Abstract
We have investigated the mRNA/protein expression of several tyrosine kinase receptors, growth factors, and p16INK4A cyclin inhibitor in cell lines derived from normal human pancreatic duct epithelium (HPDE) and compared them with those of five pancreatic ductal carcinoma cell lines. Cultured HPDE cells express low levels of epidermal growth factor receptor (EGFR), erbB2, transforming growth factor (TGF)-α, Met/hepatocyte growth factor receptor (HGFR), vascular endothelial growth factor (VEGF), and keratinocyte growth factor (KGF). They also expressed high levels of amphiregulin but did not express EGF and cripto. The expression levels were similar in primary normal HPDE cells and those expressing transfected E6E7 genes of human papilloma virus-16, but their immortalization appeared to enhance the expression of EGFR and Met/HGFR. In comparison, pancreatic carcinoma cell lines commonly demonstrated overexpression of EGFR, erbB2, TGF-α, Met/HGFR, VEGF, and KGF, but they consistently showed marked down-regulation of amphiregulin mRNA expression. In contrast to all carcinoma cell lines that showed deletions of the p16 gene, HPDE cells consistently demonstrated normal p16 genotype and its mRNA expression. This is the first report that compares the phenotypic expression of cultured pancreatic ductal carcinoma cells with epithelial cell lines derived from normal human pancreatic ducts. The findings confirm that malignant transformation of human pancreatic duct cells commonly results in a deregulation of expression of various growth factors and receptors.
Pancreatic cancer represents 5% of all cancer deaths in both men and women in North America, with one of the lowest 5-year survival rates of all cancers. 1 Over 90% of pancreatic cancers are adenocarcinomas arising from the duct epithelium. 2 The overall 5-year survival rate is only 5%, and at the time of first diagnosis, only 10 to 20% of cases are eligible for the potentially curative Whipple’s procedure. 3 Even in the latter group of patients, the 5-year survival is less than 30%. 4 Furthermore, pancreatic cancer is relatively resistant to both chemotherapy and radiotherapy, and these options are used primarily for palliation.
To date, the molecular basis of aggressive and resistant phenotypes of pancreatic cancer remains unclear. Many investigators have demonstrated oncogene activation, tumor suppressor gene inactivation, and growth factor and receptor overexpression in these tumors. 5 Some of these molecular changes do not correlate with prognosis, 6-8 whereas others have been suggested to correlate with poor patient survival. 9-15 Thus, further understanding on the functional roles of these genotypic and phenotypic changes during human pancreatic carcinogenesis and in the biology of pancreatic cancer cells may provide new clues for developing strategies to prevent and treat this disease.
Among the various phenotypic changes occurring in human pancreatic ductal adenocarcinoma, overexpression of tyrosine kinase receptors (RTKs), such as family members of epidermal growth factor receptor (EGFR) and their ligand molecules, 16 and the Met/hepatocyte growth factor receptor (HGFR) have been reported. 13, 16-28 These studies compared their expression in normal pancreas and primary pancreatic cancer tissue using total mRNA or protein extracts or by immunohistochemistry. Studies on the expression of various RTKs and/or their ligands in pancreatic carcinoma cell lines have also been published. 29, 30 These studies, however, did not compare the expression in cancer cells with that in corresponding control cell lines derived from normal human pancreatic duct epithelium, thus imposing some limitation on the ability to interpret the data.
Our laboratory 31 has recently reported a successful establishment of primarily cultured and immortal epithelial cell lines from normal human pancreatic duct epithelium (HPDE). We have further characterized the phenotype of these HPDE cell lines by comparing the expression of various growth factors, tyrosine kinase receptors, and p16INK4A cyclin inhibitor in these cells with their expression in several previously uncharacterized human pancreatic ductal adenocarcinoma cell lines.
Materials and Methods
Primary Culture and Immortalization of Human Pancreatic Duct Epithelial Cells
The explant method for establishing primary cultures of normal HPDE cells has previously been published. 31 The life span of primarily cultured HPDE cells could be prolonged by infection with LXSN16-E6E7 retroviral expression vector for the E6 and E7 genes of human papilloma virus (HPV)-16 virus. These cells, however, eventually also developed crisis at passages 9 to 12 (approximately 45 to 60 population doublings). Maintenance of these senescent cells by frequent fresh medium replacement eventually led to the emergence of immortal cell lines. These immortal lines have continuously proliferated for more than 20 passages (approximately 100 population doublings). HPDE cells were cultured routinely in keratinocyte serum-free (KSF) medium supplemented by bovine pituitary extract and epidermal growth factor (Gibco-BRL, Grand Island, NY). These HPDE cell lines do not proliferate in serum-containing medium.
Human Pancreatic Carcinoma Cell Lines
The establishment of cell lines PK-1, -8, and -9 were reported previously. 32 PK-9 was established from a primary tumor, whereas PK-1 and PK-8 were established from liver metastases of pancreatic ductal adenocarcinomas. PK-45 and PK-59 were subsequently established using the same method. They were established and routinely maintained in RPMI-1640 containing 10% (v/v) fetal bovine serum (Wisant, Quebec, Canada). These carcinoma cell lines do not proliferate in supplemented or basal KSF medium.
RNA Isolation and Northern Blot Analyses
Total cellular RNA was isolated from cultured cells using the method previously described. 33 RNA was isolated from cells that have reached confluent growth for 2 to 3 days and also from proliferating cells in cultures that were approximately 60 to 70% confluent. The medium of the cultures was always renewed 24 hours before isolation. Twenty-microgram samples of RNA were separated electrophoretically, transferred onto Hybond-N membrane in 10X SSC (20X SSC contains 2 mol/L NaCl and 0.3 mol/L sodium citrate, pH 7.0), and then cross-linked by exposure to the ultraviolet light of a transilluminator. The blots were probed with cDNA probes for EGFR, transforming growth factor (TGF)-α, erbB2/neu/HER-2, EGF, and amphiregulin, as reported previously. 33 The Met/HGFR mRNA expression was probed with an 841-bp Bg1I-XhoI insert of the plasmid POK6 containing full-length c-met cDNA. 34 Vascular endothelial growth factor (VEGF) mRNA was probed with a 3.36-kb VEGF189 cDNA. 35 Cripto mRNA was probed with an 890-bp EcoRI fragment cDNA. 36 The cDNA probe for keratinocyte growth factor (KGF) spanning nucleotides 997 to 1672 was cloned using the reverse transcriptase polymerase chain reaction (RT-PCR) technique and subcloned into pGEM-4Z plasmid. EGF mRNA was probed with a 960-bp PstI fragment of plasmid pmEGF-26F12 (American Type Culture Collection, Rockville, MD). The p16INK4A gene/mRNA was probed by a 0.8-kb XbaI-ApaI fragment of RC/CMV/p16 plasmid. Probes for the 18 S and 28 S ribosomal RNA were also obtained from American Type Culture Collection. The cDNA probes were labeled by [32P]dCTP using the oligolabeling kit from Pharmacia (Piscataway, NJ). The hybridized membranes were stringently washed at 60°C in 0.2X SSC solution containing 0.1% SDS. Membranes were exposed to Kodak XAR-5 film at −80°C for 3 to 5 days.
Southern Blot Analyses
Genomic DNA was isolated according to standard procedure. Ten micrograms of each DNA sample was digested with EcoRI. After separation in 1% agarose gel and Southern blot transfer, hybridization was carried out as previously reported. 31 The cDNA probe for c-myc was a 1.4-kb EcoRI-HindIII fragment of PKSC-myc plasmid.
Protein Immunoblot Analyses
Protein extracts were prepared from preconfluent or confluent cultures of HPDE6-E6E7 (greater than 16) and PK-9 cell lines. Before protein isolation, the HPDE cells were starved for 24 hours in basal KSF (supplement-free) medium. This was done because the KSF supplement contained EGF and bovine pituitary extract that would down-regulate the levels of various growth factor receptors. Similarly, the PK cells were starved for 24 hours in serum-free RPMI-1640 medium. After washing in ice-cold phosphate-buffered saline (PBS), the cultured cells were lysed in 1 ml of aqueous solution containing 50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 8.0), 1% Triton X-100, 10% glycerol, 150 mmol/L NaCl, 10 mmol/L EDTA, 100 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 100 μg phenylmethylsulfonyl fluoride (PMSF). After a centrifugation at 13,000 × g for 15 minutes, the supernatants were collected. Protein concentrations were determined using the BioRad protein assay kit (BioRad Canada, Oakville, Ontario, Canada).
The protein levels of EGFR and Met/HGFR were estimated using the Western blot technique. Fifty-microgram protein samples were electrophoretically separated in an 8% SDS-polyacrylamide gel and then transferred onto a polyvinylidene difluoride membrane (Boehringer Mannheim Canada, Dorval, Quebec, Canada). After blocking with a 5% skim milk solution in a buffer containing 10 mmol/L Tris/HCl (pH 7.0), 0.1% Tween 20, 2.5 mmol/L EDTA, and 50 mmol/L NaCl, the membrane was incubated with polyclonal rabbit antibodies against EGFR (sc-003) or h-Met (sc-161). These were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoreactivity was subsequently revealed using the BM chemiluminescence western blotting kit (Boehringer Mannheim Canada).
To assay the protein levels of erbB2/neu, 0.6 mg of protein lysates was immunoprecipitated with the 9G6 mouse monoclonal antibody against Neu (sc-008, Santa Cruz Biotechnology). After incubation for 1 hour at 4°C, 50 μl of protein-A-Sepharose (Pharmacia) was added, and the suspensions were rocked for 1 hour at 4°C. The antigen-antibody complex was pelleted by microcentrifugation and subsequently washed three times in the same lysis buffer. The immunoprecipitated pellet was then dissolved in 25 μl of Laemmli sample buffer and electrophoretically separated as described above. After transfer onto a polyvinylidene difluoride membrane, it was immunoblotted using the same Neu antibody, with the reaction detected by chemiluminescence.
Results
Pancreatic Duct Epithelial Cell Lines
Among the various HPDE cell lines studied, HPDE6 was the only primarily cultured cells that have not been infected with LXSN16-E6E7 retrovirus, and hence represented a truly normal HPDE cell line. The HPDE(1,4,5,6)-E6E7 cell lines were cells that were infected with LXSN16-E6E7 and expressed the E6E7 genes of HPV-16 genes. 31 The lines that were at or less than passage (P)9 represented pre-crisis cells, whereas those at P18 were post-crisis immortal cell lines. Both immortalized HPDE4-E6E7 (P18) and HPDE6-E6E7 (P18) were anchorage dependent in their growth and were nontumorigenic for 4 months in the subcutaneous tissue of SCID mice. 31
mRNA Expression in Confluent Cultures
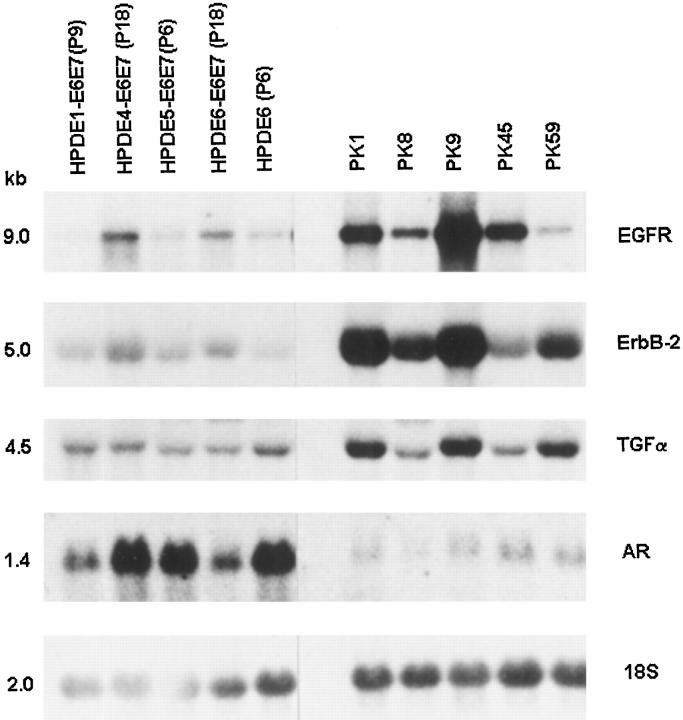
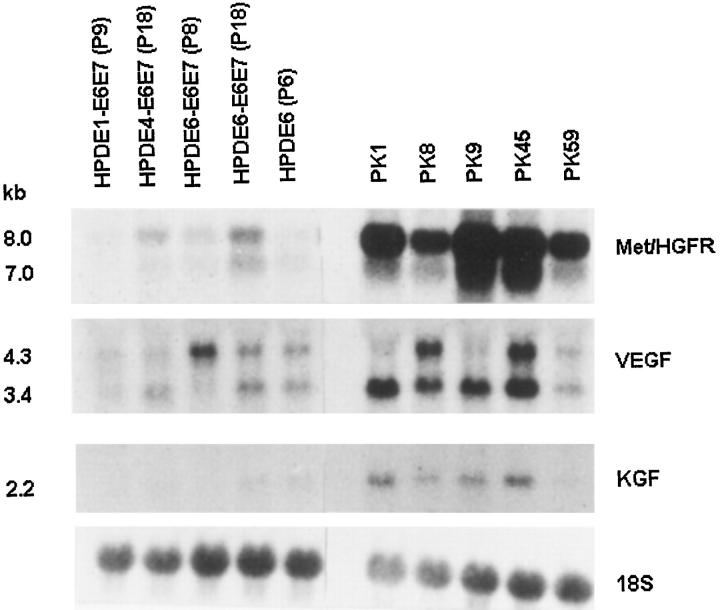
Figures 1 and 2 ▶ ▶ show the levels of mRNA expression for EGFR, erbB2, TGF-α, AR, Met/HGFR, VEGF, and KGF among the various HPDE cell lines established from different individuals. Inter-individual differences were slight, but it appears that for some genes (EGFR and Met/HGFR), the immortalized HPDE (4,6)-E6E7 (P18) cell lines showed slightly increased levels of expression as compared with their pre-immortalized (P9) cells. In turn, the expression levels in the latter cells were similar to those of normal HPDE6 cell line. These HPDE cell lines uniformly demonstrated a relatively high level of AR mRNA expression.
Figure 1.
The mRNA expression of epidermal growth factor receptor family proteins and ligands in duct epithelial cell lines derived from normal human pancreas (HPDE) and pancreatic carcinomas (PKs). HPDE6(P6), primary culture of normal pancreatic duct epithelium; HPDE1-E6E7(P9), HPDE5-E6E7(P6), pre-crisis LXSN16E6E7 (retroviral expression vector carrying the E6E7 genes of HPV16) infected cells from two separate individuals; HPDE4-E6E7(P18) and HPDE6-E6E7(P18), immortalized LXSN16E6E7 infected HPDE cells from two other individuals; PK1, PK8, PK9, PK45, and PK59, pancreatic ductal carcinoma cell lines. Hybridization with 18 S ribosomal RNA probe showed the relative loading amount of RNA samples. All samples were hybridized on a single membrane.
Figure 2.
The expression of met (hepatocyte growth factor receptor), vascular endothelial growth factor (VEGF), and keratinocyte growth factor (KGF) in HPDE and pancreatic carcinoma cell lines. (See Figure 1 ▶ for details). All samples were hybridized on a single membrane.
As compared with HPDE or HPDE-E6E7 cells, the human pancreatic ductal carcinoma cell lines showed frequent and significant up-regulation of mRNA expression for EGFR, erbB2, TGF-α, Met/HGFR, VEGF, and KGF (Figures 1 and 2) ▶ ▶ . Enhanced expression for both EGFR and TGF-α was seen in three of five carcinoma lines, and for erbB2 in four of five lines (Figure 1) ▶ . All five PK lines demonstrated overexpression of Met/HGFR (Figure 2) ▶ . Four carcinoma cell lines also demonstrated overexpression of VEGF and KGF mRNA. The overexpression of various receptors was not associated with gene amplification (Figure 3) ▶ . All five PK lines demonstrated marked and consistent down-regulation of AR as compared with the HPDE cell lines (Figure 1) ▶ . None of the HPDE and PK cell lines showed significant mRNA expression for EGF and cripto (data not shown).
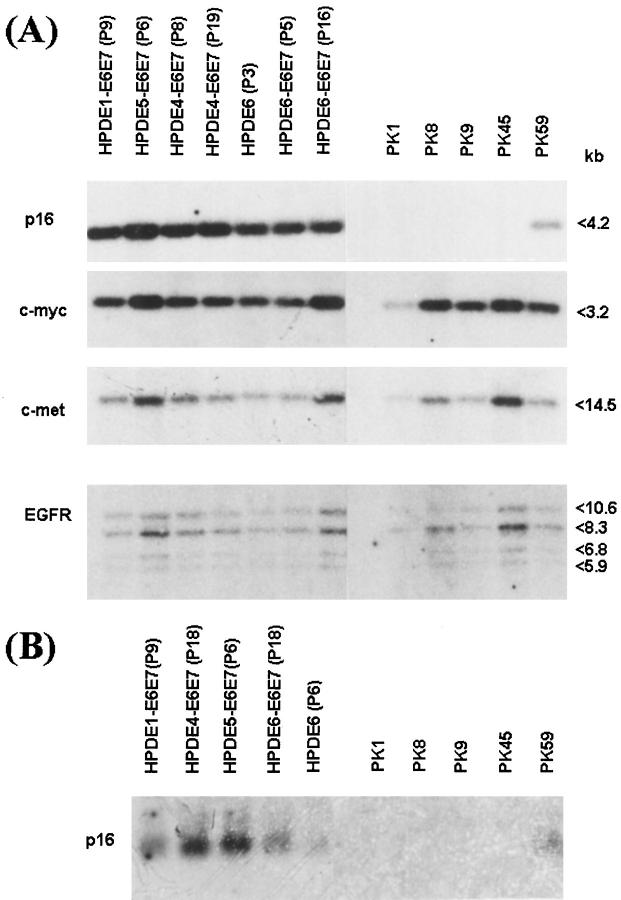
Figure 3.
The p16INK4A genotype and its expression in pancreatic ductal cell lines. A Southern blot analysis for p16 showed retention of normal p16 gene in HPDE cell lines and its homozygous deletion in four of five PK lines. The PK-59 cell line demonstrated 50% loss of hybridization signal, indicating heterozygous deletion. In contrast to p16, the hybridization signals for c-myc, c-met (hepatocyte growth factor receptor), and epidermal growth factor receptor (EGFR) genes among HPDE and adenocarcinoma (PK) cell lines were similar among these cell lines. Variability could be attributed to unequal amounts of DNA samples. B Northern blot analysis showed expression of p16 mRNA in all HPDE cell lines and in PK-59 but its absence in the other four PK cell lines with homozygous deletion of the gene.
Receptor Protein Expression
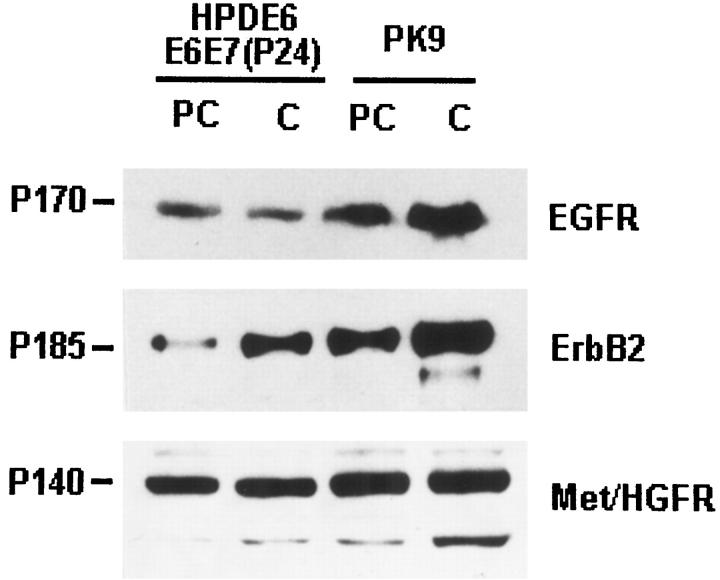
To confirm the differential expression of EGFR, erB2, and Met/HGFR at protein levels, we performed immunoblot assays on HPDE6-E6E7 (P23) and PK9 cells that were in proliferative (preconfluent) and in relatively quiescent (confluent) culture conditions (Figure 4) ▶ . The results indicated that levels of all three RTK proteins were higher in the cancer cells as compared with HPDE cells. The results were consistent with the findings at the mRNA level. Although the levels of EGFR and Met/HGFR proteins in both HPDE6-E6E7 and PK-9 cell lines were not significantly influenced by the proliferative state of the cells, those of erbB2 were lower in preconfluent as compared with postconfluent cells.
Figure 4.
Immunoblot analyses for levels of EGFR, erbB2, and Met/HGFR proteins in immortalized HPDE16-E6E6 (passage 23) and PK-9 cell lines. Studies were performed on cells in preconfluent (PC) and confluent (C) cultures. The levels of EGFR and Met/HGFR were not influenced by the culture condition, but erbB2 levels were higher in confluent cells. Note also the higher levels in PK-9 as compared with the HPDE6-E6E7 cells.
Cell Cycle Regulator Genes
A great majority of pancreatic ductal carcinomas are known to have deletion of the p16INK4A gene. 37, 38 Four of the PK carcinoma cell lines also demonstrated a homozygous deletion of the p16 gene and, the loss of its mRNA expression (Figure 3, A and B) ▶ . In contrast, all HPDE cells showed a normal p16 genotype and mRNA expression, which appeared not to be influenced by the immortalization of these cells. In the PK-59 tumor cell line, the p16 gene copy number was approximately one-half of that in HPDE cells, suggesting a heterozygous deletion. This p16 mRNA, however, remained expressed in this cell line.
In contrast to p16, the c-myc genotype was not significantly different between the HPDE and adenocarcinoma cell lines. The variation between cell lines was most likely due to slightly unequal loading of the DNA samples, as the same pattern of variation was also detected with the c-met and EGFR genes.
Discussion
A large number of cultured and xenograft human pancreatic carcinoma cell lines were established, and they have been widely used to study both the genotypic and phenotypic changes that occur in pancreatic cancer. 30, 39 In contrast, a method for the establishment of cell lines from normal human pancreatic duct epithelium (HPDE) was reported only recently. 31 These HPDE cell lines provide a chance to establish new and dynamic in vitro models for mechanistic studies of human pancreatic ductal carcinogenesis. These cells may also serve as the appropriate control non-neoplastic duct cells for comparing the phenotypic changes that occur in human pancreatic cancer cell lines. We have demonstrated here that the HPDE cell lines consistently showed low levels of expression of many growth factors and RTKs that have previously been reported to be overexpressed in human pancreatic cancers. We also demonstrated that the HPDE cells showed the normal genotype and expression of the p16INK4A cyclin inhibitor, a gene that is almost universally inactivated in pancreatic ductal carcinoma. 38 We have previously reported that these HPDE cells have wild-type Ki-ras genotype, 31 another gene that is activated by point mutation in most (>90%) pancreatic ductal carcinomas and in a significant proportion of hyperplastic pancreatic ductal lesions. 40
The expression of protein and/or mRNA for EGFR, TGF-α, erbB2, and Met/HGFR in HPDE cells is consistent with reports that indicated low levels of immunoreactivity for these proteins in the normal duct epithelium of human pancreas. 17-23, 27 In situ hybridization studies have also demonstrated the expression of mRNAs for these genes in the normal duct epithelium of human pancreas. 17, 20 Some of these studies reported enhanced immunoreactivity for EGFR, TGF-α, and Met/HGFR in ducts within areas of chronic pancreatitis. 18, 24-27 As chronic pancreatitis is most likely associated with duct cell regeneration and proliferation, the latter findings suggest a partial regulation of expression of these genes by the proliferative state in these duct cells. Except for erbB2, the expression of which was up-regulated, we were unable to detect significant differences between the mRNA and/or protein levels of EGFR, TGF-α, and Met/HGFR in relatively quiescent cells in confluent cultures, as compared with proliferative cells in preconfluent cultures. The findings were also noted in the cancer cell line studied. Unfortunately, due to the limited number of cells that we could obtain from primary cultures of HPDE6 cells before their entering senescence, we were unable to perform similar studies on these truly normal cells. It has been reported, however, that the E6E7 genes of HPV may increase the levels of EGFR protein, and this appears to be regulated at the posttranscription level. 41
Although there is a heterogeneity of overexpression for EGFR, erbB2, and TGF-α among cell lines, all five carcinoma lines we studied demonstrated overexpression as compared with HPDE cells on these genes. Furthermore, all five carcinoma cell lines also showed marked down-regulation of amphiregulin expression. The EGF family agonist genes consist of several members, including EGF, TGF-α, betacellulin, cripto, amphiregulin, and heparin-binding EGF. 36, 42 In contrast to other family members of EGF, amphiregulin is a bifunctional growth factor with predominantly a stimulatory effect on the growth of normal cells, but an inhibitory effect on tumor cells. 43, 44 Furthermore, the EGF receptor (EGFR) also has several homologous family members including erbB2, erbB3, and erbB4. 45 These erbB family receptors can inter-dimerize, but they demonstrate different binding affinities to the different receptor forms. 42 Therefore, our findings suggest that a net enhancement of the expression, hence, putatively, the activity of EGFR family autocrine loops, is a commonly occurring biochemical event during human pancreatic ductal carcinogenesis. Additional studies are required to confirm their roles in the biology of pancreatic ductal carcinomas.
These in vitro findings are consistent with the in vivo data on expression levels of EGF and EGFR family gene products in pancreatic ductal carcinoma. A strong immunostaining of cancer cells and overexpression of EGFR, EGF, heparin-binding EGF, and TGF-α mRNA and/or protein in primary cancerous as compared with normal pancreatic tissues have been reported. 17, 18, 23, 25, 27 Expression of amphiregulin mRNA in both normal and cancerous pancreatic tissue has also been reported. 46 This study also reported a positive nuclear staining in normal cells, whereas cancer cells stained cytoplasmically. The significance of this is currently unknown. We were unable to detect EGF and cripto mRNA expression in vitro in both the HPDE and PK carcinoma cell lines. Similar findings were reported by Oikawa et al 29 who failed to detect any mRNA expression of EGF, heparin-binding EGF, and cripto in all 12 carcinoma cell lines they studied. In contrast, Tsutsumi et al 22 and Friess et al 23 have reported an immunoreactivity to cripto antibody in duct epithelium of normal pancreas and chronic pancreatitis and also commonly in pancreatic ductal adenocarcinoma.
Our result indicating frequent overexpression of erbB2 among pancreatic carcinoma cell lines was similar to that reported by Oikawa et al. 29 Although normal pancreatic duct epithelium consistently demonstrates absent or very low levels of reactivity with various erbB2 antibodies, strong immunoreactivity in cancer cells was detected in 45 to 70% of ductal adenocarcinoma cases. 13, 19-21 Overexpression of erbB2 appears to be more frequent in well or moderately differentiated than poorly differentiated tumors, and it is overexpressed in an even greater (>80%) percentage of hyperplastic and preneoplastic pancreatic ductal lesions. 21 The role of erbB2 overexpression in human pancreatic ductal carcinogenesis requires additional investigations.
The overexpression of Met/HGFR appears to be common in human pancreatic cancer cell lines, which is consistent with previous reports that pancreatic ductal carcinoma cells in vivo overexpressed Met/HGFR as compared with normal duct epithelium. 26, 28 DiRenzo et al 31 have also reported expression of Met/HGFR mRNA and/or protein in all 31 pancreatic carcinoma cell lines studied, but their findings could be evaluated only in a relative term, as normal HPDE cells were not available for comparison.
The current study also demonstrated that when compared with the HPDE cells, pancreatic cancer cell lines commonly overexpress KGF and VEGF. Siddiqi et al 47 detected KGF mRNA expression by the RT-PCR method in four of seven pancreatic carcinoma cell lines, and they reported increased KGF mRNA levels in 44% of cancerous as compared with normal pancreatic tissue. In our hands, four of five cancer cell lines showed KGF mRNA overexpression. Itakura et al 48 recently reported the expression of VEGF mRNA and protein in all six human pancreatic carcinoma cell lines they studied. In situ hybridization and immunohistochemistry also demonstrated VEGF mRNA and protein overexpression in primary pancreatic ductal carcinoma cells as compared with normal duct epithelium.
The cyclin inhibitor p16INK4A/MTS-1/CDKN2 is a critical cell cycle regulator protein that is inactivated in almost all pancreatic ductal adenocarcinomas. 38 Inactivation occurs by homozygous deletion in approximately one-half of these tumors and by heterozygous deletion accompanied by intragenic mutation or methylation silencing of the remaining allele in the remaining cases. The inactivation of the p16 gene putatively leads to deregulation of the G1-phase cell cycle control. Consistent with these results, the p16 gene is also homozygously deleted in four of five PK adenocarcinoma cell lines studied, with the fifth cell line showing a heterozygous deletion.
In summary, we have demonstrated that, in contrast to pancreatic ductal carcinoma cells, the phenotypic and genotypic characteristics of HPDE cells resemble more those of normal pancreatic ductal epithelium. We believe the cultured HPDE cells and immortalized cell lines derived from them are valuable for in vitro research in human pancreatic carcinogenesis.
Acknowledgments
We thank Drs. Karen Gray (Uniformed Services University, Bethesda, MD), Joyce Slingerland (Sunnybrook Health Science Center, Toronto, Ontario, Canada), and Linda Penn (Ontario Cancer Institute) for generously providing the various cDNA plasmids and probes.
Footnotes
Address reprint requests to Dr. Ming-Sound Tsao, Ontario Cancer Institute, 610 University Avenue, Toronto, Ontario M5G 2M9, Canada. E-mail: ming_tsao@pmh.toronto.on.ca.
Supported by the Medical Research Council of Canada grant MT-14359.
References
- 1.Parker SL, Tong T, Bolden S, Wingo PA: Cancer statistics, 1997. CA Cancer J Clin 1997, 47:5-27 [DOI] [PubMed] [Google Scholar]
- 2.Kloppel G: Pathology of nonendocrine pancreatic tumours. Go VLW Dimagno EP Gardner JD Lebenthal E Reber HA Scheele GA eds. The Pancreas: Biology, Pathobiology, and Disease. 1993, :pp 871-897 Raven Press, New York [Google Scholar]
- 3.Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H: Results of resection for cancer of exocrine pancreas: a study from the French Association of Surgery. Br J Surg 1994, 81:102-107 [DOI] [PubMed] [Google Scholar]
- 4.Murr MM, Sarr MG, Oishi AJ, van Heerden JA: Pancreatic cancer. CA Cancer J Clin 1994, 44:304-318 [DOI] [PubMed] [Google Scholar]
- 5.DiGiuseppe JA, Hruban RH: Pathobiology of cancer of the pancreas. Semin Surg Oncol 1995, 11:87-96 [Google Scholar]
- 6.Motojima K, Urano T, Nagata Y, Shiku H, Tsunoda T, Kanematsu T: Mutations in the Kirsten-ras oncogene are common but lack correlation with prognosis and tumor stage in human pancreatic carcinoma. Am J Gastroenterol 1991, 86:1784-1788 [PubMed] [Google Scholar]
- 7.Hruban RH, van Mansfeld ADM, Offerhaus GJA, van Weering DHJ, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL: K-ras oncogene activation in adenocarcinoma of the human pancreas. Am J Pathol 1993, 143:545-554 [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegata NS, Sessa F, Renault B, Bonato M, Leone BE, Solcia E, Ranzani GN: K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumours progress through different genetic lesions. Cancer Res 1994, 54:1556-1560 [PubMed] [Google Scholar]
- 9.DiGiuseppe JA, Hruban RH, Goodman SN, Polak M, van den Berg FM, Allison DC, Cameron JL, Offerhous JA: Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol 1994, 101:684-688 [DOI] [PubMed] [Google Scholar]
- 10.Sinicrope FA, Evans DB, Leach SD, Cleary KR, Fenoglio CJ, Lee JJ, Abbruzzese JL: bcl-2 and p53 expression in resectable pancreatic adenocarcinomas: association with clinical outcome. Clin Cancer Res 1996, 2:2015-2022 [PubMed] [Google Scholar]
- 11.Friess H, Yamanaka Y, Buchler M, Ebert M, Berger H, Gold LI, Korc M: Enhanced expression of transforming growth factor-β isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 1993, 105:1846-1856 [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka Y, Friess H, Buchler M, Berger HG, Uchida E, Onda M, Kobrin MS, Korc MS: Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res 1993, 53:5289-5296 [PubMed] [Google Scholar]
- 13.Lei S, Appert HE, Nakata B, Domenico DR, Kim K, Howard JM: Overexpression of HER2/neu oncogene in pancreatic cancer correlates with shortened survival. Int J Pancreatol 1995, 17:15-21 [DOI] [PubMed] [Google Scholar]
- 14.Shimoyama S, Gansauge F, Gansauge S, Negri G, Oohara T, Berger HG: Increased angiogenin expression in pancreatic cancer is related to cancer aggressiveness. Cancer Res 1996, 56:2703-2706 [PubMed] [Google Scholar]
- 15.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Kloppel G, Lemoine NR: Loss of membrane E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol 1994, 174:243-248 [DOI] [PubMed] [Google Scholar]
- 16.Lemoine NR, Lobresco M, Leung H, Barton C, Hughes CM, Prigent SA, Gullick WJ, Kloppel G: The erbB-3 gene in human pancreatic cancer. J Pathol 1992, 168:269-273 [DOI] [PubMed] [Google Scholar]
- 17.Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchler M, Beger HG: Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor-alpha. J Clin Invest 1992, 90:1352-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemoine NR, Hughes CM, Barton CM, Poulsom R, Jeffery RE, Kloppel G, Hall PA, Gullick WJ: The epidermal growth factor receptor in human pancreatic cancer. J Pathol 1992, 166:7-12 [DOI] [PubMed] [Google Scholar]
- 19.Hall PA, Hughes CM, Staddon SL, Richman PI, Gullick WJ, Lemoine NR: The c-erbB-2 proto-oncogene in human pancreatic cancer. J Pathol 1990, 161:195-200 [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Kunz J, Beger HG, Korc M: Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol 1993, 24:1127-1134 [DOI] [PubMed] [Google Scholar]
- 21.Day JD, DiGiuseppe JA, Yeo C, Lai-Goldman M, Anderson SM, Goodman SN, Kern SE, Hruban RH: Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol 1996, 27:119-124 [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi M, Yasui W, Naito A, Ohashi K, Kobayashi E, Noguchi O, Horiguchi K, Okita S, Tsujiuchi T, Kitada H, Ishikawa O, Tahara E, Konishi Y: Expression of cripto in human pancreatic tumors. Jpn J Cancer Res 1994, 85:118-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friess H, Yamanaka Y, Buchler M, Kobrin M, Tahara E, Korc M: Cripto, a member of the epidermal growth factor family, is over-expressed in human pancreatic cancer and chronic pancreatitis. Int J Cancer 1994, 56:668-674 [DOI] [PubMed] [Google Scholar]
- 24.Ebert M, Yokoyama M, Kobrin MS, Friess H, Lopez ME, Buchler MW, Johnson GR, Korc M: Induction and expression of amphiregulin in human pancreatic cancer. Cancer Res 1994, 54:3959-3962 [PubMed] [Google Scholar]
- 25.Kobrin MS, Funatomi H, Friess H, Buchler MW, Stathis P, Korc M: Induction and expression of heparin-binding EGF-like growth factor in human pancreatic cancer. Biochem Biophys Res Commun 1994, 202:1705-1709 [DOI] [PubMed] [Google Scholar]
- 26.Ebert M, Yokoyama M, Friess H, Buchler MW, Korc M: Co-expression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 1994, 54:5775-5778 [PubMed] [Google Scholar]
- 27.Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR: Transforming growth factor alpha and epidermal growth factor in human pancreatic cancer. J Pathol 1991, 163:111-116 [DOI] [PubMed] [Google Scholar]
- 28.Furukawa T, Duguid WP, Kobari M, Matsuno S, Tsao M-S: Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol 1995, 147:889-895 [PMC free article] [PubMed] [Google Scholar]
- 29.Oikawa T, Hitomi J, Kono A, Kaneko E, Yamaguchi K: Frequent expression of genes for receptor tyrosine kinases and their ligands in human pancreatic cancer cells. Int J Pancreatol 1995, 18:15-23 [DOI] [PubMed] [Google Scholar]
- 30.Di Renzo MF, Olivero PM, Comoglio PM, Lemoine NR: Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 1995, 55:1129-1138 [PubMed] [Google Scholar]
- 31.Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao M-S: Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol 1996, 148:1763-1770 [PMC free article] [PubMed] [Google Scholar]
- 32.Kobari M, Hisano H, Matsuno S, Sato T, Kan M, Tachibana T: Establishment of six human pancreatic cancer cell lines and their sensitivities to anti-tumor drugs. Tohoku J Exp Med 1986, 150:231-248 [DOI] [PubMed] [Google Scholar]
- 33.Tsao M-S, Zhu H, Viallet J: Autocrine growth loop of the epidermal growth factor receptor in normal and immortalized human bronchial epithelial cells. Exp Cell Res 1996, 223:268-273 [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues GA, Naujokas MA, Park M: Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol Cell Biol 1991, 11:2962-2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung DW, Cachianes G, Kuang W-J, Goeddel DV, Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246:1306-1309 [DOI] [PubMed] [Google Scholar]
- 36.Ciccodicola A, Dono R, Obici S, Simeone A, Zollo M, Persico MG: Molecular characterization of a gene of the ‘’EGF family’ expressed in undifferentiated human NTERA2 teratocarcinoma cells. EMBO J 1989, 8:1987-1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Yan Y-X, McClure M, Nakagawa H, Fujimura F, Rustgi AK: MTS-1 (CDKN2) tumor suppressor gene deletions are a frequent event in esophagus squamous cancer and pancreatic adenocarcinoma cell lines. Oncogene 1995, 10:619-622 [PubMed] [Google Scholar]
- 38.Schutte M, Hruban RH, Geradts J, Maynard M, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff I, Schmiegel W, Baylin SB, Kern SE, Herman JG: Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 1997, 57:3126-3130 [PubMed] [Google Scholar]
- 39.Hahn SA, Seymour AB, Shamsul Hoque ATM, Schutte M, da Costa LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE: Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res 1995, 55:4670-4675 [PubMed] [Google Scholar]
- 40.Moskaluk CA, Hruban RH, Kern SE: p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res 1997, 57:2140-2143 [PubMed] [Google Scholar]
- 41.Hu G, Liu W, Mendelsohn J, Ellis LM, Radinsky R, Andreeff M, Deisseroth AB: Expression of epidermal growth factor receptor and human papillomavirus E6/E7 proteins in cervical carcinoma cells. J Natl Cancer Inst 1997, 89:1271-1276 [DOI] [PubMed] [Google Scholar]
- 42.Beerli RR, Hynes NE: Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem 1996, 271:6071-6076 [DOI] [PubMed] [Google Scholar]
- 43.Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ: Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science 1989, 243:1074-1076 [DOI] [PubMed] [Google Scholar]
- 44.Plowman GD, Green JM, McDonald VL, Neubauer MG, Disteche CM, Todaro GJ, Shoyab M: The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol 1990, 10:1969-1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Earp HE, Dawson TL, Li X, Yu H: Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implication for breast cancer research. Breast Cancer Res Treat 1995, 35:115-132 [DOI] [PubMed] [Google Scholar]
- 46.Ebert M, Yokoyama M, Kobrin MS, Friess H, Lopez ME, Buchler MW, Johnson GR, Korc M: Induction and expression of amphiregulin in human pancreatic cancer. Cancer Res 1994, 54:3959-3962 [PubMed] [Google Scholar]
- 47.Siddiqi I, Funatomi H, Kobrin MS, Friess H, Buchler MW, Korc M: Increased expression of keratinocyte growth factor in human pancreatic cancer. Biochem Biophys Res Commun 1995, 215:309-315 [DOI] [PubMed] [Google Scholar]
- 48.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Büchler MW, Korc M: Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 1997, 3:1309-1316 [PubMed] [Google Scholar]