Abstract
The 150-kd transmembrane protein CD100 is the first semaphorin protein shown to be expressed in lymphoid tissue. CD100 is present in the interfollicular T cell zones and is also expressed by B cells in the germinal centers of secondary lymphoid follicles, but not in the mantle zones. The CD100 molecule was recently cloned, and CD100 transfectants were shown to induce homotypic aggregation of human B cells and improve their viability in vitro, suggesting that CD100 may play a role in lymphocyte aggregation and germinal center formation. We studied the expression of CD100 in 138 clinical cases representing a range of lymphoproliferative disorders, to determine whether this molecule is expressed in these neoplastic processes. In general, we found CD100 expression to be common in peripheral T-cell non-Hodgkin’s lymphomas but rare in B-cell non-Hodgkin’s lymphomas. CD100 expression was not detectable in low-grade B-cell non-Hodgkin’s lymphomas, including cases of small lymphocytic lymphoma (18 cases), marginal zone lymphoma (10 cases), and mantle cell lymphoma (10 cases), as might be expected for these neoplasms that are not of follicular center cell origin. Surprisingly, we found that the vast majority of follicular lymphomas (37 of 40 cases) as well as diffuse large-cell lymphomas of B-cell type (35 cases) did not express CD100. The neoplastic cells in 3 of 11 cases of predominantly large-cell-type follicular lymphoma did express CD100. In contrast, all five cases of high-grade, small non-cleaved (Burkitt-like) B-cell lymphoma were immunoreactive for CD100 expression, as were 18 of 20 cases (90%) of malignant T cell neoplasms. Northern blot analysis of CD100 expression correlated with immunohistochemical findings. Absence of expression of CD100 by neoplastic follicular center B cells is a common feature in follicular lymphomas, but expression of CD100 by T cells is maintained in T-cell lymphoproliferative disorders.
The semaphorins are a family of secreted and transmembrane proteins with chemorepellent effects, present in the nervous system, and involved in the regulation of axonal growth. 1-3 Recently, we identified CD100 as the first semaphorin expressed in the immune system. 4 It is a 150-kd cell surface homodimer expressed on T and B lymphocytes, natural killer cells, and myeloid cells, but not on red blood cells, eosinophils, or endothelial cells. 5,6 CD100 expression increases after B cell and T cell activation. Antibody cross-linking of CD100 provides a proliferative signal to T cells in the presence of submitogenic levels of anti-CD3 or anti-CD2 antibodies, suggesting that CD100 is involved in T cell activation. 7 The CD100 protein is associated with CD45 on the surface of T cells and modulates CD45 phosphatase activity, 8 and the large cytoplasmic domain of CD100 is associated with a serine kinase. 9 CD100 can be proteolytically cleaved from the cell surface to release a soluble semaphorin. 8
Recently, we showed that CD100 transfectants induce B cells to aggregate and improve their viability in vitro, suggesting that the interaction of CD100 with its counter-receptor may have a physiological role in germinal center formation, possibly by enhancing CD23 proteolysis in centroblasts. 4 The counter-receptor for CD100 has not yet been identified; however, some other semaphorin gene family members have been shown to bind to a multisubunit receptor composed of a neuropilin gene family member plus an additional unidentified subunit. 10-12 Vaccinia and Variola poxviruses and some herpesviruses such as Alcelaphine herpesvirus express a secreted protein with homology to CD100 and other semaphorins. 1,13 It has been suggested that these may be used by the viruses to subvert the host immune response. Alcelaphine herpesvirus is a T-lymphotropic γ2 herpesvirus and causes malignant catarrhal fever in ruminants, a fatal lymphoproliferative and degenerative disease characterized by perivascular and epithelial lymphoid infiltrates, corneal opacities, and lymph node enlargement. 14 It is believed that part of the pathophysiology is due to proliferation and dysfunction of T cells. 15,16
In reactive lymphoid tissue, CD100 is present in the interfollicular T cell zones as well as in the germinal centers of secondary lymphoid follicles, including the dark and light zones, but not in the mantle zones. 4 The expression of CD100 in lymphoproliferative processes has not been studied, although CD100 is known to be expressed at high levels in lymphoid and myeloid leukemia cell lines. 5,6 We studied CD100 expression in a range of B-cell and T-cell lymphoproliferative disorders to explore the possible role of CD100 in these processes.
Materials and Methods
Specimens of hematopoietic neoplasms (138 cases) were obtained from the Department of Pathology, Brigham and Women’s Hospital, Boston, MA, in compliance with Human Research Committee guidelines. Histological and immunophenotypic findings from the routine diagnostic evaluations of specimens were reviewed. Cases were classified using the REAL classification. 17
Tissue that had been snap-frozen in dry ice/isopentane was used for the preparation of frozen sections for immunoperoxidase analysis. Frozen sections were fixed in acetone for 10 minutes, washed in phosphate-buffered saline (PBS), incubated with anti-CD100 primary mouse monoclonal antibody (clone F937G2) 18 for 1 hour at room temperature, washed in PBS, incubated with biotinylated horse anti-mouse IgG antibody (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature, washed with PBS, and then incubated with avidin/biotinylated-peroxidase complex (Vector Laboratories) for 40 minutes at room temperature, followed by reaction with diaminobenzidine/hydrogen peroxide. Frozen sections were then counterstained with 2% methyl green for 10 minutes. For a number of specimens, consecutive frozen sections were analyzed for CD100, CD20 (antibody L26, Dako Corp., Carpinteria, CA), CD3 (antibody UCHT1, Dako), CD23 (antibody MHM6, Dako), bcl-2 (antibody 124, Dako), and Ki-67 (antibody MIB-1, Immunotech, Westbrook, ME), using the above method. Cases of T-cell acute lymphoblastic leukemia (ALL) were studied by flow cytometric immunophenotypic analysis and CD100 immunoperoxidase analysis using cytocentrifuge preparations of neoplastic cells.
RNA was isolated from selected specimens, electrophoresed in 1.2% agarose, and transferred to nitrocellulose membrane. 19 The blot was hybridized overnight at 42°C in 50% formamide/6X SSPE (1X SSPE contains 0.18 mol/L NaCl, 10 mmol/L sodium phosphate, pH 7.4, 1 mmol/L EDTA), 2X Denhardt’s solution, 100 μg/ml salmon sperm DNA 19 with probe (10 6 cpm/ml). The probe consisted of two XhoI fragments encoding the entire CD100 cDNA, which were labeled with [α-32P]CTP and [α-32P]ATP by random priming. 20 After hybridization, the nitrocellulose membrane was washed to a final stringency of 2X standard saline citrate (SSC), 0.1% SDS at 65°C and was used to expose Kodak XAR-5 film with an intensifier for 3 days at −70°C.
Results
CD100 Immunoreactivity
A total of 138 cases of non-Hodgkin’s lymphomas and other hematopoietic neoplasms were examined for CD100 immunoreactivity; the results are summarized in Table 1 ▶ . Twenty cases of malignant T-cell neoplasms were studied for CD100 immunoreactivity, including 4 cases of mixed small- and large-cell lymphoma, 2 cases of large-cell lymphoma, 4 cases of Ki-1-positive anaplastic large-cell lymphoma, 5 cases of mycosis fungoides (cutaneous T-cell lymphoma), 4 cases of T-cell acute lymphoblastic leukemia (ALL), and 1 case of large granular lymphocytic leukemia, T-cell type. In all cases, neoplastic T cells were uniformly immunoreactive for CD100 with strong membrane staining, with the exception of two of the four cases of Ki-1-positive anaplastic large-cell lymphoma (Figure 1) ▶ .
Table 1.
CD100 Immunoreactivity in Non-Hodgkin’s Lymphomas and Other Neoplasms
| Tumor type | CD100 immunoreactivity* |
|---|---|
| B-cell non-Hodgkin’s lymphomas | |
| SLL/CLL | 0 /18† |
| Marginal zone | 0 /10 |
| Mantle cell | 0 /10 |
| Follicular center cell | 3 /40 |
| Predominantly small cell | 0 /19 |
| Mixed small and large cell | 0 /10 |
| Predominantly large cell | 3 /11 |
| Diffuse large cell | 0 /35‡ |
| Small non-cleaved | 5 /5 |
| T-cell non-Hodgkin’s lymphomas | 18 /20§ |
*Number of cases immunoreactive/total number of cases studied.
†Including three cases of lymphoplasmacytic lymphoma and one case of chronic lymphocytic leukemia (CLL)/prolymphocytic leukemia (PLL).
‡Including seven cases of large-cell lymphoma, immunoblastic type, and two cases of T-cell-rich B-cell lymphoma.
§Includes four cases of mixed small- and large-cell lymphoma, two cases of large-cell lymphoma, four cases of Ki-1-positive anaplastic large-cell lymphoma, two of which were negative for CD100, five cases of mycosis fungoides (cutaneous T-cell lymphoma), four cases of T-cell acute lymphoblastic leukemia (ALL), and one case of large granular lymphocytic leukemia (LGL), T cell type.
Figure 1.
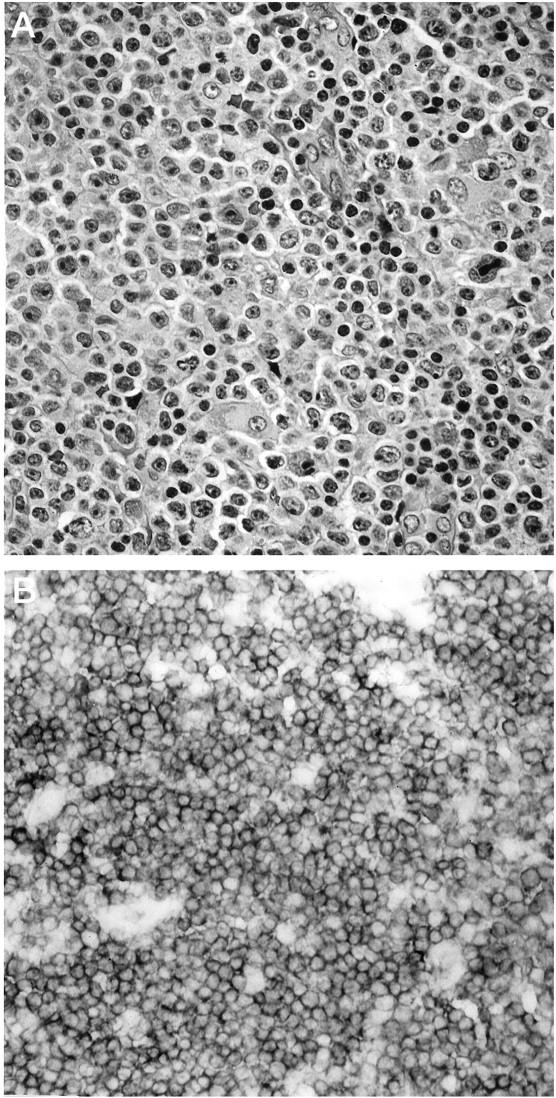
Immunoreactivity for CD100 in a case of diffuse, large-cell non-Hodgkin’s lymphoma, T-cell type. A: Representative neoplastic tissue fixed in 10% buffered formaldehyde, paraffin embedded, sectioned, and stained with H&E. B: CD100 immunoperoxidase staining of frozen neoplastic tissue. Large neoplastic cells exhibit strong, uniform surface staining for CD100.
Lower-grade B-cell lymphomas, including cases of small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL), marginal zone lymphoma, and mantle cell lymphoma, were uniformly negative for CD100. This included three cases of lymphoplasmacytic lymphoma, one case of chronic lymphocytic leukemia(CLL)/prolymphocytic leukemia (PLL), and two cases of the blastic variant of mantle cell lymphoma. In all of these cases, scattered, small, non-neoplastic T cells were immunoreactive for CD100 and served as an internal positive control (data not shown).
Forty cases of follicular non-Hodgkin’s lymphomas were studied for CD100 immunoreactivity, including predominantly small-cell type, mixed small- and large-cell type, and predominantly large-cell-type follicular lymphomas. In the final subcategory, large neoplastic cells constitute >50% of the population of the nodular neoplastic infiltrates. The vast majority of follicular lymphoma cases were nonreactive for CD100. A typical case of predominantly small-cell type follicular lymphoma is shown in Figure 2 ▶ . Neoplastic B cells were nonreactive for CD100, whereas scattered, non-neoplastic T cells (confirmed in consecutive frozen sections using pan-T cell antibody CD3) were immunoreactive for CD100. The neoplastic cells in three cases of predominantly large-cell-type follicular lymphoma were immunoreactive for CD100 with strong membrane staining (Figure 3) ▶ . Consecutive frozen sections revealed the presence of scattered, small, non-neoplastic CD3-positive T cells interspersed with neoplastic B cells that were CD20 positive and exhibited monotypic surface immunoglobulin light chain staining (data not shown).
Figure 2.
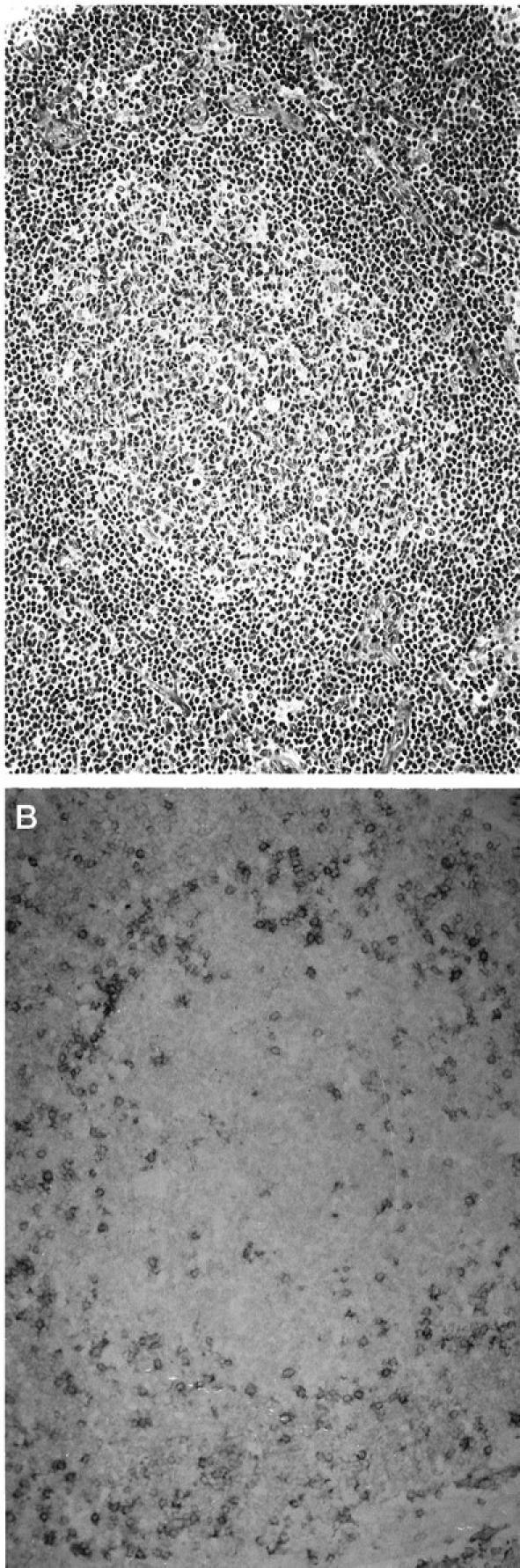
Immunoreactivity for CD100 in a case of predominantly small-cell-type follicular non-Hodgkin’s lymphoma. A: Representative neoplastic tissue fixed in 10% buffered formaldehyde, paraffin embedded, sectioned, and stained with H&E. B: CD100 immunoperoxidase staining of frozen neoplastic tissue. Neoplastic cells are nonreactive for CD100. Scattered, reactive T cells within the neoplastic follicle, as well as reactive T cells in the interfollicular zone, are strongly immunoreactive for CD100.
Figure 3.
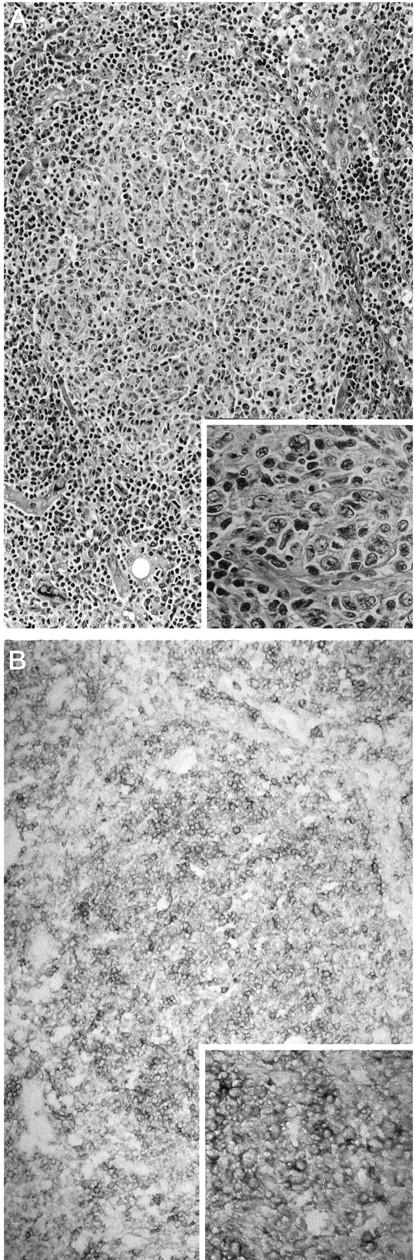
Immunoreactivity for CD100 in a case of predominantly large-cell-type follicular non-Hodgkin’s lymphoma. A: Representative neoplastic tissue fixed in 10% buffered formaldehyde, paraffin embedded, sectioned, and stained with H&E, with higher-power inset showing a mix of small cleaved and large lymphoid cells. B: CD100 immunoperoxidase staining of frozen neoplastic tissue. As seen in the higher-power inset, large neoplastic cells as well as small cleaved neoplastic cells exhibit moderate, uniform surface staining for CD100. Scattered, reactive T cells within the neoplastic follicle, as well as reactive T cells in the interfollicular zone, are strongly immunoreactive for CD100.
Forty additional cases of B-cell lymphomas were studied for CD100 immunoreactivity. The neoplastic cells in 35 cases of diffuse large-cell lymphoma, including 7 cases of immunoblastic lymphoma, and 2 cases of T-cell-rich B-cell lymphoma, large-cell type, were uniformly nonreactive for CD100. In all cases scattered, small, non-neoplastic T cells were immunoreactive for CD100. Neoplastic cells in five cases of high-grade, small non-cleaved (Burkitt-like) B-cell lymphoma were studied and found to exhibit uniform membrane staining for CD100 (Figure 4) ▶ . In addition, six cases of classic Hodgkin’s disease were examined, and the neoplastic Reed-Sternberg cells and mononuclear Reed-Sternberg cell variants in these cases were found to be nonreactive for CD100 (data not shown).
Figure 4.
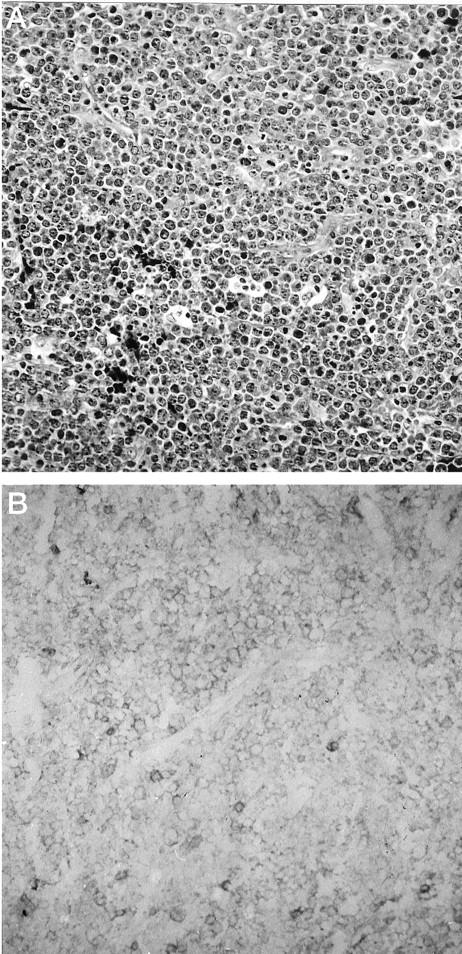
Immunoreactivity for CD100 in a case of small non-cleaved (Burkitt-like) B-cell lymphoma. A: Representative neoplastic tissue fixed in 10% buffered formaldehyde, paraffin embedded, sectioned, and stained with H&E. B: CD100 immunoperoxidase staining of frozen neoplastic tissue. Intermediate-size neoplastic cells exhibit low to moderate, uniform surface staining for CD100. Scattered, reactive T cells are strongly immunoreactive for CD100.
Northern Blot Analysis for CD100 mRNA Expression
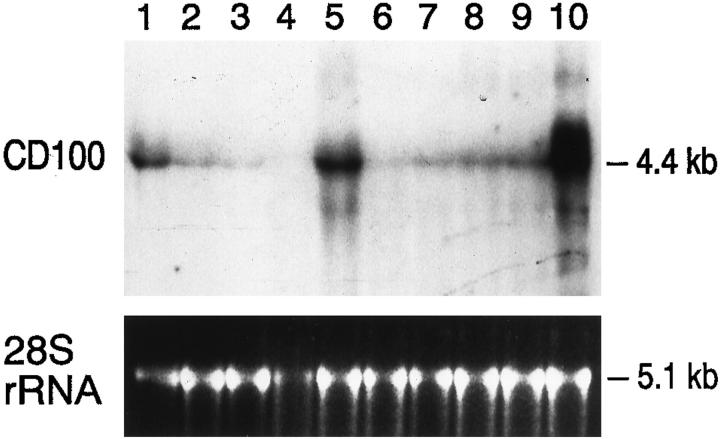
RNA was extracted from six of the above cases for Northern blot analysis; the result is shown in Figure 5 ▶ . In all cases examined, mRNA expression of CD100 reflected the pattern seen by immunoperoxidase staining. The 4.4-kb CD100 mRNA was present at a moderate level in activated T cells (lane 1) and in reactive lymphoid tissue (lane 9). Previous immunoperoxidase analysis of the latter specimen had shown the presence of numerous interfollicular T cells and germinal center B cells immunoreactive for CD100. 4 Cells from the neoplastic cell lines Rex (acute T-cell lymphoblastic leukemia; lane 2) and Raji (human Burkitt’s lymphoma; lane 3) expressed CD100 mRNA at a low level. Cases of mantle cell lymphoma (lane 4) and follicular lymphoma negative for CD100 immunoreactivity by immunoperoxidase staining (lane 6; also shown in Figure 2 ▶ ) expressed very low levels of CD100 mRNA. In contrast, CD100 mRNA was moderately to strongly expressed in a case of follicular lymphoma positive for CD100 by the immunoperoxidase method (lane 5; also shown in Figure 3 ▶ ). A case of B-cell chronic lymphocytic leukemia (lane 8) negative for CD100 expression by immunoperoxidase expressed low to moderate levels of CD100 mRNA, consistent with the presence of interspersed, reactive T cells in this tissue, which were found to be positive for CD100 by the immunoperoxidase method. The CD100 mRNA was expressed at a very high level in a representative case of peripheral T-cell lymphoma that was positive for CD100 by the immunoperoxidase method (lane 10; also shown in Figure 1 ▶ ). A case of small non-cleaved B cell lymphoma that was positive for CD100 by the immunoperoxidase method expressed CD100 mRNA at low to intermediate levels (lane 7; also shown in Figure 4 ▶ ).
Figure 5.
CD100 mRNA expression in non-Hodgkin’s lymphomas. Northern blot containing RNA prepared from 1) activated T cells; 2) Rex (acute T-cell lymphoblastic leukemia cell line); 3) Raji (human Burkitt lymphoma cell line); 4) mantle cell non-Hodgkin’s lymphoma; 5) follicular lymphoma, predominantly large-cell type; 6) follicular lymphoma, predominantly small-cell type; 7) high-grade, small non-cleaved (Burkitt-like) lymphoma; 8) chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL); 9) reactive lymph node; and 10) T-cell non-Hodgkin’s lymphoma, diffuse, large-cell type. Each lane contains 20 μg of total RNA and was probed with the 4.3-kb CD100 cDNA insert. The position of the 4.4-kb CD100 mRNA is indicated. The bottom panel shows the ethidium-bromide-stained 5.1-kb 28 S rRNA corresponding to each of the above lanes. The neoplasms studied in lanes 5, 6, 7, and 10 correspond to those shown in Figures 3, 2, 4, and 1 ▶ ▶ ▶ , respectively.
Correlation of CD100 Immunoreactivity in Follicular Lymphomas with Other Immunophenotypic Markers
Eight of the eleven cases of follicular large-cell lymphoma, including the three cases with CD100 staining of neoplastic B cells, were further analyzed for expression of CD23, bcl-2, and Ki-67 (MIB-1), a proliferation marker. All eight cases of follicular large-cell lymphoma, including the CD100-positive cases, exhibited negative staining for CD23 and positive staining for bcl-2. There was comparable MIB-1 nuclear staining of neoplastic cells in all eight cases, with no qualitative differences in intensity or frequency of staining evident in the three CD100-positive cases compared with the CD100-negative cases (data not shown).
Discussion
Previously we found that CD100, a novel leukocyte semaphorin expressed on activated B and T cells, induces B cells to aggregate and improves their viability in vitro. 4 These results were obtained using CD100 transfectants and so presumably reflect CD100 expressed on an activated T or B cell engaging a CD100 counter-receptor on B cells. We also found that CD100 was expressed by germinal center B cells, as well as germinal center associated and interfollicular T cells, but not by mantle zone B cells, suggesting that CD100 might have a role in germinal center formation. 4 Based on these results, we hypothesized that T-cell lymphoproliferative disorders, as well as B-cell lymphomas of follicular center cell origin, would express CD100.
As predicted, the vast majority of T-cell malignant neoplasms studied, 18 of 20 cases (90%), were strongly immunoreactive for CD100. The one case studied by Northern blot analysis expressed very high levels of CD100 mRNA. The two T-cell non-Hodgkin’s lymphoma cases nonreactive for CD100 were examples of Ki-1-positive anaplastic large-cell lymphoma, actually a heterogeneous group of neoplasms. 21 Two additional cases of Ki-1-positive anaplastic large-cell lymphoma studied exhibited positive staining for CD100.
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) and mantle cell lymphoma are pre-follicular center cell lymphomas, based on current models of B-cell ontogeny and studies in these neoplasms of somatic hypermutation in rearranged immunoglobulin genes. 22-24 We had previously observed that non-neoplastic B cells of the follicular mantle zone are nonreactive for CD100 by immunoperoxidase analysis. 4 Therefore, we hypothesized that these neoplasms, like peripheral blood B cells, would exhibit little or no CD100 expression. As expected, all of the cases of CLL/SLL and mantle cell lymphoma studied were nonreactive for CD100 by immunoperoxidase analysis. Two representative cases studied by Northern blot analysis similarly exhibited minimal expression of CD100 mRNA attributed to the presence of interspersed reactive T cells. Cases of marginal zone lymphoma, a set of post-follicular center cell neoplasms, based on studies of somatic hypermutation in rearranged immunoglobulin genes in non-neoplastic mantle zone cells and mantle zone lymphoma cells, 25,26 were also nonreactive for CD100.
Previously, we found that CD100 was expressed by germinal center B cells, but surprisingly, the vast majority of follicular lymphomas studied, 37 of 40 cases (93%), did not express CD100 by immunoperoxidase analysis. The three CD100-positive follicular lymphomas were of large-cell type, but eight other large-cell follicular lymphomas were CD100 negative. None of the predominantly small-cell-type and mixed small- and large-cell-type follicular lymphomas expressed CD100, based on immunoperoxidase analysis. Northern blot analysis of a follicular lymphoma case negative for CD100 by immunoperoxidase confirmed the absence of CD100 mRNA whereas Northern blot analysis of a CD100-positive follicular lymphoma confirmed abundant expression of CD100 mRNA. The absence of CD100 mRNA expression in follicular lymphoma cases negative by immunoperoxidase indicates that the cells are not synthesizing a secreted form of CD100.
We found that all five cases of high-grade, small non-cleaved (Burkitt-like) B lymphoma expressed CD100, although at only a low to moderate level, based on Northern blot and immunoperoxidase analysis. This was similar to the level of expression seen in the Raji Burkitt’s lymphoma cell line in our Northern blot analysis. A recent study of immunoglobulin VH gene somatic mutation in high-grade, small non-cleaved B lymphoma suggest that these neoplasms are derived from early centroblasts or follicular center cells that have partially differentiated into memory B cells. 26 Based on these results, the state of follicular center cell differentiation of small non-cleaved cell lymphomas (very early or very late in follicular center cell development) may result in fairly low-level expression of CD100. The presence of CD100 staining in small non-cleaved B-cell lymphoma as well as a subset of follicular large-cell (centroblastic) lymphomas raises the possibility that both lymphoma types may be derived from a similar, immature follicular center cell.
The presence of CD100 staining in all cases of small non-cleaved (Burkitt-like) lymphoma but no other diffuse B-cell non-Hodgkin’s lymphomas suggests that CD100 staining may be a useful marker for evaluating cases of diffuse lymphoma in which the differential diagnosis includes small non-cleaved (Burkitt-like) lymphoma. In these instances, diffuse large-B-cell lymphoma is often in the differential diagnosis and has been found to be uniformly negative for CD100 staining based on our study of a large number of cases.
The lack of expression of CD100 in follicular and diffuse large-cell lymphomas leads to the hypothesis that either the normal B-cell counterpart of these tumors does not express CD100 or that loss of expression confers an advantage in growth or metastatic mobility. Expression of CD100 in the germinal center has been examined only by immunohistochemistry. 4 Careful analysis of CD100 expression by flow cytometry, correlated with that of markers characteristic of various stages of germinal center development such as CD10, CD23, CD38, CD44, CD77, IgM, and IgD would show whether CD100 is expressed only at certain developmental stages. Alternatively, if CD100 expression is found at all stages of germinal center development, this would suggest that loss of CD100 is advantageous to the tumor. Loss of expression is also characteristic of tumor suppressor genes, and two semaphorin family members are candidate tumor suppressor genes. Both genes map to the 3p21.3 locus that is frequently homozygously deleted in small-cell lung carcinoma, and their expression is down-regulated in these tumors. 27 The chromosomal location of CD100 is not yet known, but Southern blotting of tumor sample DNAs would indicate whether the CD100 sequence is deleted in follicular and diffuse large-cell lymphomas.
Loss of CD100 expression in B-cell non-Hodgkin’s lymphomas might lead to decreased adhesivity and increased mobility and metastatic potential, consistent with widespread disease that may be observed in follicular lymphoma and diffuse large-cell lymphoma. This hypothesis might be tested by transfection of CD100 into cell lines derived from follicular and diffuse large-cell lymphomas, followed by examination of their metastatic potential in Nude or SCID mice.
Only exceptional cases of large-cell-type follicular lymphoma expressed CD100, raising the possibility that retained CD100 expression in follicular lymphomas may be specifically associated with centroblastic (large-cell) differentiation and may in some fashion contribute to proliferation of the neoplastic centroblasts. We have previously shown that CD100 stimulates B-cell viability, 4 and other semaphorin genes have also been shown to be involved in cell survival. 28 Human semaphorin E was isolated on the basis of its capacity to confer resistance to cell killing by cis-platinum and is aberrantly overexpressed in recurrent squamous cell carcinomas. 28 In addition, semaphorin E is overexpressed in the synovial cells of patients with rheumatoid arthritis. 29 However, in our study, CD100-positive and CD100-negative cases of follicular large-cell lymphoma exhibited similar staining for the proliferation marker Ki-67 (MIB-1), suggesting that they have comparable growth rates regardless of differences in CD100 expression. Also, there was no difference in the expression of the anti-apoptotic protein bcl-2 between CD100-positive and CD100-negative follicular large-cell lymphomas. It will be useful to study additional cases of predominantly large-cell-type follicular lymphoma to confirm this finding and to further analyze CD100-positive cases for clues to the role of CD100 in the behavior of these neoplasms. It will also be interesting to determine whether there is any clinical consequence of CD100 expression in predominantly large-cell-type follicular lymphomas, compared with CD100-negative cases.
Using CD100 transfectants, we found that CD100 induces B cells to aggregate and improves their viability in vitro. 4 CD100 could synergize with CD40 ligand (CD40L) to stimulate B cells. Binding of CD40L to CD40 induces B cell expression of multiple proteins, including CD23. Co-engagement of CD100 counter-receptor by CD100 prevented this expression of CD23 but not of other CD40-induced proteins. If the CD100 signal was delivered after CD23 was expressed, CD23 was lost from the cell surface, suggesting an active process, perhaps proteolytic cleavage. These results were obtained using CD100 transfectants and so presumably reflect CD100 expressed on an activated T or B cell engaging a CD100 counter-receptor on B cells. This supported a model in which CD100 affects CD40L-mediated B cell aggregation and survival by enhancing the proteolysis of CD23, resulting in enhanced B cell aggregation and in vitro viability. 30 In previous reported immunoperoxidase studies, neoplastic B cells in cases of high-grade, small non-cleaved cell lymphoma were nonreactive for CD23, 31,32 and we have found these lymphomas to be consistently immunoreactive for CD100, consistent with the above model. Similarly, although the neoplastic cells in cases of follicular lymphoma may be immunoreactive for CD23, 33-35 by immunoperoxidase analysis we found that all three cases of CD100-positive follicular lymphoma were nonreactive for CD23. We also found that five cases of CD100-negative follicular large-cell lymphoma were also negative for CD23. These findings suggest that the above model of CD100-mediated proteolysis of CD23 as a mechanism of enhanced B-cell viability may also hold true in a subset of B-cell lymphoproliferative disorders.
The absence of CD100 expression in follicular lymphomas was the most surprising result, based on the positive expression of CD100 in germinal center B cells. As expression of CD100 allows the cell to stimulate adhesion, the loss of CD100 should reduce this capacity thereby avoiding a T-cell anti-tumor response. Indeed, follicular lymphomas are poor antigen-presenting cells (APCs), 36 as are other CD100-negative lymphomas such as CLL. In contrast, CD100-positive lymphomas such as high-grade, small non-cleaved (Burkitt-like) B lymphoma or lymphoblastoid cell lines are excellent APCs. The expression of co-stimulatory molecules such as B7–1 and B7–2 is important for this increased APC capacity, but the expression of CD100 may contribute. In vivo studies introducing murine CD100 into B-cell lymphomas will test the role of CD100 in B-cell APC function and the capacity to stimulate an anti-tumor response.
In conclusion, we have studied the expression of a novel transmembrane semaphorin protein expressed in B and T cells that we find is also expressed in a majority of T-cell non-Hodgkin’s lymphomas and a subset of B-cell non-Hodgkin’s lymphomas. Preliminary evidence suggests that this molecule may function to enhance B-cell viability both in normal lymphoid tissue and in neoplastic states.
Footnotes
Address reprint requests to Dr. David M. Dorfman, Department of Pathology, Brigham and Women’s Hospital, Boston, MA 02115. E-mail: dmdorfman@bics.bwh.harvard.edu.
Supported by National Institutes of Health grants CA40216–11 and AI35225–03.
References
- 1.Kolodkin AL, Matthes DJ, Goodman CS: The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 1994, 75:1389-1399 [DOI] [PubMed] [Google Scholar]
- 2.Luo Y, Raible D, Raper JA: Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993, 75:217-222 [DOI] [PubMed] [Google Scholar]
- 3.Marx J: Helping neurons find their way. Science 1995, 268:971-973 [DOI] [PubMed] [Google Scholar]
- 4.Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ: Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci USA 1996, 93:11780-11785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bougeret C, Mansur I-G, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L: Increased surface expression of a newly identified 150-kd dimer early after human T lymphocyte activation. J Immunol 1992, 148:318-323 [PubMed] [Google Scholar]
- 6.Herold C, Dastot H, Roy J-P, Bensussan A, Boumsell L: CD100 defines a newly identified 150-kd human lymphocyte surface structure. Schlossman SF Boumsell L Gilks W Harlan JM Kishimoto T Morimoto C Ritz J Shaw S Silverstein R Springer T Tedder T Todd RF eds. Leukocyte Typing V: White Cell Differentiation Antigens. 1995, :pp 288-292 Oxford University Press, Oxford [Google Scholar]
- 7.Herold C, Bismuth G, Bensussan A, Boumsell L: Activation signals are delivered through two distinct epitopes of CD100, a unique 150 kd human lymphocyte surface structure previously defined by BB18 mAB. Int Immunol 1994, 7:1-8 [DOI] [PubMed] [Google Scholar]
- 8.Herold C, Elhabazi A, Bismuth G, Bensussan A, Boumsell L: CD100 is associated with CD45 at the surface of human T lymphocytes: role in homotypic adhesion. J Immunol 1996, 157:5262-5268 [PubMed] [Google Scholar]
- 9.Elhabazi A, Lang V, Herold C, Freeman GJ, Bensussan A, Boumsell L, Bismuth G: The human semaphorin-like leukocyte surface molecule CD100 associates with a serine kinase activity. J Biol Chem 1997, 272:23515-23520 [DOI] [PubMed] [Google Scholar]
- 10.He Z, Tessier-Lavigne M: Neuropilin is a receptor for the axonal chemorepellant semaphorin III. Cell 1997, 90:739-751 [DOI] [PubMed] [Google Scholar]
- 11.Kolodkin AL, Levengood DV, Rowe EG, Tai Y-T, Giger RJ, Ginty DD: Neuropilin is semaphorin III receptor. Cell 1997, 90:753-762 [DOI] [PubMed] [Google Scholar]
- 12.Feiner L, Koppel AM, Kobayashi H, Raper JA: Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron 1997, 19:539-545 [DOI] [PubMed] [Google Scholar]
- 13.Ensser A, Fleckenstein B: Alcelaphine herpesvirus type I has a semaphorin-like gene. J Gen Virol 1995, 76:1063-1067 [DOI] [PubMed] [Google Scholar]
- 14.Plowright W, Ferris RD, Scott GR: Blue wildebeest and the aetiological agent of bovine malignant catarrhal fever. Nature 1960, 188:1167-1169 [DOI] [PubMed] [Google Scholar]
- 15.Reid HW, Buxton D: Malignant catarrhal fever of deer. Proc R Soc Edinburgh 1984, 82B:261-273 [Google Scholar]
- 16.Roizman B, Desrosiers RC, Fleckenstein B, Lopez C, Minson C, Studdert MJ: The family Herpesviridae: an update. Arch Virol 1992, 132:425-447 [DOI] [PubMed] [Google Scholar]
- 17.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 18.Carriere D, Arcier JM, Derocq JM, Fontane C, Richer G: Antigenic modulation induced by four monoclonal antibodies adsorbed on gold particles (specificity anti-CD4, anti-CD5, anti-CD7, and anti-150-kd antigen): relationship between modulation and cytotoxic activity of immunotoxins. Exp Cell Res 1989, 182:114-128 [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, ed 2. Cold Spring Harbor NY, Cold Spring Harbor Laboratory Press 1989
- 20.Feinberg AP, Vogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 1983, 132:6-13 [DOI] [PubMed] [Google Scholar]
- 21.Penny RJ, Blaustein JC, Longtine JA, Pinkus GS: Ki-1-positive large cell lymphomas, a heterogeneous group of neoplasms: morphologic, immunophenotypic, genotypic, and clinical features of 24 cases. Cancer 1991, 68:362-373 [DOI] [PubMed] [Google Scholar]
- 22.Ahmed R, Gray D: Immunological memory and protective immunity: understanding their relation. Science 1996, 272:54-60 [DOI] [PubMed] [Google Scholar]
- 23.Wagner SD, Martinelli V, Luzzatto L: Similar patterns of VH gene usage but different degrees of somatic mutation in hairy cell leukemia, prolymphocytic leukemia, Waldenstrom’s macroglobulinemia, and myeloma. Blood 1994, 83:3647-3653 [PubMed] [Google Scholar]
- 24.Hummel M, Tamaru J, Kalvelage B, Stein H: Mantle cell (previously centrocytic) lymphomas express VH genes with no or very little somatic mutations like the physiologic cells of the follicle mantle. Blood 1994, 84:403-407 [PubMed] [Google Scholar]
- 25.Dunn-Walters D, Isaacson PG, Spencer J: Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med 1995, 182:559-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamaru J, Hummel M, Marafioti T, Kalvelage B, Leoncini L, Minacci C, Tosi P, Wright D, Stein H: Burkitt’s lymphomas express VH genes with a moderate number of antigen-selected somatic mutations. Am J Pathol 1995, 147:1398-1407 [PMC free article] [PubMed] [Google Scholar]
- 27.Sekido Y, Bader S, Latif F, Chen J-Y, Duh F-M, Wei M-H, Albanesi JP, Lee CC, Lerman MI, Minna JD: Human semaphorins A (V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci USA 1996, 93:4120-4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada T, Endo R, Gotoh M, Hirohashi S: Identification of semaphorin E as a non-MDR drug resistance gene of human cancers. Proc Natl Acad Sci USA 1997, 94:14713-14718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangasser-Stephan K, Dooley S, Welter C, Mutschler W, Hanselmann RG: Identification of human semaphorin E gene expression in rheumatoid synovial cells by mRNA differential display. Biochem Biophys Res Commun 1997, 234:153-156 [DOI] [PubMed] [Google Scholar]
- 30.Bourget I, Di Berardino W, Breittmayer J-P, Grenier-Brossette N, Plana-Prades M, Bonnefoy J-Y, Cousin J-L: CD20 monoclonal antibodies decrease interleukin-4-stimulated expression of the low-affinity receptor for IgE (Fc(RII/CD23) in human B cells by increasing the extent of its cleavage. Eur J Immunol 1995, 25:1872-1876 [DOI] [PubMed] [Google Scholar]
- 31.Stein H, Lennert K, Feller AC, Mason DY: Immunohistological analysis of human lymphoma: correlation of histological and immunological categories. Adv Cancer Res 1984, 42:67-147 [DOI] [PubMed] [Google Scholar]
- 32.Pallesen G: The distribution of CD23 in normal human tissues and in malignant lymphomas. McMichael AJ eds. Leukocyte Typing III: White Cell Differentiation Antigens. 1987, :pp 383-386 Oxford University Press, Oxford [Google Scholar]
- 33.Williamson JMS, Grigor I, Smith MEF, Holgate CS, Quirke P, Bird CCC: Cluster differentiation antigen expression, proliferative activity and clinical stage in centroblastic centrocytic lymphomas. J Pathol 1986, 150:51-59 [DOI] [PubMed] [Google Scholar]
- 34.Schuurman H-J, van Baarlen J, Huppes W, Lam BW, Verdonck LF, van Unnik JAM: Immunophenotyping of non-Hodgkin’s lymphomas: lack of correlation between immunophenotype and cell morphology. Am J Pathol 1987, 129:140-151 [PMC free article] [PubMed] [Google Scholar]
- 35.Salter DM, Krajewski AS, Cunningham S: Activation and differentiation antigen expression in B-cell non-Hodgkin’s lymphoma. J Pathol 1988, 154:209-222 [DOI] [PubMed] [Google Scholar]
- 36.Schultze JS, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, Gribben JG, Nadler LM: Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci (USA) 1995, 92:8200-8204 [DOI] [PMC free article] [PubMed] [Google Scholar]