current concepts of myeloproliferative disorders in which, although abnormal clonal proliferation may result in the predominance of a single lineage, more than one cell line may appear to be involved when there is transformation of a multipotent stem cell.
Several lines of evidence make it clear that the ζ-globin/v-Ha-ras transgene is involved in leukemogenesis in Tg.AC mice. First, a similar disease has never been seen in the parental FVB/N strain of mouse. 23 Second, a high level of transgene expression is invariably present in leukemic tissues, whereas expression is generally undetectable or low in normal tissues. 9 Furthermore, in situ hybridization studies with a transgene-specific probe produce a signal localized to the leukemic infiltrating cells in the liver 9 (and data not shown). No viral particles have been seen in electron microscopic examination of leukemic cells (data not shown).
Our current working hypothesis is that transgene activation targets accumulation of the activated ras protein to erythroid progenitor cells resulting in abnormal clonal proliferation, as evidenced by the known ability of ras-containing oncogenic viruses to induce a malignant phenotype in erythroid cells, both in vivo and in vitro. 24 Most of the erythroleukemias observed to date in the Tg.AC mouse have a mixture of cells at different stages of differentiation. Attempts to culture cells from erythroleukemic tissues were largely unsuccessful because the cells rapidly terminally differentiated in culture, even in the presence of supplemental interleukin-3 (data not shown). These observations are in agreement with our findings of large numbers of metarubricytes in leukemic mice as well as with other studies where it has been shown that Ha-ras infection of hematopoietic cells confers a proliferative advantage to cells of erythroid lineage although not blocking their capacity to differentiate. 25,26 Therefore, we conclude that overexpression of the v-Ha-ras transgene results in neoplastic transformation of erythroid progenitor cells and confers a proliferative advantage but not maturation arrest.
It has been shown that the start of transcription in cell lines derived from cutaneous malignancies in Tg.AC mice is dependent upon the ζ-globin promoter of the transgene construct (Cannon et al, submitted for publication). To determine whether transgene expression was also dependent upon the ζ-globin promoter in erythroleukemia, we undertook S1 protection experiments of RNA from leukemic spleen and liver tissue. Because we detected a protected product that is identical to the endogenous ζ-globin gene promoter, we can conclude that activation of transgene expression is dependent upon the ζ-globin promoter of the transgene construct and that initiation of transcription is not due to the activity of the transcriptional domain of another gene flanking the transgene integration site in chromosome 11.
In view of the fact that the ras transgene expression is driven by the ζ-globin promoter, it was of interest to examine both normal adult hematopoietic organs as well as tissues from animals with erythroleukemia for the presence of erythroid-specific transcription factors that function to regulate globin gene expression and that could thereby act as a transcriptional activator of the transgene. GATA-1, a member of the GATA family of nuclear DNA-binding transcription factors, has emerged as an important regulator of normal erythropoiesis, 17,20,22 including erythroid differentiation and globin gene regulation. Particularly noteworthy in regards to Tg.AC mice, the transgene ζ-globin promoter contains GATA consensus binding sites. Therefore, in addition to immunostaining for GATA-1 as a nuclear marker of erythroid lineage, the expression of this gene was also examined by RT-PCR as a potential regulator of transgene expression. Those results demonstrated co-expression of GATA-1 and endogenous murine ζ-globin in nonleukemic hematopoietic tissues of both Tg.AC and FVB/N mice. In addition, analogous to its expression in normal erythropoiesis, GATA-1 was also expressed in erythroleukemic infiltrates of the liver and spleen, consistent with previous findings in erythroleukemia cells. 27-29 There is also emerging evidence that suggests that GATA-1 functions as an anti-apoptotic factor to protect developing erythroid cells. 30 The possibility exists, then, that overexpression of GATA-1 in neoplastic erythroid cells acts as a survival mechanism in conjunction with oncogenic ras-driven proliferation in the development of erythroleukemia in these mice.
The tissue of origin of erythroleukemia in Tg.AC mice is unclear. Liver origin is suggested by the fact that this organ is the most consistently involved and severely affected in all cases examined. However, our selection of cases based on hepatomegaly obviously biased the phenotype observed toward those mice with marked liver involvement. Bone marrow or spleen origin is suggested by the RT-PCR detection in these tissues of all the proposed necessary molecular ingredients for leukemogenesis in these mice, ie, transgene, GATA-1, and ζ-globin gene. Elucidation of the histogenesis of erythroleukemia in Tg.AC mice awaits development of a reproducible model to induce the disease such that mechanistic studies of its initiation and progression can be performed. Attempts thus far have been unsuccessful. Induction studies using blood loss and erythropoietin treatments have been attempted with the rationale that physiological stimulation of benign erythropoiesis may ultimately lead to transgene expression and aberrant proliferation of erythroid cells. Apparently these methods did not inflict all the genetic or epigenetic events necessary for malignant transformation or did not target the susceptible progenitor cell population, perhaps one in which ζ-globin rather than adult hemoglobin is being expressed. Despite its sporadic spontaneous occurrence, however, erythroleukemia in the Tg.AC transgenic mouse offers potential as a novel murine model for the study of normal and abnormal erythropoiesis as a mouse model of myeloid neoplasia rarely seen in other strains.
Acknowledgments
We gratefully acknowledge the critical reviews of Drs. Morrow Thompson, Greg Travlos, Jean Louis Klein, and Theodora Devereux, the necropsy assistance of Beth Gaul and Ralph Wilson, and the excellent work of the NIEHS Histology Laboratory. In addition, we thank Dr. Raymond Tennant for the Tg.AC mice used in this study.
Footnotes
Address reprint requests to Dr. Mahler, NIEHS, Mail Drop B3-06, P.O. Box 12233, Research Triangle Park, NC 27709. E-mail: mahler@niehs.nih.gov.
G. Farris’s current address: Los Alamos National Laboratory, Los Alamos, NM 87545.
D. Malarkey’s current address: College of Veterinary Medicine, Department of Microbiology, Pathology, and Parasitology, North Carolina State University, Raleigh, NC 27695.
References
- 1.Pattengale PK: Tumors of the lymphohaematopoietic system. Turusov V Mohr U eds. Pathology of Tumors in Laboratory Animals. 1994, :pp 651-670 France, International Agency for Research on Cancer, Lyon [Google Scholar]
- 2.Frith CH, Ward JM, Chandra M: The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol Pathol 1993, 21:206-218 [DOI] [PubMed] [Google Scholar]
- 3.Fredrickson TN: Erythroleukemia, mouse. Jones TC Ward JM Mohr U Hunt RD eds. Monographs on Pathology of Laboratory Animals: Hematopoietic System. 1990, :pp 205-211 Springer-Verlag, Berlin [Google Scholar]
- 4.Frith CH, McConnell RF, Johnson AN: Erythroleukemia in a mouse. Lab Animal Sci 1990, 40:418-419 [PubMed] [Google Scholar]
- 5.Cuthbertson RA, Klintworth GK: Transgenic mice: a gold mine for furthering knowledge of pathobiology. Lab Invest 1988, 58:484-502 [PubMed] [Google Scholar]
- 6.Pattengale PK, Stewart TA, Leder A, Sinn E, Muller W, Tepler I, Schmidt E, Leder P: Animal models of human disease: pathology and molecular biology of spontaneous neoplasms occurring in transgenic mice carrying and expressing activated cellular oncogenes. Am J Pathol 1989, 135:39-61 [PMC free article] [PubMed] [Google Scholar]
- 7.Leder A, Kuo A, Cardiff RD, Sinn E, Leder P: v-Ha-ras transgene abrogates the initiation step in mouse skin carcinogenesis. Proc Natl Acad Sci USA 1990, 87:9178-9182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardiff RD, Leder A, Kuo A, Pattengale PK, Leder P: Multiple tumor types appear in a transgenic mouse with a ras oncogene. Am J Pathol 1993, 142:1199-1207 [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen LA, Trempus CS, Mahler JF, Tennant RW: Association of tumor development with increased cellular proliferation and transgene overexpression, but not c-Ha-ras mutations, in v-Ha-ras transgenic Tg.AC mice. Carcinogenesis 1996, 17:1825-1833 [DOI] [PubMed] [Google Scholar]
- 10.Spalding JW, Momma J, Elwell MR, Tennant RW: Chemically induced skin carcinogenesis in a transgenic mouse line (Tg.AC) carrying a v-Ha-ras gene. Carcinogenesis 1993, 14:1335-1341 [DOI] [PubMed] [Google Scholar]
- 11.Hansen LA, Tennant RW: Focal transgene expression associated with papilloma development in v-Ha-ras transgenic Tg.AC mice. Mol Carcinogen 1994, 9:143-154 [DOI] [PubMed] [Google Scholar]
- 12.Suurmeijer AJ, Boon ME: Notes on the application of microwaves for antigen retrieval in paraffin and plastic tissues sections. Eur J Morphol 1993, 31:144-150 [PubMed] [Google Scholar]
- 13.Trempus CS, Haseman JK, Tennant RW: Decreases in phorbol ester-induced papilloma development in v-Ha-ras transgenic Tg.AC mice during reduced gene dosage of bcl-2. Mol Carcinogen 1997, 20:68-77 [DOI] [PubMed] [Google Scholar]
- 14.Chung SW, Cai Y, Nyein R, Brizzolara E, de Riel K, Wong P: Endogenous growth factor patterns modulate hematopoietic lineage development. Oncogene 1994, 9:3527-3533 [PubMed] [Google Scholar]
- 15.Leder A, Weir L, Leder P: Characterization, expression, and evolution of the mouse embryonic zeta-globin gene. Mol Cell Biol 1985, 5:1025-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orkin SH: Globin gene regulation and swiching: circa 1990. Cell 1990, 63:665-672 [DOI] [PubMed] [Google Scholar]
- 17.Orkin SH: GATA-binding transcription factors in hematopoietic cells. Blood 1992, 80:575-581 [PubMed] [Google Scholar]
- 18.Simon MC, Pevny L, Wiles MV, Keller G, Costantini F, Orkin SH: Rescue of erythroid development in gene targeted GATA-1 mouse embryonic stem cells. Nature Genet 1992, 1:92-98 [DOI] [PubMed] [Google Scholar]
- 19.Weiss MJ, Keller G, Orkin SH: Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev 1994, 8:1184-1197 [DOI] [PubMed] [Google Scholar]
- 20.Pevny L, Lin C-S, D’Agati V, Simon MC, Orkin SH, Costantini F: Development of hematopoietic cells lacking transcription factor GATA-1. Dev Suppl 1995, 121:163-172 [DOI] [PubMed] [Google Scholar]
- 21.Pevny L, Simon MC, Robertson E, Klein WH, Tsai S-F, D’Agati V, Orkin SH, Costantini F: Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for the transcription factor GATA-1. Nature 1991, 349:257-260 [DOI] [PubMed] [Google Scholar]
- 22.Simon MC: Gotta have GATA. Nature Genet 1995, 11:9-11 [DOI] [PubMed] [Google Scholar]
- 23.Mahler JF, Mann P, Takaoka M, Maronpot RR: Spontaneous lesions of the FVB/N mouse. Toxicol Pathol 1996, 24:710-716 [DOI] [PubMed] [Google Scholar]
- 24.Pierce JH, Aaronson SA: Myeloid cell transformation by ras containing murine sarcoma viruses. Mol Cell Biol 1985, 5:667-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pharr PN, Ogawa M: Enhancement of the proliferation of murine fetal liver erythroid progenitors by infection with Harvey sarcoma virus. Leukemia 1990, 4:210-215 [PubMed] [Google Scholar]
- 26.Maher J, Baker D, Dibb N, Roberts I: Mutant ras promotes haemopoietic cell proliferation or differentiation in a cell-specific manner. Leukemia 1996, 10:83-90 [PubMed] [Google Scholar]
- 27.Ekert H, Sievers EL, Tan A, Martin DIK, Smith FO, Barnard D, Bernstein ID: GATA-1 is expressed in acute erythroblastic leukemia. Br J Haematol 1994, 86:410-412 [DOI] [PubMed] [Google Scholar]
- 28.Guerrasio A, Saglio G, Rosso C, Alfarano A, Camaschella C, Lo Coco F, Biondi A, Rambaldi A, Nicolis S, Ottolenghi S: Expression of GATA-1 mRNA in human myeloid leukemic cells. Leukemia 1994, 8:1034-1038 [PubMed] [Google Scholar]
- 29.Anagnou NP, Yuan TY, Lim E, Helder J, Wieder S, Glaister D, Marks B, Wang A, Colbert D, Deisseroth A: Regulatory factors specific for adult and embryonic globin genes may govern their expression in erythroleukemia cells. Blood 1995, 65:705-712 [PubMed] [Google Scholar]
- 30.Weiss MJ, Orkin SH: Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci USA 1995, 92:9623-9627 [DOI] [PMC free article] [PubMed] [Google Scholar]