Abstract
Degradation of extracellular matrix (ECM) proteins in the aorta is a critical step for the development of atherosclerosis. Expression of matrix metalloproteinase (MMP)-12 (macrophage elastase), an elastin-degrading proteinase in the MMP family, was investigated in the thoracic aorta of rabbits fed a 1% cholesterol-containing diet for 16 weeks. In the atherosclerotic lesions, MMP-12 was produced abundantly at both the mRNA and protein levels, whereas no expression was observed in the normal rabbit aortas. The principal source of MMP-12 was macrophage foam cells (MFCs) that had infiltrated the atherosclerotic intima; this was demonstrated in both in vitro culture studies of MFCs purified from atherosclerotic lesions and immunohistochemical studies of aortic lesions. Additional biochemical studies using recombinant rabbit MMP-12 revealed that MMP-12 digested elastin, type IV collagen, and fibronectin and also activated MMP-2 and MMP-3. Expression of MMP-12 by human macrophage cell lines was increased by stimulation with acetylated low-density lipoprotein, implying augmentation of MMP-12 production during foam cell formation. Increased expression of MMP-12 in atherosclerotic lesions, concomitant with foam cell generation, which triggers the acceleration of ECM breakdown, is likely to be a critical step in the initiation and progression of the atherosclerotic cascade.
One of the earliest events in the atherogenic cascade is characterized by adherence of monocytes to endothelial cells and their subsequent migration into the subendothelial space. After they take up a large amount of lipid and transform into macrophage foam cells (MFCs), accumulation of these MFCs creates the first recognizable lesion of atherosclerosis, the fatty streaks. 1-5 With time and lesion progression, such fatty streaks expand to form more complicated, raised lesions called atheromatous plaques. During the events in atherogenesis, MFCs are considered to work as scavengers of oxidized lipoproteins, generators of cytokines, synthesizers of reactive oxygen species, and the sources of extracellular matrix (ECM)-degrading proteinases. 1-5 Expression of the activity of the ECM-degrading proteinases results in at least two major pathological features of atherosclerosis. First, smooth muscle cells (SMCs) are liberated, migrate from the media into the intima, and proliferate to form a massive intimal lesion, which may take the final appearance of the advanced, or complicated, occlusive lesion of atherosclerosis. And second, plaque destabilization occurs, in particular, ECM breakdown in the fibrous cap of atherosclerotic plaques, which contributes to plaque rupture and thrombosis. 3,6-8 Among ECM proteins, lysis of elastin in the aorta has considerable correlation to progression of atherosclerosis, 9-11 suggesting that augmented turnover of elastin mediated by elastase activity is implicated in the pathogenesis of atherosclerosis.
Matrix metalloproteinases (MMPs) are the most commonly observed ECM-degrading metal-dependent proteinases in atherogenic lesions. 6-8,12-16 To date, at least 14 enzymes have been identified in humans. MMPs serve as key enzymes for the homeostasis of ECM in the physiological state. 17 However, once their activities are abnormally exerted, they are known to cause various kinds of pathological events other than atherosclerosis, such as tumor metastasis, 18,19 osteoarthritis, and rheumatoid arthritis. 20,21
MMP-12 (macrophage elastase) was first identified as an elastolytic metalloproteinase secreted by inflammatory macrophages. 22 Mouse and human MMP-12 cDNAs were both cloned from a macrophage cDNA library and found to share 64% homology at the amino acid level. 23,24 Besides elastase activity, MMP-12 shows broad substrate specificity on ECM proteins such as fibronectin, laminin, vitronectin, type IV collagen, and heparan sulfate while having no proteolytic activity on type I collagen. 25 Accordingly, MMP-12 not only digests elastin but also degrades the basement membrane, which allows macrophages to penetrate into injured tissue during inflammation. 26
Although there are substantial numbers of reports on the localization of several MMPs in atherosclerotic lesions, little is known about the roles of MMP-12 in the pathogenesis of atherosclerosis. In this study, using immunohistochemical and biochemical methods, the expression of MMP-12 and its roles in atherosclerosis were examined in the aortas of rabbits fed a cholesterol-containing diet. The results present new evidence that MMP-12 plays a pivotal role in the development of atherosclerosis.
Materials and Methods
Cholesterol-Fed Rabbits
Male Japan white rabbits weighing 2.0 to 2.5 kg (10 weeks old initially) were fed a 1% cholesterol-containing diet for 16 weeks, as described previously. 27 Age-matched control rabbits were fed a standard laboratory chow. Serum cholesterol levels were measured at the beginning and end of 16 weeks of the diet using Cholesterol E-test Wako (Wako Pure Chemical Industries, Osaka, Japan). After sacrifice, the blood was washed out of the carcass with phosphate-buffered saline, and the descending thoracic aorta was removed to use for total RNA preparation with ISOGEN (Nippongene, Toyama, Japan), Western blot analysis, and MFC isolation. Three rabbits from each group were used for Northern blot and Western blot analysis. Each sample was tested at least three times for each analysis. For immunohistochemistry, the aorta was fixed by perfusion of periodate-lysine-paraformaldehyde (PLP), removed en bloc, and immersed in the PLP fixative for 4 hours. The aorta was opened longitudinally, and samples of the descending thoracic aorta were embedded in paraffin for immunohistochemical studies. The sections were also stained with hematoxylin and eosin and van Gieson’s elastin.
Catalytic Domain of Rabbit MMP-12 (MMP-12cat) Expressed in Escherichia coli
An E. coli expression plasmid for the catalytic domain of rabbit MMP-12 (MMP-12cat) was obtained as follows. Two oligonucleotides, 5′- CCCCCATGGCTAGCTTCAAAACCATGCCAGGGAG-3′ (forward primer) and 5′- GGGGGATCCCTATGGCATGGGTTGATGCTGCTGCT-3′ (reverse primer), were synthesized to encode the protein from Phe95 to Pro267, which is the catalytic domain of rabbit MMP-12. Using full-length cDNA of rabbit MMP-12 cloned from a rabbit osteoclast cDNA library (GenBank accession number AB006779, M. Kobori, manuscript in preparation), the polymerase chain reaction was carried out with the oligonucleotide primers, and the reaction product was ligated into the NcoI-BamHI site of an E. coli expression plasmid, pET-3d (Novagen, Madison, WI). E. coli strain BL21 (DE3) pLysS (Novagen) was transformed with the MMP-12cat expression plasmid, and MMP-12cat-containing E. coli inclusion bodies were refolded as described previously. 28 The refolded MMP-12cat was loaded onto a heparin-Sepharose (Pharmacia LKB, Uppsala, Sweden) column, eluted with buffer A (50 mmol/L Tris/HCl, pH 7.5, 5 mmol/L CaCl2, 0.05 mmol/L ZnCl2, 0.05% Brij-35) containing 0.75 mol/L NaCl and then dialyzed against buffer A. The protein concentration was assessed with a protein assay kit (BioRad, Richmond, CA) according to the manufacturer’s recommendations. Amino-terminal amino acid sequencing analysis was performed using protein sequencer model 473 (Perkin Elmer, Norwalk, CT).
Antibodies to Rabbit MMPs
Recombinant rabbit MMP-1, -3, and -9 were obtained as described previously. 28 Mouse polyclonal antibody (Ab) against rabbit MMP-12 was raised using MMP-12cat as an antigen. For affinity purification, 500 μl of the immunized mouse serum was affinity absorbed to a 1-ml Sepharose 4B (Pharmacia) column coupled with 2 mg of MMP-12cat and eluted with 5 ml of 50 mmol/L glycine/HCl (pH 2.7). Specificity to other MMPs was eliminated from the affinity-purified anti-MMP-12 polyclonal Ab by continuous passage through Sepharose 4B columns coupled with rabbit MMP-1, -3, or -9, respectively and the unbound solution (25 ml) was used as the specific anti-rabbit MMP-12 polyclonal Ab. A mouse monoclonal anti-rabbit MMP-3 Ab, MP1807, was obtained using the recombinant precursor form of MMP-3 (pro-MMP-3) as an immunizing antigen. A mouse monoclonal anti-human MMP-2 Ab (Fuji Chemical Industries, Toyama, Japan) was used to detect rabbit MMP-2. 29
Isolation of Aortic Cells and MFCs
For the isolation of aortic cells from whole aortas, thoracic aortas were cut into small pieces and then digested with an enzyme mixture containing 450 U/ml collagenase (Worthington Biochemical Co., Freehold, NJ), 4.7 U/ml elastase (Elastin Products Co., Owensville, MO), 1 mg/ml soybean trypsin inhibitor (Wako Pure Chemical Industries) and 2 mmol/L CaCl2 in Hanks’ balanced salt solution (Gibco-BRL, Gaithersburg, MD) for 1 hour. The dissociated cells were filtered with a nylon membrane and suspended at 5.0 × 10 5 cells/ml in a serum-free medium, Media I (Immuno-Biologic Laboratories, Gunma, Japan). After 5 days of incubation, Western blot analysis 28 using anti-MMP-12 polyclonal Ab was performed on conditioned medium. Purification of MFCs was performed according to the method described by Galis et al. 12 Purified MFCs were suspended at 2.0 × 10 5 cells/ml in Media I, and Western blot analysis was performed on 12 μl of the conditioned medium collected after 3 days of culture. For identification of the MFCs, 2.5 × 10 3 cells were smeared on glass slides, fixed with 100% ethanol, and stained with a specific antibody to rabbit macrophages, RAM-11. 30
Transient Expression of Rabbit MMPs in COS-7 Cells
For constructing mammalian cell expression plasmids for rabbit MMP-2, -3, and -12, each full-length cDNA was inserted into expression vector pEF-BOS-dhfr, and transient expression of the MMPs in COS-7 cells (CRL-1651, American Type Culture Collection (ATCC), Rockville, MD) was performed as described previously. 28 Also, the full-length cDNA fragments of rabbit MMP-1 and -9 were used as probe DNAs for Northern blot analysis. 29
Immunohistochemistry
Five-micron paraffin-embedded sections were prepared and mounted on glass slides. Serial sections were deparaffinized with xylene and a graded series of ethanol. Endogenous peroxidase activity was blocked by immersing the slides in 0.3% H2O2 for 10 minutes at room temperature. After blocking with 10% rabbit serum for 30 minutes at room temperature, each section was incubated overnight at 4°C with purified anti-rabbit MMP-12 polyclonal Ab diluted 1:50 or 1:500 in PBS containing 1% bovine serum albumin, anti-rabbit macrophage monoclonal (M)Ab, RAM-11, diluted 1:12,000, and anti- muscle actin MAb, HHF-35 (Enzo Biochemicals, Farmingdale, NY), diluted 1:100. The immunoreactivity was detected by the HISTOFINE SAB-PO(M) kit (Nichirei Co., Tokyo, Japan) following the manufacturer’s instructions. Briefly, the slides were incubated with species-appropriate biotinylated secondary antibodies for 30 minutes, followed by peroxidase-conjugated streptavidin for 30 minutes at room temperature. The reaction was visualized with 3-amino-9-ethyl-carbazole (Dako Japan Co., Kyoto, Japan). The sections were counterstained with hematoxylin. Omission of primary antibodies and staining with type- and class-matched irrelevant immunoglobulin served as negative controls.
Double immunostaining was carried out for the identification of the cells producing MMP-12. Anti-rabbit MMP-12 polyclonal Ab was used for first primary Ab. After the first immunostaining procedure described above, the sections were rinsed with 0.1 mol/L glycine/HCl buffer (pH 2.2) for 1 hour to remove immunocomplexes and were incubated overnight at 4°C with the second primary Ab, RAM-11. Thereafter, the slides were incubated with species-appropriate biotinylated secondary antibodies for 30 minutes, followed by alkaline-phosphatase-conjugated streptavidin (Nichirei) for 30 minutes at room temperature. The reaction of the second primary Ab was visualized with fast blue (Nichirei).
Degradation of ECM Proteins by MMP-12cat
Type I, III, and V collagens from human placenta were purchased from Sigma Chemical Co. (St. Louis, MO), type II collagen of calf joints was purchased from Yagai Research Center (Yamagata, Japan), and type IV collagen of Engelbreth-Holm-Swarm mouse tumor was from Becton Dickinson (San Jose, CA). Purification of human plasma fibronectin and the proteolytic activity of MMP-12cat on the ECM proteins were performed as described previously. 24 Briefly, 6 μg/ml MMP-12cat was incubated with 0.2 μg/ml type I, type II, type III, type IV, or type V collagens at 25°C for 25 hours or with 0.2 μg/ml fibronectin at 37°C for 25 hours. The reaction products were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and silver stained.
Effect of AcLDL on the Expression of MMP-12 from Macrophages
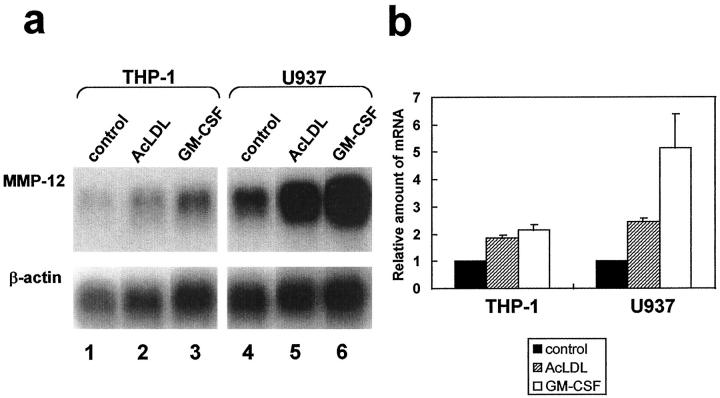
Human cell lines U937 (ATCC CRL-1593) and THP-1 (ATCC TIB-202), both of which have the ability to differentiate into macrophages, were cultured in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (Gibco). For induction of macrophages, 1 × 10 7 U937 cells or 5 × 10 6 THP-1 cells were incubated in the medium containing 100 ng/ml phorbol 12-myristate 13-acetate (PMA) in 15-cm dishes. After 3 days of stimulation with PMA, the adherent cells were maintained in the same medium without PMA for an additional 3 days and used as a source of macrophages. To obtain acetylated low-density lipoprotein (AcLDL), bovine LDL (d = 1.02 to 1.06) (Yagai Research Center) was used according to the method described previously. 31 The differentiated U937 and THP-1 cells were incubated in RPMI-1640 with or without 35 μg of protein/ml of AcLDL or 50 ng/ml human granulocyte macrophage-colony stimulating factor (GM-CSF) (PeproTech, London, UK) for 24 hours, and mRNA was isolated using ISOGEN and Oligotex-dt30 (Takara, Kyoto, Japan) to blot on a nylon membrane by 2 μg each. Human MMP-12-cDNA 24 for use as a probe DNA was obtained by a polymerase chain reaction using human adult lung cDNA (Clontech, Palo Alto, CA) as template DNA. The amounts of mRNA expression were quantified with a bio-imaging analyzer, BAS2000 (Fuji Photo Film Co., Tokyo, Japan).
Results
During feeding of the cholesterol diet, the average plasma cholesterol concentration rose from 26.9 ± 2.1 mg/dl to 2735.4 ± 52.1 mg/dl (mean ± SEM; n = 10; P < 0.01). On gross examination of animals fed the cholesterol diet for 16 weeks, the arch and the upper half of the descending portion of the thoracic aorta were almost totally covered by an atherosclerotic process that variably also involved the surface of the remainder of the vessels. Microscopically, in advanced lesions, the intimal surface was elevated to form plaque lesions that contained a mixture of MFCs and SMCs embedded in a variable amount of ECM.
Northern Blot Analysis of Rabbit Aorta
As shown in Figure 1 ▶ , MMP-12 was present in the whole aorta with raised intima from cholesterol-fed rabbits (Figure 1a ▶ , lane 1). A much stronger signal for MMP-12 was present in the atherosclerotic intimas (Figure 1b ▶ , lanes 2 and 3) than in the whole aorta with raised intima (Figure 1b ▶ , lane 1), whereas no MMP-12 signal was found in the aortas of normal rabbits (Figure 1, a and b ▶ , lanes 4 and 5). MMP-1, -3, and -9 were also present only in the cholesterol-fed rabbits (Figure 1, c, e, and f) ▶ ; on the other hand, MMP-2 was detected in the aortas of both normal and cholesterol-fed rabbits (Figure 1d) ▶ .
Figure 1.
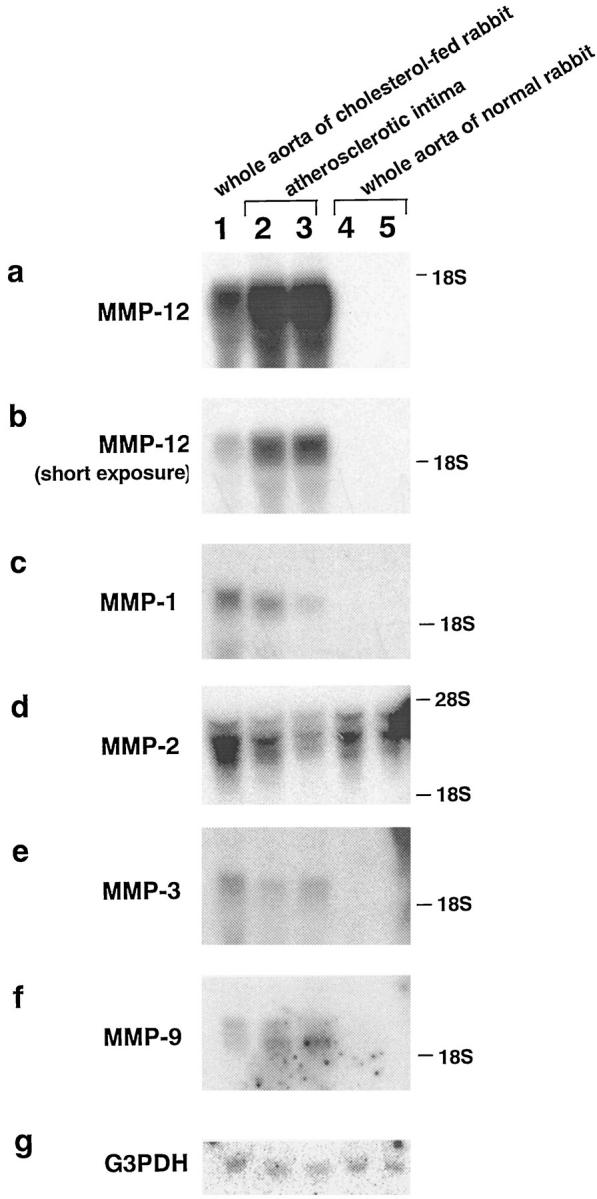
Northern blot analysis of MMPs in aortas of normal and cholesterol-fed rabbits. Total RNA was isolated from the whole aorta of a cholesterol-fed rabbit (lane 1), atherosclerotic intima of a cholesterol-fed rabbit (lanes 2 and 3), and the whole aorta of normal rabbits (lanes 4 and 5). Total RNA (30 μg each) was blotted on a nylon membrane, and full-length cDNA fragments of rabbit MMP-1, -2, -3, -9, and -12 and human glyceraldehyde 3-phosphate dehydrogenase (G3PDH) were used as probes. The hybridized membranes were exposed to x-ray films for 1 day (a and d), 3 days (c, e, and f), or 5 hours (b). This is a representative result of three independent experiments.
Expression of MMP-12 by the Aortic Cells from Cholesterol-Fed Rabbits
To evaluate the protein expression of MMP-12 and its existing form (Figure 2) ▶ , Western blot analysis was carried out on the conditioned medium of dissociated cells from the whole aortas. As shown in Figure 3 ▶ , MMP-12 was produced by the cells from a whole aorta with a raised intima (lane 2) at a molecular weight of 45 kd, whereas the cells from a lesion-negative whole aorta of a cholesterol-fed rabbit (lane 1) had no such ability. Secretion of MMP-12 was also undetectable for the cells from the whole aorta of a normal rabbit (lane 3). In comparison with COS-7-expressed MMP-12 with (lane 5) or without (lane 4) previous treatment with trypsin, the size of 45 kd detected in lane 2 corresponded to the activated form of MMP-12 (active MMP-12). On the other hand, Western blot analysis for MMP-3 in the same sample revealed that MMP-3 totally kept its latency (data not shown), indicating that MMP-12 in lane 2 was not artificially activated during the extraction procedure.
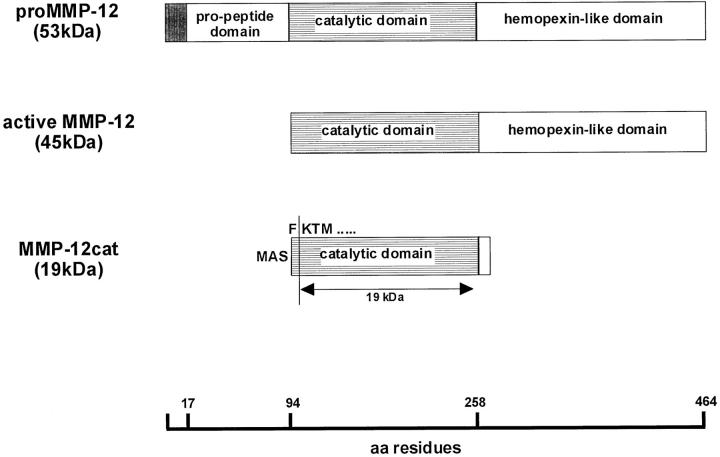
Figure 2.
A schematic illustration of rabbit MMP-12. The latent form of rabbit MMP-12 (top), the active form of MMP-12 (middle), and the E. coli-expressed catalytic domain of MMP-12 (bottom) are illustrated. The meshed box in pro-MMP-12 indicates the signal peptides. In the structure of MMP-12cat, MAS represents starting amino acids Met, Ala, and Ser added at the amino terminus to be expressed in E. coli. The arrow shows the 19-kd MMP-12cat, which was obtained by purification. In the amino-terminal amino acid sequence of the 19-kd MMP-12cat, Met, Ala, Ser, and Phe were cleaved and the enzymes were started with Lys.
Figure 3.
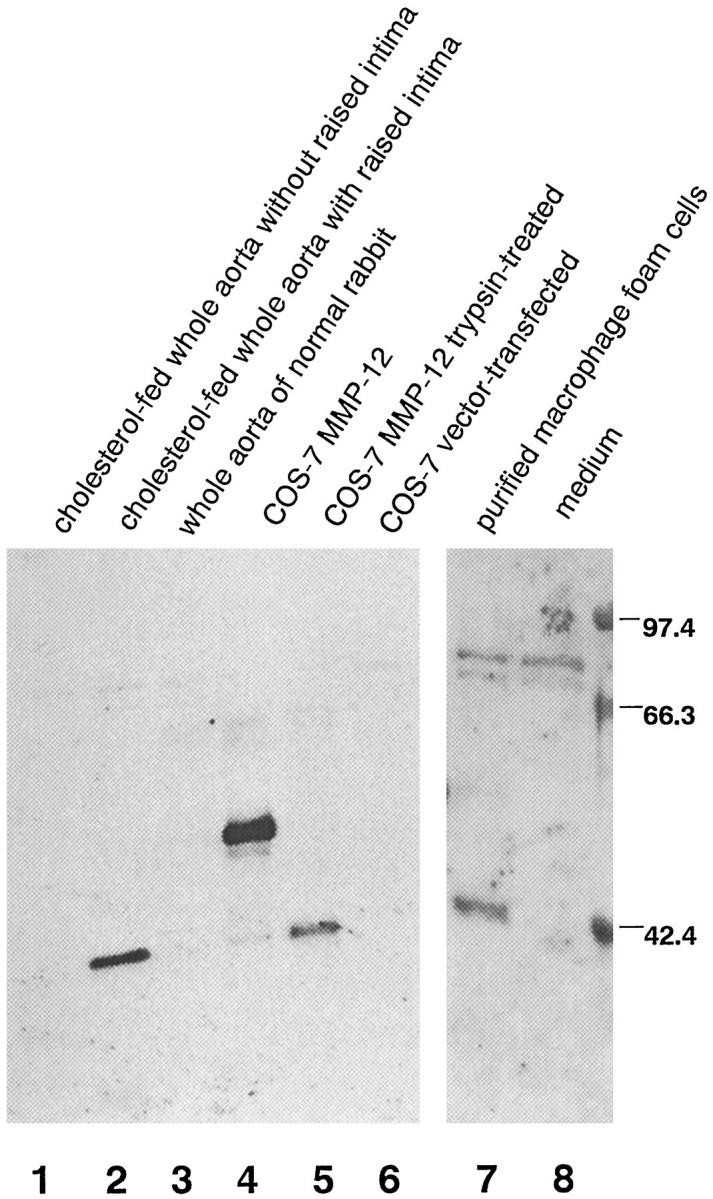
MMP-12 expression by cells from aorta with atherosclerotic intima. The conditioned medium of dissociated cells from a cholesterol-fed rabbit’s whole aorta without raised intima (lane 1) and with raised intima (lane 2) and cells from the whole aorta of a normal rabbit (lane 3) were subjected to Western blot analysis with an anti-MMP-12 polyclonal Ab. Also analyzed was the conditioned medium of purified MFCs from the intima of an atherosclerotic aorta (lane 7). Rabbit pro-MMP-12 expressed by COS-7 cells (lane 4), active MMP-12 (lane 5), which was trypsin-treated conditioned medium of MMP-12-expressed COS-7 cells, conditioned medium of vector-transfected COS-7 cells (lane 6), and medium incubated without cells (lane 8) were used as control samples. This is a representative result of three independent experiments.
To examine whether MFCs produce MMP-12, the cells were purified from the aortas of cholesterol-fed rabbits by enzymatic digestion and discontinuous density-gradient centrifugation. More than 95% of the cells obtained were identified as macrophage-derived cells by their reactivity with anti-rabbit macrophage MAb RAM-11 (data not shown). Similar to the results for dissociated cells from the whole aorta, MFCs secreted 45-kd MMP-12, which was considered to be active MMP-12 (Figure 3 ▶ , lane 7). This demonstrated that, in the aorta of cholesterol-fed rabbits, MFCs have the ability to generate MMP-12, which is readily activated after its secretion.
Immunohistochemistry
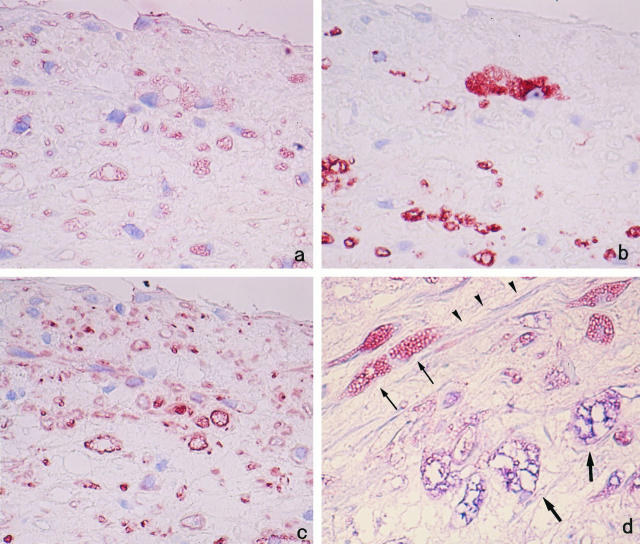
The atherosclerotic lesion in the thoracic aorta contained RAM-11-positive round or foamy cells (MFCs) and HHF-35-positive cells surrounded by various amounts of connective tissue matrix (Figure 4, a–c) ▶ . In raised lesions, RAM-11-positive MFCs tended to cluster in the deeper intima, whereas HHF-35-positive cells were generally spindle-shaped and tended to be located in superficial lesions, although there was a considerable variation from region to region and from lesion to lesion.
Figure 4.
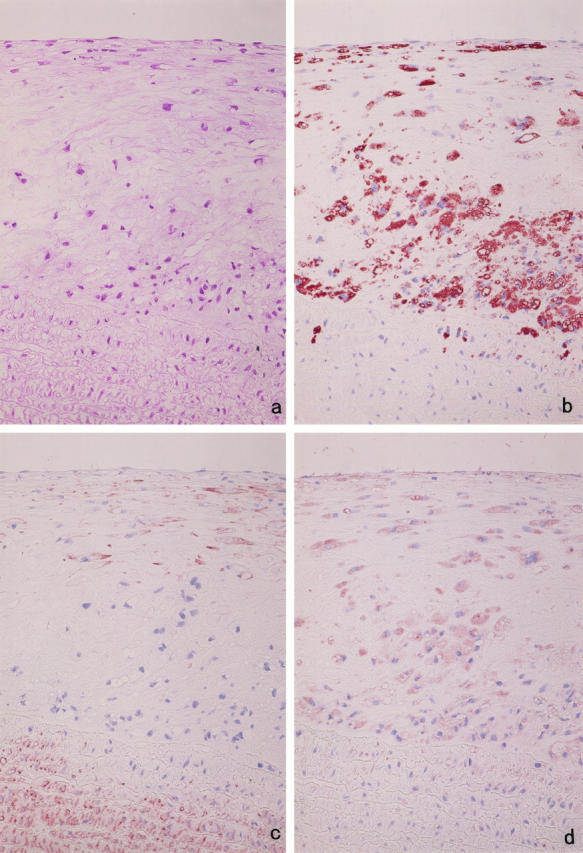
Immunohistochemical localization of MMP-12. Serial sections of cholesterol-fed rabbit aorta were stained with hematoxylin and eosin (a) and immunostained with anti-rabbit macrophage MAb RAM-11 (b), anti-muscle actin MAb HHF-35 (c), and anti-rabbit MMP-12 polyclonal Ab (d). The sections indicate that most of the RAM-11-positive MFCs are stained with anti-MMP-12 Ab. Counterstaining with hematoxylin (b to d); original magnification, ×200.
Purified anti-MMP-12 Abs diluted in 1:50 and 1:500 were tested for determination of optimal concentration. There was no cellular staining in the atherosclerotic aorta in the 1:500 dilution, although identical results were obtained in the 1:50 dilution in which MMP-12-expressing cells localized exclusively to the cells present in the intimal lesion area. The aorta from normal rabbits did not stain at all for MMP-12 (data not shown).
Examination of serial sections demonstrated that RAM-11-positive MFCs were the principal cells expressing MMP-12 (Figure 4d) ▶ . The staining property of MMP-12-positive cells was, however, variable; in particular, the large vacuolated MFCs often stained weakly or appeared negative, possibly due to abundance of intracytoplasmic fat vacuoles. In addition, staining of serial sections and double immunostaining indicated that a number of SMCs contained immunoreactive MMP-12 in some areas populated by spindle- or stellate-shaped HHF-35-positive cells (Figure 5) ▶ . Medial SMCs had no immunoreactivity with anti-MMP-12 Ab.
Figure 5.
Cellular localization of MMP-12 in superficial region of the intima. Serial sections were immunostained by anti-MMP-12 Ab (a), anti-macrophage Ab RAM-11 (b), and anti-muscle actin Ab HHF-35 (c). In addition to RAM-11-positive MFCs, HHF-35-positive SMCs are variably stained with anti-MMP-12 Ab. Counterstaining was with hematoxylin. Double immunostaining was performed for MMP-12 and the RAM-11 (d). MMP-12-positive macrophages are stained red-blue (thick arrows), whereas MMP-12-positive SMCs are stained red only (thin arrows). Note also double-negative cells (MMP-12-negative SMCs, arrowheads). Original magnification, ×400 (a to c) and ×1000 (d).
Enzymatic Activity of MMP-12
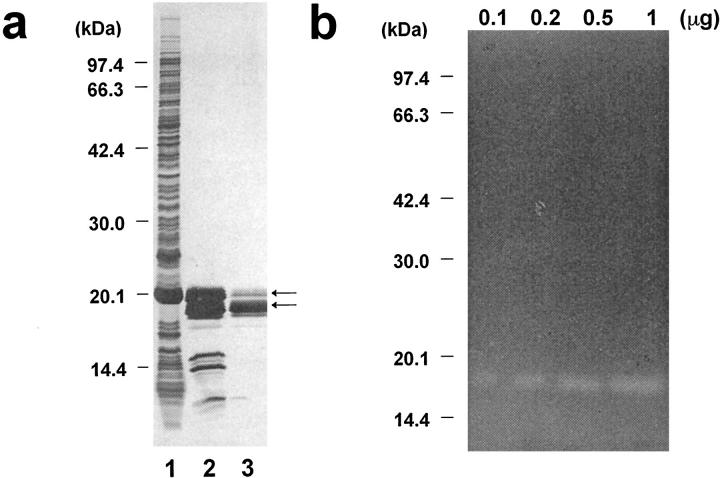
To examine the functions of MMP-12 at the site of atherosclerotic lesions in vivo, MMP-12cat (Figure 2) ▶ was manufactured in the E. coli expression system. After being refolded from the inclusion body, autolytic cleavage occurred on some portion of the MMP-12cat, and two sizes of molecules, 20.5 and 19 kd, were obtained (Figure 6a ▶ , lane 2). Amino-terminal amino acid sequencing analysis revealed that Met, Ala, and Ser, which were amino-terminally tagged amino acids, and Phe were removed, and both types of MMP-12cat had the same amino acid sequence (K-T-M-P-G-R-P). This result suggested that proteolysis occurred at the 10- to 15-amino-acid upper region of the carboxyl-terminal end of MMP-12cat, although the purified 19-kd molecule retained elastase activity (Figure 6b) ▶ .
Figure 6.
E. coli expression of catalytic domain of MMP-12. a: Purification of MMP-12cat, analyzed by SDS-PAGE and silver staining. Lane 1, whole E. coli extract; lane 2, MMP-12cat refolded from the inclusion body; lane 3, MMP-12cat purified on a heparin-Sepharose column. The arrows indicate two sizes, 20.5 and 19 kd, of MMP-12cat. b: Elastin zymography of MMP-12cat, with 0.1, 0.2, 0.5, and 1 μg of purified MMP-12cat applied to κ-elastin containing 10% SDS-PAGE gel. Elastin zymography 25 was carried out by incubating the gel at 37°C for 72 hours.
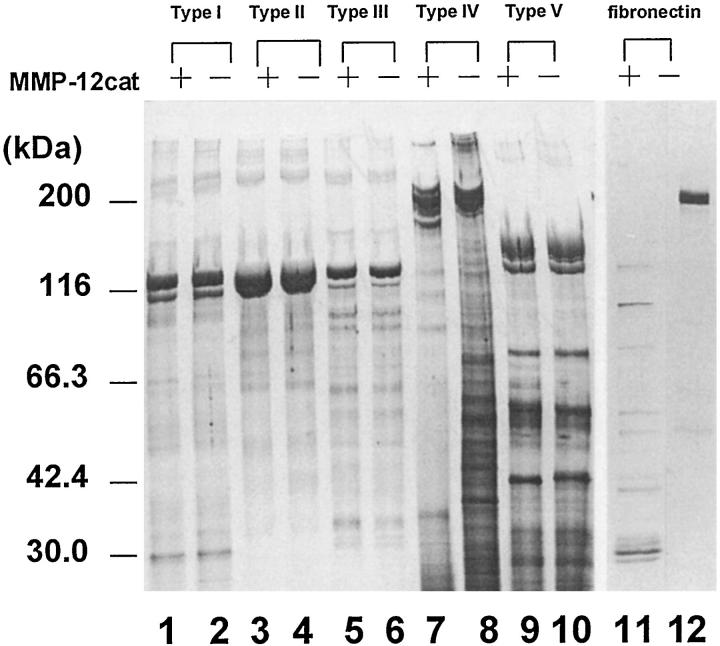
MMP-12cat was able to cleave type IV collagen (Figure 7 ▶ , lane 7) and also digested fibronectin (lane 11), although none of the other collagens were cleaved. MMP-12 also had the ability to activate pro-MMP-2 and pro-MMP-3 in a time- and concentration-dependent manner (Figure 8) ▶ . In the comparison of Figure 7, a and b ▶ , MMP-12cat’s activity on pro-MMP-2 was likely to be much less than that on pro-MMP-3. The concentration of pro-MMP-3 in the conditioned medium was 990 ng/ml, as determined by a double-Ab sandwich ELISA system, 32 which indicates that pro-MMP-3 was incubated with a 3 to 30 times larger molar amount of MMP-12cat in this experiment.
Figure 7.
Degradation of ECM proteins by MMP-12cat. Type I (lanes 1 and 2), type II (lanes 3 and 4), type III (lanes 5 and 6), type IV (lanes 7 and 8), and type V (lanes 9 and 10) collagens and fibronectin (lanes 11 and 12) were incubated with MMP-12cat (lanes 1, 3, 5, 7, 9, and 11) or alone (lanes 2 , 4, 6, 8, 10, and 12). The reaction products were analyzed by SDS-PAGE under reducing conditions and silver stained.
Figure 8.
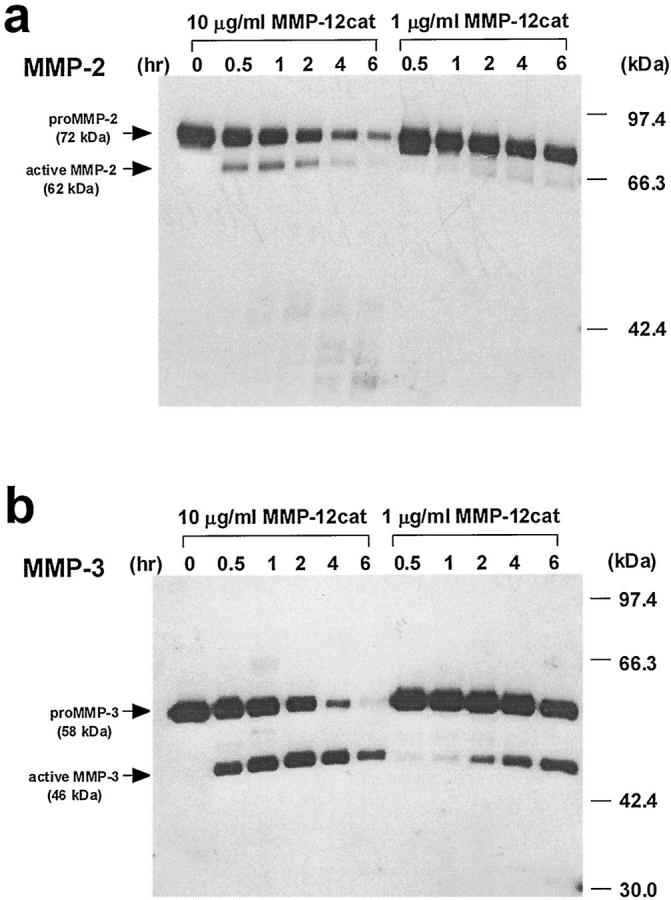
Time- and concentration-dependent pro-MMP-2 and -3 activation by MMP-12cat. a: Activation of pro-MMP-2 by MMP-12cat. Five microliters of conditioned medium of rabbit MMP-2-expressing COS-7 cells was reacted with 10 or 1 μg/ml MMP-12cat in reaction buffer (2 μmol/L pepstatin A, 2 mg/ml Pefabloc, and 1 μg/ml leupeptin in buffer A). The reactions were quenched at 0, 0.5, 1, 2, 4, or 6 hours with EDTA at a final concentration of 50 mmol/L. The reaction products were analyzed by Western blot using an anti-human MMP-2 MAb. The pro-MMP-2 (72 kd) and active MMP-2 (62 kd) are indicated by arrows. b: Activation of pro-MMP-3 by MMP-12cat. Conditioned medium of COS-7 cells containing rabbit MMP-3 was reacted with MMP-12cat, and an anti-rabbit MMP-3 MAb, MP1807, was used for Western blot analysis. The pro-MMP-3 (58 kd) and active MMP-3 (46 kd) are indicated. This is a representative experiment of two performed.
Effect of AcLDL Stimulation on MMP-12 Expression
Finally, the effect of AcLDL on the production of MMP-12 by the macrophage cell lines was studied by Northern blot analysis. The expression of MMP-12 was strengthened by incubation with AcLDL or GM-CSF in both macrophage-differentiated U937 and THP-1 (Figure 9a) ▶ . The relative amounts of mRNA for MMP-12, standardized with the amount of mRNA for β-actin, indicated that incubation with 35 μg of protein/ml of AcLDL doubled the expression of MMP-12 in both cell lines (Figure 9b) ▶ . Incubation with GM-CSF, which is known to increase the transcriptional level of MMP-12 and to enhance the mRNA stability of MMP-12, 33 augmented MMP-12 expression by approximately five times in U937 and approximately two times in THP-1.
Figure 9.
MMP-12 expression is enhanced by stimulation with AcLDL. a: Northern blot analysis of MMP-12 expression by macrophage-differentiated THP-1 (lanes 1 to 3) and U937 (lanes 4 to 6) cells under stimulation with AcLDL (lanes 2 and 5) or GM-CSF (lanes 3 and 6). Radiolabeled human MMP-12 cDNA (top) and human β-actin cDNA (bottom) were used as probe DNAs. This is a representative experiment of three performed. b: The relative amounts of mRNA for MMP-12 were standardized with the amount of mRNA for β-actin and are indicated as 1 for the amount of MMP-12 in each cell line under normal conditions. The values are the mean ± SD of triplicate cultures.
Discussion
Northern blot analysis demonstrated that MMP-12 was expressed in the cholesterol-fed rabbits’ aortas but not in the normal rabbits’ aortas. In comparison, MMP-2 was constitutively expressed in both the normal and cholesterol-fed rabbits’ aortas. These results are in good agreement with the previous finding that MMP-2 was ubiquitously present in both non-atherosclerotic and atherosclerotic human aortas. 6 Taken together with our previous finding that rabbit MMP-2 was expressed widely in several tissues under normal conditions, 29 the activity of MMP-2 is presumably regulated by the endogenous inhibitor of MMPs, tissue inhibitor of metalloproteinases (TIMPs), under normal conditions.
Western blot analysis demonstrated that MMP-12 was also expressed in the atherosclerotic aortas at the protein level. The ability to produce MMP-12 by MFCs, which are the predominant cell population in early atherosclerotic lesions, was also ascertained for MFCs purified directly from atherosclerotic lesions. MMP-12 was detected as a 45-kd active form both in the conditioned medium of MFCs and dissociated cells from the whole aorta. Two possibilities would be raised for the activation process of MMP-12 at the atherosclerotic lesions. First, a serine proteinase, plasmin, which acts as a common activator for most MMPs, 3 may play a role in the process. However, MMP-3 was detected as a latent form in our experiment, which is similar to the study by Galis et al 12 in which MMP-1 and -3 in the conditioned medium of purified rabbit MFCs kept their latency. Thus, in this situation, MMP-12 may be more sensitive to the proteolytic activity of plasmin than MMP-3. Second, it may be due to the result of self-activation. A number of reports demonstrated that human and mouse MMP-12 was autolytically processed into an activated form. 23,24 It is therefore likely that, without exogenous stimuli, MMP-12 has the greater propensity for self-activation than other MMPs.
MMP-2 and -9, which also have elastase activity, were reported to be present in atherosclerotic lesions. 7,8,16,34,35 Our study with gelatin zymography confirmed that both MMP-2 and -9 were produced by atherosclerotic arterial cells and were actually present in the conditioned medium (data not shown). It seems likely that the coordinated expression of MMP-2, -9, and -12 is responsible for the elastin degradation in atherosclerotic lesion.
Unlike MMP-2, which has been shown in the intima and the media of aortas with atherosclerotic lesions, 7 our immunohistochemical study demonstrated that MMP-12 was restricted to the intima. This suggests that SMCs in the media of the aorta have no ability to produce MMP-12. The reactivity of the rabbit macrophage-specific Ab indicated that the major cell type expressing MMP-12 was macrophage-derived foam cells; this concept was also supported by the result of Western blot analysis of the conditioned medium from purified MFCs. Besides MFCs, small numbers of SMCs that had migrated into intima were also stained with anti-MMP-12 Ab. It is well known that synthetic-state SMCs, which have migrated into the intima, and contractile-state SMCs, which localize in the media, have considerable differences in the molecules they produce, such as cytokines and ECM components. 1 Accordingly, MMP-12 might be one of those molecules that can be produced by SMCs in the synthetic state.
The previous study with in situ hybridization indicated that expression of MMP-12 was confined to only a few cells in human carotid atherosclerotic lesions, 14 which is inconsistent with our immunohistochemical study finding that MMP-12 was widely expressed in the cells of the rabbit atherosclerotic intima. The reason for the difference is not yet clear, but it may depend on the disparity in the developmental stage of atherosclerosis. Our rabbit hypercholesterolemia model represents an early-middle stage of atherosclerosis, in which MFCs, the principal source of MMP-12, were the major component cells of the intima. Taking these observations together, it is likely that, in atherosclerotic lesions, substantial amounts of MMP-12, which tend to be easily activated, are expressed not in the media but in the intima of the aorta mainly by the MFCs.
Our study of the recombinant MMP-12cat demonstrated that, besides elastin-degrading activity, MMP-12cat cleaved type IV collagen and fibronectin, which are components of the basement membrane. 36 MMP-12 is also reported to digest laminin, entactin, and heparan sulfate, all of which constitute basement membranes. 26 Therefore, MMP-12 not only reduces the elasticity of the aorta by the digestion of elastin but also may facilitate the influx of inflammatory cells from the blood into the aorta by breaking down basement membranes underlying the endothelium.
In addition to ECM-degrading activity, MMP-12 had the ability to activate pro-MMP-2 and pro-MMP-3. Pro-MMP-3 was activated by incubation with a 3 to 30 times larger molar amount of MMP-12cat. Accordingly, in atherosclerotic lesions, it is likely that MMP-12 functions as an activator of pro-MMP-3, which in turn activates other pro-MMPs, such as MMP-1 or -9, 37,38 and leads to the degradation of a variety of ECM proteins, including collagen types I, III, IV, and V and gelatin. MMP-12 also activated pro-MMP-2. Membrane-type MMPs are considered to play pivotal roles in activating pro-MMP-2 in vivo. 39 However, the previous demonstration that MMP-1 also activates pro-MMP-2 40 suggests that activation of pro-MMP-2 by membrane-type MMPs is not a unique pathway. MMP-12 may also be implicated in the activation of pro-MMP-2 in the atherosclerotic intima. In the case of MMP-2, however, not only pro-MMP-2 but also its activated form decreased with time (Figure 8a) ▶ . This may be caused by self-cleavage of the molecule in active MMP-2. The similar phenomenon was reported by Bergmann et al 41 that human MMP-2 tended to be degraded by the autolytic processing.
AcLDL, similar to oxidized LDL, is known to be taken up by scavenger receptors and causes generation of foam cells. 5 A number of reports have shown that modified LDL induces release of elastase from macrophages. 42,43 To our knowledge, our result is the first demonstration that expression of MMP-12 is transcriptionally modulated by AcLDL. Increased expression of MMP-12 may be caused by activation of mitogen-activated protein (MAP) kinase 44 and formation of a transcription complex, AP-1, 45 the recognition sequence of which, the so-called tumor promoter-recognition element (TRE), exists in the 5′-flanking region of the human MMP-12 gene. 46 Expression of MMP-12 by macrophages is enhanced by GM-CSF, 34 which is released from endothelial cells by stimulation with modified LDL. 47 Hence, in atherosclerotic lesions, modified LDL may be an inducer for MMP-12 expression not only indirectly by causing endothelial cells to secrete GM-CSF but also directly by stimulating macrophages in the course of foam cell formation. Even though additional study is needed, MMP-12 expression from SMC-derived foam cells in the intima may also be related to intracellular lipid accumulation.
In summary, the current study demonstrated the following points. First, diet-induced hypercholesterolemia induced increased expression of MMP-12 by MFCs accumulated in atherosclerotic intimas. Second, MMP-12 had the ability to digest several ECM proteins and to activate other pro-MMPs. Third, MMP-12 expression by MFCs was enhanced by stimulation with modified LDL. These results strongly suggest that increased expression of MMP-12, concomitant with foam cell generation, which triggers the acceleration of ECM breakdown, is a critical step in the initiation and progression of cholesterol-induced atherogenesis. Thus, additional studies will be designed to elucidate the implication of MMP-12 in the pathogenesis of human atherosclerosis.
Acknowledgments
We are grateful to Dr. Shigekazu Nagata for providing pEF-BOS and Dr. Toyohiro Tsukada for providing RAM-11. We also thank Dr. Jianglin Fan for his helpful discussion.
Footnotes
Address reprint requests to Dr. Shun-ichiro Matsumoto, Biomedicine Laboratories, Institute for Drug Discovery Research, Yamanouchi Pharmaceutical Co., Ltd., 21, Miyukigaoka, Tsukuba-shi Ibaraki 305-8585, Japan. E-mail: matsumot@yamanouchi.co.jp.
Supported in part by a grant from the Japan Society for the Promotion of Sciences (JSPS-RFTF96100202) (to T. Watanabe).
References
- 1.Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801-809 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Tokunaga O, Fan J, Shimokama T: Atherosclerosis and macrophages. Acta Pathol Jpn 1989, 39:473-486 [DOI] [PubMed] [Google Scholar]
- 3.Dollery CM, McEwan JR, Henney AM: Matrix metalloproteinases and cardiovascular disease. Circ Res 1995, 77:863-868 [DOI] [PubMed] [Google Scholar]
- 4.Holvoet P, Collen D: Oxidized lipoproteins in atherosclerosis and thrombosis. FASEB J 1994, 8:1279-1284 [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989, 320:915-924 [DOI] [PubMed] [Google Scholar]
- 6.Galis ZS, Sukhova GK, Lark MW, Libby P: Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994, 94:2493-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Li L, Zielke HR, Cheng L, Xiao R, Crow MT, Stetler-Stevenson WG, Froehlich J, Lakatta EG: Increased expression of 72-kd type IV collagenase (MMP-2) in human aortic atherosclerotic lesions. Am J Pathol 1996, 148:121-128 [PMC free article] [PubMed] [Google Scholar]
- 8.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V: Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques: potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 1995, 92:1565-1569 [PubMed] [Google Scholar]
- 9.Tsujii T, Katayama K, Naito I, Seno S: The circulating α1-antitrypsin-elastase complex attacks the elastic lamina of blood vessels: an immunohistochemical study. Histochemistry 1988, 88:443-451 [PubMed] [Google Scholar]
- 10.Landi A, Bihari-Varga M, Kellar L, Mezey Z, Gruber É: Elastase-type enzymes and their relation to blood lipids in atherosclerotic patients. Atherosclerosis 1992, 93:17-23 [DOI] [PubMed] [Google Scholar]
- 11.Katsuda S, Okada Y, Nakanishi I: Abnormal accumulation of elastin-associated microfibrils during elastolysis in the arterial wall. Exp Mol Pathol 1990, 52:13-24 [DOI] [PubMed] [Google Scholar]
- 12.Galis ZS, Sukhova GK, Kranzhöfer R, Clark S, Libby P: Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci USA 1995, 92:402-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henney AD, Wakeley PR, Davies MJ, Foster K, Hembry R, Murphy G, Humphries S: Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 1991, 88:8154-8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC: Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA 1996, 93:9748-9753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC: Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. J Clin Invest 1995, 96:318-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikkari ST, Hoyhtya M, Isola J, Nikkari T: Macrophages contain 92-kd gelatinase (MMP-9) at the site of degenerated internal elastic lamina in temporal arteritis. Am J Pathol 1996, 149:1427-1433 [PMC free article] [PubMed] [Google Scholar]
- 17.Matrisian LM: The matrix-degrading metalloproteinases. Bioessays 1992, 14:455-463 [DOI] [PubMed] [Google Scholar]
- 18.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S: Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284:67-68 [DOI] [PubMed] [Google Scholar]
- 19.McDonnell S, Matrisian LM: Stromelysin in tumor progression and metastasis. Cancer Metastasis Rev 1990, 9:305-319 [DOI] [PubMed] [Google Scholar]
- 20.Vincenti MP, Clark IM, Brinckerhoff CE: Using inhibitors of metalloproteinases to treat arthritis: easier said than done? Arthritis Rheum 1994, 37:1115-1126 [DOI] [PubMed] [Google Scholar]
- 21.Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, Pelletier JP: Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest 1994, 70:807-815 [PubMed] [Google Scholar]
- 22.Banda MJ, Werb Z: Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J 1981, 193:569-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro SD, Griffin GL, Gilbert DJ, Jenkins NA, Copeland NG, Welgus HG, Senior RM, Ley TJ: Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem 1992, 267:4664-4671 [PubMed] [Google Scholar]
- 24.Shapiro SD, Kobayashi DK, Ley TJ: Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 1993, 268:23824-23829 [PubMed] [Google Scholar]
- 25.Chandler S, Cossin J, Lury J, Wells G: Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumor necrosis factor-α fusion protein. Biochem Biophys Res Commun 1996, 228:421-429 [DOI] [PubMed] [Google Scholar]
- 26.Gronski TJ, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD: Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 1997, 272:12189-12194 [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Hirata M, Yoshikawa Y, Nagafuchi Y, Toyoshima H, Watanabe T: Role of macrophages in atherosclerosis. Lab Invest 1985, 53:80-90 [PubMed] [Google Scholar]
- 28.Matsumoto S, Katoh M, Saito S, Watanabe T, Masuho Y: Identification of soluble type of membrane-type matrix metalloproteinase-3 formed by alternatively spliced mRNA. Biochim Biophys Acta 1997, 1354:159-170 [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto S, Katoh M, Watanabe T, Masuho Y: Molecular cloning of rabbit matrix metalloproteinase-2 and its broad expression at several tissues. Biochim Biophys Acta 1996, 1307:137-139 [DOI] [PubMed] [Google Scholar]
- 30.Tsukada T, Rosenfeld M, Ross R, Gown AM: Immunocytochemical analysis of cellular components in atherosclerotic lesions: use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis 1986, 6:601-613 [DOI] [PubMed] [Google Scholar]
- 31.Murakami M, Horiuchi S, Takata K, Morino Y: Distinction in the mode of receptor-mediated endocytosis between high density lipoprotein and acetylated high density lipoprotein: evidence for high density lipoprotein receptor-mediated cholesterol transfer. J Biochem 1987, 101:729-741 [DOI] [PubMed] [Google Scholar]
- 32.Saito S, Katoh M, Masumoto M, Matsumoto S, Masuho Y: Collagen degradation induced by the combination of IL-1α and plasminogen in rabbit articular cartilage explant culture. J Biochem 1997, 122:49-54 [DOI] [PubMed] [Google Scholar]
- 33.Kumar R, Dong Z, Fidler IJ: Differential regulation of metalloelastase activity in murine peritoneal macrophages by granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor. J Immunol 1996, 157:5104-5111 [PubMed] [Google Scholar]
- 34.Katsuda S, Okada Y, Okada Y, Imai K, Nakanishi I: Matrix metalloproteinase-9 (92-kd gelatinase/type IV collagenase equals gelatinase B) can degrade arterial elastin. Am J Pathol 1994, 145:1208-1218 [PMC free article] [PubMed] [Google Scholar]
- 35.Okada Y, Katsuda S, Okada Y, Nakanishi I: An elastinolytic enzyme detected in the culture medium of human arterial smooth muscle cells. Cell Biol Int 1993, 17:863-869 [DOI] [PubMed] [Google Scholar]
- 36.Laurie GW, Leblond CP, Martin GR: Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol 1982, 95:340-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H: Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry 1990, 29:10261-10270 [DOI] [PubMed] [Google Scholar]
- 38.Ogata Y, Enghild JJ, Nagase H: Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 1992, 267:3581-3584 [PubMed] [Google Scholar]
- 39.Sato H, Seiki M: Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem 1996, 119:209-215 [DOI] [PubMed] [Google Scholar]
- 40.Crabbe T, O’Connell JP, Smith BJ, Docherty AJP: Reciprocated matrix metalloproteinase activation: a process performed by interstitial collagenase and progelatinase A. Biochemistry 1994, 33:14419-14425 [DOI] [PubMed] [Google Scholar]
- 41.Bergmann U, Tuuttila A, Stetler-Stevenson WG, Tryggvason K: Autolytic activation of recombinant human 72 kilodalton type IV collagenase. Biochemistry 1995, 34:2819-2825 [DOI] [PubMed] [Google Scholar]
- 42.Hartung HP, Kladetzky RG, Hennerici M: Chemically modified low density lipoproteins as inducers of enzyme release from macrophages. FEBS Lett 1985, 186:211-214 [DOI] [PubMed] [Google Scholar]
- 43.Rouis M, Nigon F, Lafuma C, Hornebeck W, Chapman MJ: Expression of elastase activity by human monocyte-macrophages is modulated by cellular cholesterol content, inflammatory mediators, and phorbol myristate acetate. Arteriosclerosis 1990, 10:246-255 [DOI] [PubMed] [Google Scholar]
- 44.Kusuhara M, Chait A, Cader A, Berk BC: Oxidized LDL stimulates mitogen-activated protein kinases in smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol 1997, 17:141-148 [DOI] [PubMed] [Google Scholar]
- 45.Hahn AWA, Ferracin F, Bühler FR, Pletscher A: Modulation of gene expression by high and low density lipoproteins in human vascular smooth muscle cells. Biochem Biophys Res Commun 1991, 178:1465-1471 [DOI] [PubMed] [Google Scholar]
- 46.Belaaouaj A, Shipley JM, Kobayashi DK, Zimonjic DB, Popescu N, Silverman GA, Shapiro SD: Human macrophage metalloelastase: genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem 1995, 270:14568-14575 [DOI] [PubMed] [Google Scholar]
- 47.Rajavashisth TB, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman AM, Lusis AJ: Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 1990, 344:254-257 [DOI] [PubMed] [Google Scholar]