Abstract
Intestinal epithelial cells derive from stem cells at the base of the crypt and migrate along the crypt-lumen axis. Their life is terminated as they reach the luminal surface where they detach and are shed. Intestinal epithelial cells show evidence of apoptosis in the region of shedding, and cell death is thought to resemble a form of apoptosis called detachment-induced cell death, or anoikis. Human intestinal epithelial cells die rapidly in vitro due to loss of anchorage during isolation, making primary culture of these cells a goal that has not yet been reached. However, the molecular mechanisms underlying this process of anoikis are largely unknown. In this study, a novel protocol for the rapid, temperature-controlled isolation of highly purified human colonic epithelial cells from surgical specimens is described. Using this method, early molecular events of anoikis in nontransformed epithelial cells were studied. Intestinal epithelial cells were isolated at the beginning of the apoptotic cascade, before the activation of caspase 3 family members and cleavage of poly(ADP-ribose) polymerase and DNA fragmentation. Elucidating the molecular mechanisms of detachment-induced cell death may facilitate the establishment of long-term primary cultures of human intestinal epithelial cells and enhance our understanding of homeostasis in the intestinal epithelium.
Apoptosis is critically important to development, tissue homeostasis, and the pathogenesis of various diseases. 1,2 A family of cysteine proteases, recently classified as caspases 1 to 10, 3 are key intracellular mediators of apoptosis. However, the sequence of events that mediate the proteolytic apoptotic cascade has not been defined. Caspase 3, a 32-kd protease, is highly expressed in the human intestinal epithelium 4 and known to be involved in the amplification and execution of apoptosis in other cell types, triggered by various stimuli, such as Fas/Fas-L, tumor necrosis factor-α, granzyme B, chemotherapeutic agents, and growth factor deprivation. 5-7 Poly(ADP-ribose) polymerase (PARP), an enzyme involved in genome surveillance and DNA repair, is a substrate of caspase 3 and other members of its subfamily. 8 PARP cleavage is a commonly assessed molecular event to demonstrate caspase activation and represents an early molecular hallmark of programmed cell death. 6
Intestinal epithelial cells (IECs) derive from stem cells at the base of the crypt and migrate along the crypt-villus axis as they differentiate. On reaching the luminal surface after 3 to 5 days, they detach and are shed. 9 As IECs show evidence of DNA fragmentation and morphological features of apoptosis in the region of shedding, programmed cell death is thought to play an integral role in completing the life cycle of these cells. 10 A form of apoptosis, referred to as detachment-induced cell death, or anoikis, 11 is likely to contribute to this process, as the configuration of the basement membrane, the expression of transforming growth factor-β, and the composition of integrins at the luminal surface create conditions that promote loss of anchorage and anoikis. 12-14
Research on apoptosis of human IECs has predominantly been carried out using adenocarcinoma cell lines; however, these transformed cells cannot be considered ideal models for studies of physiological cell death. Apoptosis in IEC lines can be induced by a variety of triggers, including interferon-γ, tumor necrosis factor-α/Fas, 15 transforming growth factor-β, 14 short-chain fatty acids, 16 p53, 17 cyclooxygenase 2, 18 and bak 19 as well as detachment from extracellular matrix (ECM) 14 and loss of cell-cell contact. 20,21
Studies on apoptosis in nontransformed human IECs have been largely performed using immunohistochemical techniques. In the colon, bcl-2, a known inhibitor of apoptosis, is highly expressed in the region of the stem cells and is undetectable at the luminal surface. 22,23 No expression of bcl-2 is detected in the small intestine of humans 24 or mice. 25 The expression of bak, a promoter of apoptosis, displays an inverse pattern to that of bcl-2, with strongest expression at the luminal surface of the colon. 19 Recently, the susceptibility to Fas-mediated apoptosis has also been demonstrated in freshly isolated human IECs. 26 Progress of studies on human IECs, however, has been limited by the fact that freshly isolated IECs die of apoptosis within hours of isolation, caused by loss of ECM anchorage or cell-cell contact. 27,28 Thus, human IECs undergoing anoikis in vitro may represent a useful model to study the molecular mechanisms of cell death in IECs at the luminal surface.
In this report, a novel protocol, specifically designed to elucidate early molecular events of apoptosis in nontransformed human IECs, is described. Using this method, highly purified intact intestinal crypts can be isolated before activation of caspase 3 family members and cleavage of intracellular caspase substrates. Elucidating the molecular mechanisms of anoikis in IECs may enhance our understanding of homeostasis in the intestinal epithelium as well as facilitate long-term cultures of human nontransformed IECs.
Materials and Methods
Surgical Specimens
Surgical specimens were obtained from patients undergoing large bowel resection for colon cancer. Macroscopically normal tissue, at least 10 cm from the tumor margins, was studied. All specimens were processed within 1 hour of the resection.
Isolation of IECs
IECs were isolated by three existing protocols (methods A, B, and C) and one newly designed protocol (method D), characterized in this report.
Method A
This method was carried out according to the protocol of Youngman et al. 29 After washing the specimen in Ca2+-, Mg2+-free, Hanks’ balanced salt solution (CMF-HBSS; BioWhittaker, Walkersville, MD), the mucosa was dissected from the submucosa and cut into strips. These were washed at room temperature in 10 mmol/L dithiothreitol (DTT; Fisher Biotech, Fair Lawn, NJ) for 30 minutes, followed by two 90-minute washes in 1 mmol/L EDTA (Sigma Chemical Co., St. Louis, MO). Cells liberated from both washes were harvested by centrifugation at 500 × g for 5 minutes at room temperature, followed by a 30-minute incubation in 3 mg/ml dispase (Boehringer Mannheim, Indianapolis, IN) and 1 mg/ml DNase (Worthington, Freehold, NJ) at 37°C. Cells were harvested by centrifugation and washed twice with CMF-HBSS, followed by further purification with a 50% Percoll gradient (Pharmacia Biotech, Uppsala, Sweden). After centrifugation for 20 minutes at 300 × g, purified epithelial cells were harvested at the interphase and washed three times in CMF-HBSS.
Method B
This method was carried out according to the protocol of Gibson et al. 30 After washing the specimen in CMF-HBSS, the epithelial cell layer was scraped from the submucosa with a glass slide and finely minced using a crossed scalpel technique, followed by a 90-minute incubation at 37°C in 1.2 U/ml dispase (Boehringer Mannheim) and 50 U/ml collagenase type IV (Worthington). After passing through a 21-gauge needle, the digest was washed in CMF-HBSS and filtered through a 300- 400-μm mesh to remove undigested tissue fragments. The cells were then washed twice in CMF-HBSS, separating crypts from single cells by repeated centrifugations at 75 × g for 2 to 3 minutes.
Method C
This method was carried out according to the protocol of Whitehead et al. 31 Tissue was washed several times in 0.1 mol/L phosphate-buffered saline (PBS; BioWhittaker), and the mucosa was dissected from the submucosa, followed by a 30-minute wash in 0.04% sodium hypochloride. The mucosal strips were then cut into 1-cm 2 pieces and incubated for 1.5 hours at 22°C in 1 mmol/L EDTA, 1 mmol/L EGTA (Sigma) and 0.5 mmol/L dithiothreitol in PBS. Crypts were then liberated by vigorous hand shaking of the beaker. This procedure was repeated six times with 10-ml aliquots of PBS. Liberated crypts were harvested by centrifugation and washed once in PBS.
Method D
Specimens were thoroughly rinsed in CMF-HBBS. Mucus and debris were removed by gently tapping the tissue with paper towels between rinses. The mucosa was dissected from the submucosa, cut into strips that were washed in 10 mmol/L dithiothreitol for 30 minutes at room temperature, and then incubated for 60 minutes in 1 mmol/L EDTA in CMF-HBBS at 4°C. Epithelial cells were detached as intact crypts by 10 vigorous shakes of the vessel. The cells suspended in EDTA were immediately passed through filtering cylinders prepared by taping a piece of 80-μm nylon mesh (Nytex, Tetko, Elmsford, NY) to a plastic ring (5 cm diameter, 2.5 cm height). This allowed single cells to pass through while retaining the crypts. The mesh was rinsed with 20 ml of Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, NY) and inverted, and the purified crypts were backwashed from the cylinder with 15 ml of keratinocyte serum-free medium (K-SFM; Gibco BRL), supplemented with 2.5% penicillin-streptomycin-fungizone (PSF; BioWhittaker) and 1% gentamicin (BioWhittaker). To confirm the importance of anchorage for epithelial cell survival, in some experiments, surgical specimens were divided in half. From one half (“early”), IEC crypts were isolated immediately and purified IECs were maintained in a polypropylene container at 37°C. The other half of the tissue (“late”) was incubated intact for 5 hours under the same conditions as the isolated crypts. At the end of this period, IECs were isolated from the second half of the tissue.
Ethidium Bromide/Acridine Orange Stain
Ethidium bromide/acridine orange staining was performed as described. 32 Briefly, 2 μl of a combined dye of 100 μg/ml acridine orange (Sigma) and 100 μg/ml ethidium bromide (Sigma) were added to 20 μl of the cell suspension, and 5 μl of the stained cell suspension was rapidly transferred to a glass slide for immediate analysis using an ultraviolet fluorescence microscope (Olympus-BH2, Olympus, Lake Success, NY). Cells were scored into four categories: (C1, cells with large, green, noncondensed nuclei as non-apoptotic, viable cells; C2, cells with green nuclei that showed signs of nuclear condensation or nuclear bead formation as apoptotic, viable cells; C3, cells with red/orange nuclei that showed signs of nuclear bead formation as apoptotic, nonviable cells; and C4, cells with large red nuclei that did not show signs of nuclear condensation or bead formation as necrotic cells. At least 200 cells/sample were counted and scored. The apoptotic index (percent) was calculated by dividing the sum of apoptotic cells (C2 + C3) × 100 by the total number of cells scored.
DNA Extraction and Electrophoresis
DNA extraction was performed as described. 33 Freshly isolated IECs were pelleted by centrifugation and lysed with 0.4 ml of DNA extraction buffer (10 mmol/L Tris/HCl, pH 8.0 (Boehringer Mannheim), 0.1 mol/L EDTA, pH 8.0, and 0.5% sodium dodecyl sulfate (SDS; Boehringer Mannheim)), followed by gentle pipetting. Proteinase K (200 μg/ml; Gibco BRL) was added, and samples were incubated overnight at 42°C. An equal volume of ultra-pure phenol (Gibco BRL) was added and gently agitated for 30 minutes, followed by centrifugation for 5 minutes at 14,000 rpm. The upper aqueous phase was carefully harvested, and an equal volume of chloroform/isoamyl alcohol (Sigma) was added. After 30 minutes of gentle agitation and centrifugation for 5 minutes at 14,000 rpm, the upper, aqueous phase was harvested and incubated for 1 hour at 37°C after addition of 50 μg/ml RNAse (Sigma). An equal volume of a 1:1 mixture of phenol and chloroform/isoamyl alcohol was added. The samples were gently agitated for 30 minutes and centrifuged as above. The upper phase was again harvested, and DNA was precipitated by adding a 2.5-fold volume of ice-cold 100% ethanol. DNA was harvested by centrifugation for 10 minutes at 14,000 rpm, and the supernatant was decanted. DNA was washed twice with 1 ml of 70% ethanol, followed by centrifugation. The DNA was resuspended in 20 μl of TE buffer (10 mmol/L Tris/HCl, 1 mmol/L EDTA, pH 8.0) and quantified spectrophotometrically. Two micrograms of DNA was fractionated on a 1.6% agarose gel (Gibco BRL) by electrophoresis, stained with 0.5 μg/ml ethidium bromide (Sigma), and visualized under ultraviolet light.
Fluorogenic Substrate Assays
Synthetic tetrapeptide substrates, specifically cleaved by members of the caspase 3 and caspase 1 subfamilies, respectively, were added to IEC cytosol. N-Acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC; Biomol Research Laboratories, Plymouth Meeting, PA) was used as a substrate specific for caspase 3 family members, and N-acetyl-Tyr-Val-Ala-Asp-4-methyl-coumaryl-7-amide (YVAD-AMC; Biomol) served as a substrate for caspase 1 family members. 34 Freshly isolated IECs were lysed in 0.25 ml of lysis buffer (100 mmol/L HEPES, 10% sucrose, 0.1% 3-[(3-cholanidopropyl)dimethyl-ammonio]-1-propanesulfonate (CHAPS), 1 mmol/L phenylmethanesulfonyl fluoride (PMSF), 10 mmol/L dithiothreitol (DEVD-AMC fluorogenic assay only), 100 μmol/L pepstatin, 100 μmol/L leupeptin, 1 mmol/L EDTA at pH 6.8), followed by a 30-minute incubation on ice. Lysis was completed by two 10-second sonication pulses (Sonicator XL, Heat Systems, Farmingdale, NY). Cellular debris was removed by centrifugation, and the cytosolic extract was stored at −20°C. Protein content of the lysates was determined by the BioRad protein assay (BioRad, Hercules, CA) according to the manufacturer’s instructions. Twenty micrograms of cytosolic protein and 50 μmol/L DEVD-AMC or YVAD-AMC were incubated in a total volume of 500 μl in 100 mmol/L HEPES, 10% sucrose, 0.1% CHAPS, pH 6.8, for 30 minutes on an orbital shaker at 37°C. Samples were diluted with lysis buffer to a final volume of 1 ml immediately before measurement of fluorescence by fluorospectrophotometry (Perkin Elmer LS-3; excitation, 380 nm; emission, 480 nm; slit width, 3.0). For inhibition of caspase 3 family activity, 20 μg of protein from lysates was incubated with 150 μmol/L DEVD-CHO (Biomol) for 20 minutes at 37°C before incubation with the fluorescent substrate. Standards containing 0 to 5000 pmol of AMC were used to determine the amount of fluorochrome released. Measurements were recorded over the linear range of the assay. Bovine serum albumin (50 μg) served as negative control. As a positive control, a monocytic cell line (U937) was treated with lipopolysaccharide (100 μg/ml) to induce caspase 1 activation. Eight hours after stimulation, cytosol from U937 cells was extracted by the same protocol used for IECs and tested for caspase 1 activity.
Western Blotting
Fifty micrograms of cytosolic protein was fractionated on an 8 to 14% SDS-polyacrylamide gel and electrotransferred to Immobilon p15 membranes (Millipore Corp., Bedford, MA). Membranes were blocked overnight at 4°C with 5% milk in 0.1% Tween-20/Tris-buffered saline (TBS; Fisher), followed by incubation with the mouse monoclonal primary antibodies (anti caspase-3 at 1:2000; Transduction Laboratories, Lexington, KY; and 1 μg/ml anti-PARP35) for 60 minutes at ambient temperature. Membranes were washed six times with 0.1% Tween-20/TBS and then incubated for 1 hour with horseradish-peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:5000; Transduction Laboratories), washed again, and incubated with chemiluminescent substrate (Super Signal, Pierce, Rockford, IL) for 5 minutes. The membranes were exposed to film (Amersham, Arlington Heights, IL).
Immunohistochemistry
Freshly isolated IECs were suspended in PBS, and 50 μl of the cell suspension was transferred to glass slides (Superfrost Plus, Fisher). The slides were air dried for 10 minutes, fixed for 10 minutes in ice-cold acetone, and incubated for 10 minutes in 3% hydrogen peroxide to block endogenous peroxidase activity. All staining was performed using the Dako Autoimmunostainer (Dako, Carpinteria, CA). Slides were rinsed between each step twice for 5 minutes with TBS (Dako). The following primary antibodies were used: anti-CD3 at 1:500 for 20 minutes (Ao452, Dako), a pan-cytokeratin cocktail (anti-AE1/AE3) at 1:25 for 10 minutes (MU071-UC, BioGenex, San Ramon, CA), and anti-CD68, a macrophage marker at 1:500 for 10 minutes (M814, Dako). After two rinses with TBS, slides were incubated with a universal secondary antibody solution of biotinylated anti-rabbit and anti-mouse immunoglobulin (LSAB-2, Dako) for 10 minutes, rinsed with TBS, and labeled with horseradish-peroxidase-conjugated streptavidin (LSAB-2, Dako) for 10 minutes. Slides were rinsed with TBS and developed with diaminobenzidine chromogen substrate (Dako) for 5 minutes in hydrogen peroxide and counterstained with hematoxylin and eosin (H&E). Human tonsillar tissue served as a positive control. Percentage of positive staining was determined by scoring 200 intact single cells from three different specimens.
Electron Microscopy
Freshly isolated IECs were fixed with glutaraldehyde and suspended in agar. IECs were post-fixed with OsO4, dehydrated with a series of ethanol washes, rinsed with propylene oxide, and embedded in LX-112 (Ladd Research Industries, Burlington, VT), which polymerized at 65°C. Samples were thin sectioned (80 nm), counterstained with uranyl acetate and lead citrate, and examined with a JEOL 100CX transmission electron microscope.
Terminal Deoxynucleotidyl Transferase (TdT) Nick End Labeling (TUNEL)
Terminal deoxynucleotidyl transferase (TdT) nick end labeling was performed using the Apoptag-plus kit (Oncor, Gaithersburg, MD) according to the manufacturer’s instructions. IECs were fixed on glass slides (Superfrost Plus) in 4% paraformaldehyde. Bovine aortic endothelial cells, treated with 25 μmol/L ketocholesterol for 24 hours to induce apoptosis, 36 were processed simultaneously as positive controls.
Results
Prevalence of Apoptosis in Freshly Isolated IECs
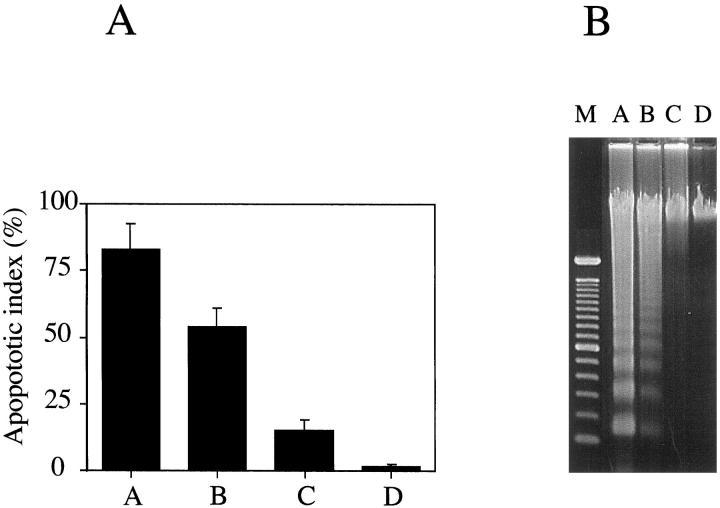
Colonic IECs were isolated in strict accordance to three existing protocols (methods A to C) and the newly developed protocol (method D), characterized in these studies. Although the viability of the cells isolated by all these protocols, as assessed by trypan blue staining, is greater than 95%, the degree of apoptosis varied considerably. Isolation of IECs using method A requires 6 hours and yields greater than 80% apoptotic cells when stained with acridine orange/ethidium bromide (Figure 1A) ▶ . This high level of apoptosis was confirmed by analyzing the DNA from isolated IECs by gel electrophoresis (Figure 1B) ▶ . IECs isolated using method A proved to be unsuitable for the study of early events in apoptosis. Therefore, the degree of apoptosis was determined in IECs isolated by two other protocols (methods B and C).
Figure 1.
Apoptosis in freshly isolated IECs. Human intestinal epithelial cells were isolated from normal colonic mucosa according to three existing isolation protocols (method A, n = 3; method B, n = 3; method C, n = 3) and a newly developed protocol (method D, n = 5). A: Apoptotic index of IECs immediately after isolation. Data shown are the mean ± SEM. Letters refer to the method used. B: DNA was extracted from IECs immediately after isolation and analyzed by electrophoresis. M, molecular mass marker; letters above other lanes refer to the method used.
The second protocol (method B) is 3 hours shorter than protocol A and uses mechanical disruption to remove the epithelium from the tissue. As shown in Figure 1, A and B ▶ ▶ , the cells obtained using protocol B were less apoptotic (apoptotic index = 53% versus 83% as determined for protocol A) with less DNA fragmentation. These data suggested that reducing the processing time is critical to decrease the degree of apoptosis in IEC. Using method C, IECs could be isolated within 2 hours at room temperature. The apoptotic index of these cells was 15% with little detectable DNA fragmentation (Figure 1, A and B) ▶ ▶ . The lower apoptotic index in IECs isolated using method C suggested that an additional reduction in both the time and temperature of isolation further decreases the rate of apoptosis.
Development of a New IEC Isolation Protocol
To develop a new procedure to isolate non-apoptotic colonic IECs, the time required for isolation, the temperature at which the protocol was performed, and the use of enzymes were systematically minimized. The final protocol (method D, as described in Materials and Methods) evolved by first reducing the time of EDTA incubation outlined in method A, then lowering the temperature of the tissue and eliminating the protease digestions as recommended in method C, and finally adding a filtration step to rapidly harvest and purify colonic crypts. A comparative study of each protocol is detailed in Table 1 ▶ .
Table 1.
Comparison between the Individual Isolation Protocols
| Isolation method* | Apoptotic index (%) | Duration of protocol (hours) | Time at 37°C (hours) | Time at 25°C (hours) | Time at 4°C (hours) | Chelating agent | Mechanical disruption | Enzymatic digestion |
|---|---|---|---|---|---|---|---|---|
| A | 84 | 5.2 | 0.5 | 4.7 | 0 | + | + | + |
| B | 53 | 2.5 | 1.5 | 1.0 | 0 | − | + | + |
| C | 18 | 2.0 | 0 | 2.0 | 0 | + | − | − |
| D | 2 | 1.0 | 0 | 0 | 1.0 | + | − | − |
*Isolation protocols as described in Materials and Methods.
Colonic IECs were purified at 4°C, immediately after detachment from the intestinal mucosa. An apoptotic index of 1.5 ± 0.5% and no evidence of DNA fragmentation indicated negligible apoptosis in these cells compared with that of the other procedures (Figure 1, A and B) ▶ ▶ . Figure 1 ▶ compares the four methods for the degree of apoptosis as determined by acridine orange/ethidium bromide staining (Figure 1A) ▶ and gel electrophoresis of epithelial cell DNA (Figure 1B) ▶ . An important observation derived from these data is that DNA fragmentation is a late event in IEC apoptosis.
Loss of Anchorage Induces Apoptosis of IECs
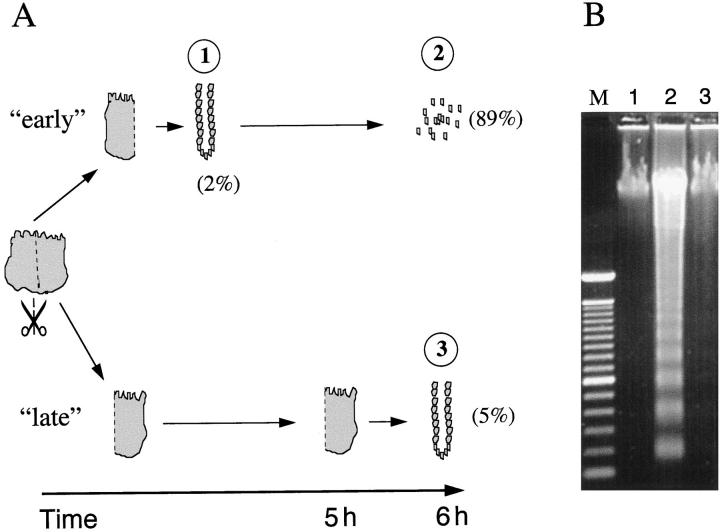
To confirm the critical role of anchorage for epithelial cell survival, surgical specimens were divided in half. From one half (“early”), IEC crypts were isolated immediately and maintained in a polypropylene container at 37°C. The other half of the tissue was incubated intact for 5 hours under the same conditions. At the end of this period, IECs were isolated from the second half of the tissue (“late”). The degree of IEC apoptosis was assessed by acridine orange/ethidium bromide stain and DNA electrophoresis. As shown in Figure 2A ▶ , epithelial cells, purified immediately (“early”), displayed an apoptotic index of 2%. Five hours later, 89% of these IECs were apoptotic. In contrast, when IECs remained anchored during the incubation period, 95% of these cells remained viable and non-apoptotic (“late”). These observations were confirmed by DNA electrophoresis (Figure 2B) ▶ .
Figure 2.
ECM anchorage protects IECs from anoikis. A: Surgical specimens were split in half. IECs from one half were isolated immediately (method D) and subsequently incubated at 37°C (“early”). Tissue from the other half was incubated intact at 37°C, allowing IECs to maintain anchorage. After 5 hours, IECs from the second half (“late”) were isolated using the same protocol. The apoptotic index is indicated by the numbers in parentheses (%). The circled numbers correspond to the lanes of the gel in B. B: DNA, extracted after immediate processing (“early”) of the tissue (lane 1, IECs immediately after isolation; lane 2, IECs 6 hours after isolation) and after delayed processing (“late”) of the tissue (lane 3), was analyzed by electrophoresis (lane M, molecular mass marker). Data are representative of three experiments.
Confirmation of Lack of Apoptosis in IECs Isolated by Method D
IECs isolated using method D were further analyzed for evidence of apoptosis by TUNEL assay. No staining was detected, demonstrating lack of fragmented DNA (data not shown). Electron microscopy assessing the morphology and ultrastructure of IECs confirmed the lack of apoptosis. In particular, the large, oval nuclei located at the basolateral surface revealed no signs of chromatin condensation at the nuclear membrane or formation of apoptotic bodies (Figure 3A) ▶ . IECs were preserved as a single layer of columnar-shaped cells. Major organelles (mitochondria, endoplasmic reticulum, and lysosomes), microvilli, tight junctions, and desmosomes were perfectly preserved (Figure 3B) ▶ .
Figure 3.
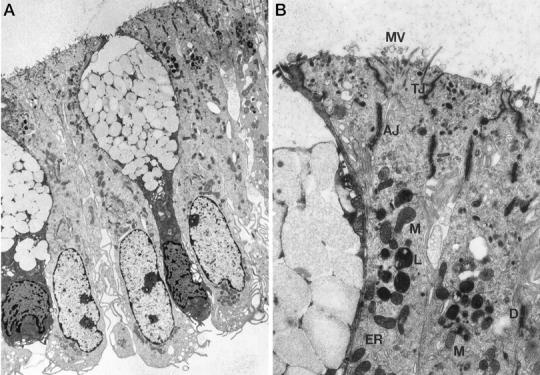
Lack of apoptosis in IECs isolated according to method D as determined by electron microscopy. A: Longitudinal cross section of purified crypt epithelial cells examined by transmission electron microscopy (magnification, ×6000). Note the fully preserved columnar shape of tightly joined colonocytes, the goblet shape of the mucus-containing cells, and the preserved basolateral location of the nuclei. B: Ultrastructure (magnification, ×48,000) of IECs, showing full preservation of cell-cell junctions, including tight junctions (TJ), adherent junctions (AJ), desmosomes (D), microvilli (MV), and organelles such as endoplasmic reticulum (ER), lysosomes (L), and mitochondria (M).
Characterization of the Cell Population Isolated by the New Protocol
Light microscopy examination of the isolated epithelium revealed tubular structures, typical of colonic crypts, formed by a single layer of columnar cells lining the crypt lumen (Figure 4A) ▶ . As the distribution of apoptotic IECs varies along the crypt axis, the isolation of selected areas of a crypt might give an unrepresentative estimate of apoptosis. After isolation, mucosal strips were fixed and stained with H&E. Microscopic examination showed that crypts were removed from the lamina propria in their entire length (Figure 4B) ▶ . To assess the purity of cells isolated using the new protocol, the percentage of epithelial cells, T cells, and macrophages was determined by immunohistochemistry. IECs were positive for cytokeratin and represented greater than 97% of the cells isolated (data not shown). Contamination by CD3+ T cells was less than 2%, and none of the cells stained positive for the macrophage marker CD68 (data not shown).
Figure 4.
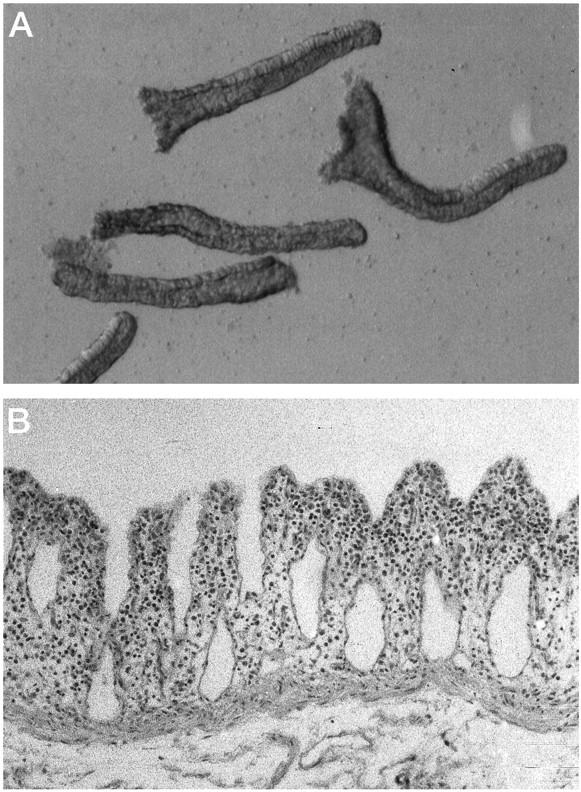
Characterization of the colonic epithelial cells isolated by method D. IECs were isolated using method D and analyzed by light microscopy. A: Magnification, ×100. Note the fully preserved tubular structures, typical of colonic crypts, formed by a single layer of columnar cells lining the central lumen. B: After removal of the epithelium, mucosal strips were stained with H&E (magnification, ×100). Note the absence of the entire crypt, leaving behind intact lamina propria.
Method D Yields IECs before the Activation of Caspases
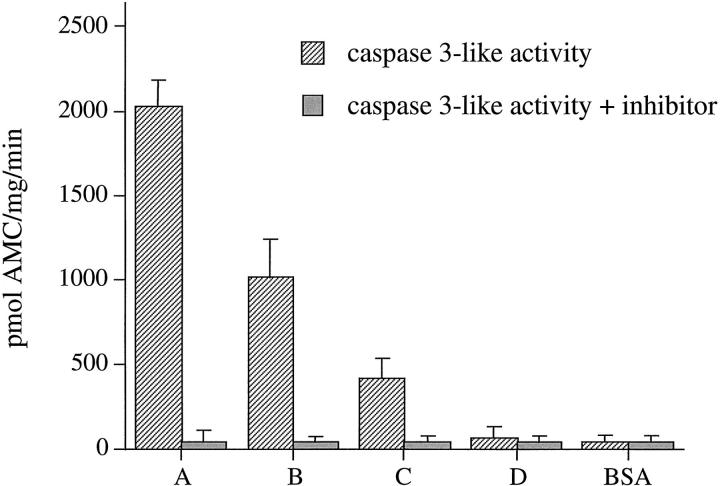
DNA fragmentation represents a relatively late event during the execution phase of apoptosis. 37 In contrast, activation of caspases initiates the apoptotic cascade and amplifies the death signal, thereby representing an early event. DEVD-AMC is a synthetic, fluorescent substrate specific for the caspase 3 subfamily. On cleavage, fluorescence is released, reflecting proteolytic activity. 38 To study the activation of caspases in IECs isolated using the four methods described above, cytosolic extracts were prepared and the enzymatic activity of caspase 3 family members was determined. IECs purified using methods A to C contained high levels of caspase-3-like enzymatic activity, whereas the caspase-3-like enzymatic activity in IECs isolated using the new method showed only background levels (Figure 5) ▶ . To confirm the specificity of DEVD-AMC cleavage, an inhibitor of caspase-3-like proteases (DEVD-CHO) was added to lysates before incubation with the substrate. Absence of fluorescence indicated that caspase-3-like specific cleavage of DEVD-AMC was totally inhibited (Figure 5) ▶ . These findings confirm that method D yields non-apoptotic IECs before caspase activation. Furthermore, they demonstrate that caspase-3-like cysteine proteases are activated in IECs undergoing anoikis. To determine whether IECs isolated by method D contain activated caspase 1 family members, the fluorogenic assay was performed using YVAD-AMC, a synthetic substrate of the caspase 1 family. YVAD-AMC was not cleaved when incubated with cytosol from IECs isolated by any method, suggesting that members of the caspase 1 family are not involved in IEC anoikis (Figure 6) ▶ . As a positive control, cytosolic protein from LPS-stimulated U937 was used. As active caspase 1 is present in stimulated monocytic cells, the substrate was readily cleaved, and this activity was inhibited by YVAD-CHO, a selective caspase 1 inhibitor.
Figure 5.
Activation of caspase 3 family cysteine proteases in apoptotic IECs. IECs were purified using the four methods (A to D) and cytosolic protein was extracted immediately after isolation. Cytosolic protein was incubated with DEVD-AMC. Fluorescence was measured by fluorospectrophotometry (striped bars). Background fluorescence was determined by incubating bovine serum albumin (BSA) with DEVD-AMC. Specificity of the assay for the caspase 3 family was confirmed by preincubating the samples with 150 μmol/L DEVD-CHO (a caspase 3 family inhibitor) before the addition of DEVD-AMC (solid bars).
Figure 6.
IEC anoikis does involve the activation of caspase 1 family cysteine proteases. Cytosolic extracts were prepared and the fluorescence assay performed as described in Figure 5 ▶ but using instead YVAD-AMC (striped bars ). Cytosolic protein from the human monocytic cell line U937 stimulated with LPS was used as positive control. Enzymatic activity was inhibited by the addition of 150 μmol/L YVAD-CHO (solid bars).
Non-Apoptotic IECs Contain Inactive Caspase 3
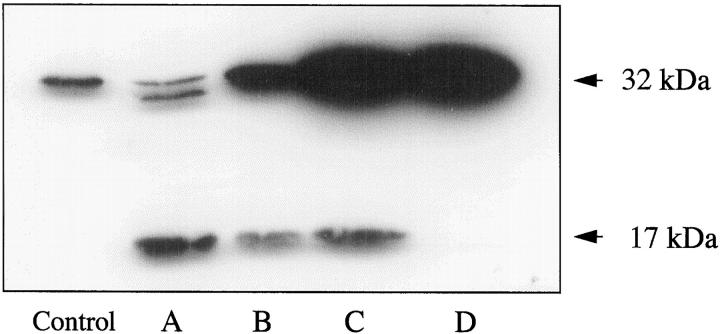
Although the fluorescence assay described above indicated the activation of various members of the caspase 3 subfamily, we performed Western blot analysis to assess in particular the activation of caspase 3, which is abundantly expressed in the intestinal epithelium. The inactive 32-kd proenzyme of caspase 3 is specifically cleaved during apoptosis to 17- and 12-kd fragments that reassemble to form the active enzyme. As shown in Figure 7 ▶ , the 17-kd cleavage product was readily detectable in lysates from methods A, B, and C, whereas in lysates of IECs isolated by method D only the intact, non-activated 32-kd form of caspase 3 was detectable, demonstrating that method D yielded IECs before activation of caspase 3. These findings also indicate the involvement of caspase 3 in IEC anoikis. Furthermore, they show that caspase 3 activation is an early event as IECs from method C display marked cleavage of caspase 3 within 2 hours after detachment.
Figure 7.
Activation of caspase 3 in IECs undergoing anoikis. Cytosolic protein from IECs isolated by the four methods was analyzed by Western blotting for caspase 3. Uncleaved caspase 3 migrates at 32 kd. Activation of caspase 3 results in the appearance of the 30-, 20-, and 17-kd cleavage products. The control was provided with the antibody (32 kd). Letters below other lanes refer to the isolation method used.
Lack of PARP Cleavage in IECs Isolated Using Method D
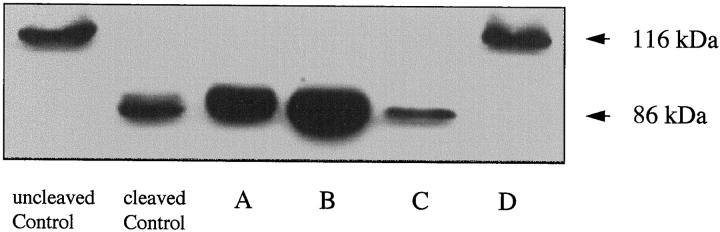
PARP is a 116-kd protein, which is cleaved during apoptosis by members of the caspase 3 family of cysteine proteases to 85- and 31-kd fragments. To study the biological activity of caspase 3 family members in freshly isolated IECs, lysates of IECs, isolated according to the four methods, were assessed by Western blot for evidence of PARP cleavage. In agreement with the data presented above, PARP cleavage was readily detected in lysates from IECs isolated using methods A and B. Cleavage of PARP was markedly less evident in IECs purified using method C and undetectable in lysates of IECs isolated by method D (Figure 8) ▶ .
Figure 8.
Cleavage of PARP in apoptotic IECs. Cytosolic protein was analyzed by Western blotting for PARP. Uncleaved PARP migrates at 116 kd, whereas cleaved PARP migrates at 85 kd. Uncleaved control was non-apoptotic Jurkat cells; cleaved control was Fas-induced apoptotic Jurkat cells. Letters below other lanes refer to the isolation method used.
Discussion
In this report, colonic IECs were isolated using four separate methods and assessed for evidence of apoptosis. IECs purified by existing protocols display obvious signs of apoptosis at the end of the isolation procedure, making them unsuitable to study early molecular events of the apoptotic cascade. However, using a new isolation technique described in this report, IECs were isolated before caspase activation, thereby providing a novel model to elucidate the sequence of caspase activation in nontransformed human intestinal epithelial cells. Intestinal epithelial cells undergo apoptosis in vivo as they reach the intestinal lumen and detach from the basement membrane. Detachment-induced apoptosis triggered in vitro may therefore represent a model of these physiological events. The ability to isolate non-apoptotic, morphologically intact IECs enabled us to explore early molecular events that initiate and propagate apoptosis.
Our study demonstrates that anoikis in IECs involves the rapid activation of caspase 3 family members and, in particular, caspase 3. We were unable to detect caspase 1 family activity in the four populations of IECs isolated, suggesting that anoikis involves the select activation of distinct caspases. The lack of caspase 1 family activation is seemingly contradictory to the results of two previous reports, which suggest that members of the caspase 1 family of cysteine proteases may be involved in mammary epithelial cell apoptosis 39 and programmed cell death of the intestinal epithelial tumor cell line HT-29. 40 The conclusions drawn from those studies, however, are based on indirect evidence, using cytokine response modifier A (CrmA) and Boc-aspartyl(benzyl) chloromethylketone (BACMK) to inhibit apoptosis. At the time these investigations were reported, CrmA and BACMK were thought to be specific inhibitors of caspase 1. However, subsequent studies demonstrate that CrmA also inhibits caspase 3, 7, and 8. 41-43 Although caspase 1 has been shown to induce apoptosis when overexpressed in fibroblasts 44 and mediates Fas-induced apoptosis in W4 cells, 45 other investigators have not been able to confirm these findings. 46 In addition, caspase-1-deficient mice develop normally. 47 Therefore, the existence of multiple caspase activation pathways seems likely. 48 Notably, IECs isolated by the new method displayed no evidence of PARP cleavage even though cleavage of PARP is considered to be a particularly early molecular event of apoptosis. 6,49 Ongoing studies employ this new method to characterize the kinetics of activation for various caspases and cleavage of caspase substrates in IECs undergoing anoikis. 50
Epithelial cells isolated by the new protocol are fully preserved as whole crypts by light and electron microscopy, displaying intact junctional complexes and organelles without evidence of degeneration, ischemia, or apoptosis. Contamination with T cells, macrophages, and red blood cells is effectively avoided by using an 80-μm mesh, which retains only intact crypts. The high purity, as judged by morphological examination, is confirmed by immunohistochemistry. Importantly, the isolation procedure does not involve any centrifugation, a technique that we observed to be disruptive to the delicate structure of the intestinal crypts. We could not perform flow cytometry to assess purity or evidence of apoptosis because our method isolates intact crypts, which are not suitable for single-cell analysis.
The degree of apoptosis in IECs correlated strongly with the length of time required to purify the cells. This observation is not surprising, as the initial step of IEC isolation inherently involves the detachment of the epithelial cells, which initiates the apoptotic cascade. In this study, we provide additional evidence that detachment is the pivotal trigger of IEC apoptosis during isolation as prolonged anchorage to the mucosa can effectively prevent apoptosis for several hours despite lack of blood supply and incubation of the tissue at 37°C. Furthermore, our data indicate that not only the duration of the protocol but also the temperature during cell isolation is of crucial significance to determine the degree of apoptosis. IECs isolated using method C are less apoptotic than those purified by method B (16% versus 53%), even though method C is only 30 minutes shorter. The decreased degree of apoptosis using method C probably reflects the fact that these cells were never incubated at 37°C. Both observations led to the design of a new protocol to purify IECs within the shortest possible time and at a low temperature (method D).
As changes in Ca2+ metabolism have been shown to induce apoptosis, 51 we specifically addressed this issue by studying a protocol that does not employ chelating agents (method B). IECs isolated by mechanical disruption underwent extensive apoptosis within 5 hours of detachment (>90%). Ongoing experiments using dispase in the absence of EDTA to detach IECs also confirm this observation, demonstrating that loss of anchorage, not the technique of isolation, is the critical trigger of IEC apoptosis in vitro. 52
The data in this report underscore that assays based on DNA fragmentation, such as oligonucleosome electrophoresis and TUNEL assays, are useful only to detect late stages of apoptosis. Notably, caspase 3 and PARP were already cleaved in IECs isolated using method C, even though these cells showed no DNA fragmentation. Therefore, caution is warranted when classifying cells as non-apoptotic on the basis of assays assessing DNA fragmentation alone. It should also be noted that the viability-based trypan blue stain cannot be used to identify apoptotic cells, as IECs isolated by method A (extensive DNA fragmentation and 83% apoptotic cells by acridine orange/ethidium bromide stain) were >95% viable according to trypan blue stain.
In summary, the new method described in this report provides an in vitro model to explore the mechanisms underlying homeostasis of the intestinal epithelium in vivo, as apoptosis due to loss of anchorage is likely to play a pivotal role in the physiological life cycle of IECs, preventing their ectopic growth after shedding.
Acknowledgments
We thank Dr. Susanne Mohr, Ms. Kelly Fergusson, Ms. Rui-Zhen Wang, and Mr. Joseph Polak for technical assistance. We also thank the Departments of Surgery and Pathology, University Hospitals of Cleveland, and the Colon and Rectal Surgery Department of the Cleveland Clinic Foundation for providing surgical specimens.
Footnotes
Address reprint requests to Dr. Alan D. Levine, Department of Medicine, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106-4952. E-mail: adl4@po.cwru.edu.
Supported by grants DK30399 and DK50984 from NIH (C. Fiocchi) and grants 1523/1–1 and 1523/1–2 from the Deutsche Forschungsgemeinschaft, Bonn, Germany (J. Grossmann).
References
- 1.White E: Life, death, and the pursuit of apoptosis. Genes Dev 1996, 10:1-15 [DOI] [PubMed] [Google Scholar]
- 2.Thompson C: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 3.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J: Human ICE/CED-3 protease nomenclature. Cell 1996, 87:171. [DOI] [PubMed] [Google Scholar]
- 4.Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC: Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res 1997, 57:1605-1613 [PubMed] [Google Scholar]
- 5.Erhardt P, Cooper GM: Activation of the CPP32 apoptotic protease by distinct signaling pathways with differential sensitivity to Bcl-xL. J Biol Chem 1996, 271:17601-17604 [DOI] [PubMed] [Google Scholar]
- 6.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW: CPP32/apopain is a key interleukin 1β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem 1996, 271:1841-1844 [DOI] [PubMed] [Google Scholar]
- 7.Stefanis L, Park DS, Yan CY, Farinelli SE, Troy CM, Shelanski ML, Greene LA: Induction of CPP32-like activity in PC12 cells by withdrawal of trophic support: dissociation from apoptosis. J Biol Chem 1996, 271:30663-30671 [DOI] [PubMed] [Google Scholar]
- 8.Orth K, O’Rourke K, Salvesen GS, Dixit VM: Molecular ordering of apoptotic mammalian CED-3/ICE-like proteases. J Biol Chem 1996, 271:20977-20980 [DOI] [PubMed] [Google Scholar]
- 9.Potten CS, Allen TD: Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res 1977, 60:272-277 [DOI] [PubMed] [Google Scholar]
- 10.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisch SM, Francis H: Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994, 124:619-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaulieu JF: Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci 1992, 102:427-436 [DOI] [PubMed] [Google Scholar]
- 13.Probstmeier R, Martini R, Schachner M: Expression of J1/tenascin in the crypt-villus unit of adult mouse small intestine: implications for its role in epithelial cell shedding. Development 1990, 109:313-321 [DOI] [PubMed] [Google Scholar]
- 14.Wang CY, Eshleman JR, Willson JKV, Markowitz S: Both transforming growth factor-β and substrate release are inducers of apoptosis in a human colon adenoma cell line. Cancer Res 1995, 55:5101-5105 [PubMed] [Google Scholar]
- 15.Abreu-Martin MT, Vidrich A, Lynch DH, Targan SR: Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-α and ligation of Fas antigen. J Immunol 1995, 155:4147-4154 [PubMed] [Google Scholar]
- 16.Heerdt BG, Houston MA, Augenlicht LH: Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 1994, 54:3288-3293 [PubMed] [Google Scholar]
- 17.Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J: Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci USA 1992, 89:4495-4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujii M, DuBois RN: Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995, 83:493-501 [DOI] [PubMed] [Google Scholar]
- 19.Moss SF, Agarwal B, Arber N, Guan RJ, Krajewska M, Krajewski S, Reed JC, Holt PR: Increased intestinal Bak expression results in apoptosis. Biochem Biophys Res Commun 1996, 223:199-203 [DOI] [PubMed] [Google Scholar]
- 20.Hermiston ML, Gordon JI: In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 1995, 129:489-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates RC, Buret A, van Helden DF, Horton MA, Burns GF: Apoptosis induced by inhibition of intercellular contact. J Cell Biol 1994, 125:403-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B: bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res 1995, 55:237-241 [PubMed] [Google Scholar]
- 23.Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ: BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 1991, 88:6961-6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu QL, Poulsom R, Wong L, Hanby AM: Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol 1993, 169:431-437 [DOI] [PubMed] [Google Scholar]
- 25.Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Nakayama K, Hickman JA: Differential expression of bcl-2 in intestinal epithelia: correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci 1995, 108:2261-2271 [DOI] [PubMed] [Google Scholar]
- 26.Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer P, Moeller P: CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role for ulcerative colitis. Gastroenterology 1997, 113:160-167 [DOI] [PubMed] [Google Scholar]
- 27.Strater J, Wedding U, Barth TFE, Koretz K, Elsing C, Moller P: Rapid onset of apoptosis in vitro follows disruption of β1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology 1996, 110:1776-1784 [DOI] [PubMed] [Google Scholar]
- 28.Grossmann J, Fiocchi C, Levine AD: Extracellular matrix (ECM) orchestrates death and survival of human intestinal epithelial cells (IEC). Gastroenterology 1997, 112:A987 [Google Scholar]
- 29.Youngman KR, Simon PL, West GA, Cominelli F, Rachmilewitz D, Klein JS, Fiocchi C: Localisation of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology 1993, 104:749-758 [DOI] [PubMed] [Google Scholar]
- 30.Gibson PR, van de Pol E, Maxwell LE, Gabriel A, Doe WF: Isolation of colonic crypts that maintain structural and metabolic viability in vitro. Gastroenterology 1989, 96:283-291 [DOI] [PubMed] [Google Scholar]
- 31.Whitehead RH, Brown A, Bhathel PS: A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro 1986, 23:436-442 [DOI] [PubMed] [Google Scholar]
- 32.Duke RC, Cohen JJ: Morphological and biochemical assays of apoptosis. Colgan JE Kruisbeek AM Margulies DH Shevach EM Strober W eds. Current Protocols in Immunology. 1992, :pp 3.17.11-13.17.16 Greene Publishing and Wiley-Interscience, New York [Google Scholar]
- 33.Sambrook J, Fritsch EF, Maniatis T: Commonly used techniques in molecular cloning. Molecular Cloning. 1989, :pp E3-E11 NY, Cold Spring Harbor Laboratory Press, Cold Spring Harbor [Google Scholar]
- 34.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, Elliston KO, Ayala JM, Casano FJ, Chin J, Ding GJ-F, Egger LA, Gaffney EP, Limjuco G, Palyha OC, Raju SM, Rolando AM, Salley JP, Yamin T-T, Lee TD, Shively JE, MacCross M, Mumford RA, Schmidt JA, Tocci MJ: A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 1992, 356:768-774 [DOI] [PubMed] [Google Scholar]
- 35.Ranjit GB, Cheng MF, Mackay W, Whitacre CM, Berger JS, Berger NA: Poly(adenosine diphosphoribose) polymerase in peripheral blood leukocytes from normal donors and patients with malignancies. Clin Cancer Res 1995, 1:223-234 [PubMed] [Google Scholar]
- 36.Lizard G, Deckert V, Dubrez L, Moisant M, Gambert P, Lagrost L: Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am J Pathol 1996, 148:1625-1638 [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser A, Evan G: A license to kill. Cell 1996, 85:781-784 [DOI] [PubMed] [Google Scholar]
- 38.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin T-T, Yu VL, Miller DK: Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376:37-43 [DOI] [PubMed] [Google Scholar]
- 39.Boudreau N, Sympson CJ, Werb Z, Bissell MJ: Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 267:891-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunthert AR, Strater J, von Reyher U, Henne C, Joos S, Koretz K, Moldenhauer G, Krammer PH, Moller P: Early detachment of colon carcinoma cells during CD95(APO-1/Fas)-mediated apoptosis: de-adhesion from hyaluronate by shedding of CD44. J Cell Biol 1996, 134:1089-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tewari M, Quan LT, O’Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM: Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 1995, 81:801-809 [DOI] [PubMed] [Google Scholar]
- 42.Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He WW, Dixit VM: ICE-LAP3, a novel mammalian homologue of the Caenorhabditis elegans cell death protein Ced-3 is activated during Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem 1996, 271:1621-1625 [DOI] [PubMed] [Google Scholar]
- 43.Muzio M, Salvesen G, Dixit V: FLICE induced apoptosis in a cell-free-system. J Biol Chem 1997, 272:2952-2956 [DOI] [PubMed] [Google Scholar]
- 44.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J: Induction of apoptosis in fibroblasts by IL-1β-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 1993, 75:653-660 [DOI] [PubMed] [Google Scholar]
- 45.Enari M, Talanian RV, Wong WW, Nagata S: Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature 1996, 380:723-726 [DOI] [PubMed] [Google Scholar]
- 46.Smith D, McGuire M, Tocci M, Thiele D: IL-1β convertase (ICE) does not play a requisite role in apoptosis induced in T lymphoblasts by Fas-dependent or Fas-independent CTL effector mechanism. J Immunol 1997, 158:163-170 [PubMed] [Google Scholar]
- 47.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA: Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 1995, 267:2000-2003 [DOI] [PubMed] [Google Scholar]
- 48.Sarin A, Wu M, Henkart PA: Different interleukin-1β converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med 1996, 184:2445-2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371:346-347 [DOI] [PubMed] [Google Scholar]
- 50.Grossmann J, Mohr S, Laptena EG, Fiocchi C, Levine AD: Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am J Physiol 1998, 274:G1117-G1124 [DOI] [PubMed] [Google Scholar]
- 51.Nicotera P, Zhivotovsky B, Orrenius S: Nuclear calcium transport and the role of calcium in apoptosis. Cell Calcium 1994, 16:279-288 [DOI] [PubMed] [Google Scholar]
- 52.Grossmann J, Latella G, Fiocchi C, Levine AD: Loss of anchorage leads to extensive apoptosis of human intestinal epithelial cells (IEC), independent of their location on the crypt-villus axis or the mode of isolation. Gastroenterology 1998, 114:A375 [Google Scholar]