Abstract
p27Kip1 is a cyclin-dependent kinase inhibitor that regulates the decision to enter S phase or withdraw from the cell cycle. In resting cells, the level of p27Kip1 provides an inhibitory threshold above which G1 cyclin D/E/cyclin-dependent kinases accumulate before activation; however, in cycling cells, p27Kip1 protein is sequestered by high levels of active cyclin D/cyclin-dependent kinase 4 complexes. As a group, the cyclin-dependent kinase inhibitors have been proposed to act as tumor suppressor genes, and several members have been implicated in the pathogenesis of a variety of human cancers. We examined p27Kip1 expression in 116 non-Hodgkin’s lymphomas including 50 cases of MCL (40 typical and 10 blastic variants), 21 follicular lymphomas, 20 diffuse large B-cell lymphomas, 16 chronic lymphocytic leukemias, 8 marginal zone B-cell lymphomas, and 1 splenic marginal zone lymphoma, and correlated its expression with that of the proliferation marker Ki67 (MiB1) and with p53. p27Kip1gene structure was analyzed by Southern blot in the group of MCLs. In all cases of non-Hodgkin’s lymphoma other than MCL, p27Kip1 expression was inversely related to the proliferation index as measured by Ki67. In contrast, in typical MCL, p27Kip1 expression was negative in 35 of 40 (88%) cases, irrespective of the proliferative rate (median 15%; range 2 to 90%). Paradoxically, in the blastic variant of MCL, 8 of 10 (80%) cases showed expression of p27Kip1, despite a high proliferation rate (median 60%; range 32 to 100%). However, the staining in most of the cases was less intense than in the reactive T lymphocytes. Deletions of p27Kip1gene were not found in any of the 25 cases examined. p53 expression was found in 15 of 50 cases of MCL: 7 of 10 (70%) in the blastic variant and 8 of 40 (20%) in the typical MCL (70% vs. 20%, P < 0.0045). These results demonstrate that MCLs, in contrast to other non-Hodgkin’s lymphomas and normal lymphoid tissue, fail to correlate p27Kip1 expression with the proliferation rate. This peculiar uncoupling of p27Kip1 protein expression from the proliferation rate may be related to the high levels of cyclin D1 expressed in MCL and is likely to have profound effects on cell cycle regulation and contribute to the pathogenesis of MCL.
The cell cycle is regulated by a family of cyclin-dependent kinases (CDKs) and their regulatory subunit cyclins. These CDK/cyclin complexes are activated and inactivated at specific time points during the cell cycle in response to internal and external demands. 1 The kinase activity of CDKs can be inhibited by a group of CDK inhibitors that bind to cyclin-CDK complexes and block progression through the cell cycle. 2 Two major classes of CDK inhibitors have been identified. p15INK4b, p16INK4a, and p18INK4c specifically inhibit CDK4 and CDK6, whereas p21Waf1, p27Kip1, and p57Kip2 can bind to and inhibit a broad range of CDK-cyclin complexes.
p27Kip1 is a protein of 198 amino acids, the function of which is crucial both for progression from G1 into S phase and for exit from the cell cycle. 3 p27Kip1 is present in large amounts in quiescent cells, and the level declines when cells proliferate in response to mitogenic signals. 4 Recent studies suggest that p27Kip1 mediates G1 arrest induced by transforming growth factor β, rapamycin, cAMP, contact inhibition, and serum deprivation. 3-7 The development of multiple organ hyperplasia and pituitary tumors in p27Kip1 knockout mice suggests that the loss of p27Kip1 disturbs the balance between cell cycle activators and inhibitors and leads to an alteration in the balance between proliferating and nonproliferating cells, underscoring the important role of p27Kip1 as a negative cell cycle regulator. 5,8-10 p27Kip1 regulates progression from G1 into S phase by binding and inhibiting the cyclin E/CDK2 complex, the activity of which is required for entry into S phase. 11,12 Regulation of p27Kip1 protein occurs primarily through posttranscriptional mechanisms. In addition to ubiquitination, which leads to the degradation of p27Kip1 protein, p27Kip1 is regulated at the translational level and by noncovalent sequestration mediated by cyclin D1, which prevents inhibition of the cyclin E-CDK2 complex. 3,7,12-15
As a CDK inhibitor, p27Kip1has been considered a potential candidate tumor suppressor gene. However, in contrast to p53 and p16INK4a, no homozygous deletions and only rare mutations of the p27Kip1gene have been found in cell lines or in human tumors. 16-19 Although genetic abnormalities of p27Kip1have not been detected, recent reports have shown that reduced expression of p27Kip1 protein correlates with poor survival in breast and colorectal carcinoma patients. 20-23 For this reason we wished to study its range of expression in lymphoid tissues and lymphomas. We paid particular regard to mantle cell lymphoma (MCL) because of its deregulation of cyclin D1, a critical G1 cyclin that interacts normally with p27Kip1. In addition, the correlation of p27Kip1 expression with the proliferation marker Ki67 and the expression of p53 was analyzed.
Materials and Methods
Tissue Samples
Formalin- or B5-fixed and paraffin-embedded biopsies from 106 non-Hodgkin’s lymphomas (NHLs) were selected from the files of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, and 10 cases from the files of the Pathology Department of the Hospital Clinic of Barcelona. Some of these cases have been the subject of previous studies. 24-26 All cases were classified according to the revised European-American lymphoma classification. 27 The cases in this study included 50 cases of MCL, of which 40 cases were classified as typical MCL, and 10 cases were classified as aggressive or blastic variants of MCL, 28-30 21 cases of follicle center cell lymphoma, 20 cases of diffuse large B-cell lymphoma (DLBCL), 16 cases of chronic lymphocytic leukemia (CLL), 8 cases of marginal zone B-cell lymphoma, and 1 case of splenic marginal zone lymphoma. In addition, different samples of reactive lymphoid tissue including tonsils (5 cases) and lymph nodes (3 cases) were analyzed.
Immunohistochemistry
All cases had been previously immunophenotyped in paraffin sections with the following monoclonal antibodies: CD5 (clone 4C7; Novocastra, Newcastle, UK; dilution 1:50), CD20 (DAKO, Carpinteria, CA; dilution 1:200), CD43 (Leu 22; Becton Dickinson, Mountain View, CA; dilution 1:50), CD23 (Novocastra; dilution 1:40), and cyclin D1 (clone P2D11F11; Novocastra; dilution 1:10) and with the following polyclonal antibodies: CD3 (DAKO; dilution 1:100) IgM (DAKO; dilution 1:800), IgD (DAKO; dilution 1:800), Kappa (DAKO; 1:28,000), and Lambda (DAKO; 1:30,000). The expression of p27Kip1 was investigated on paraffin-embedded tissue sections with the monoclonal antibody Kip-1 (Transduction Laboratories, Lexington, KY; dilution 1:1,000), as well as p53 with the monoclonal antibody DO7 against the wild-type/mutant p53 (DAKO; dilution 1:50) and the MIB-1 antibody against the Ki67 nuclear proliferation antigen (Immunotech, Westbrook, ME; dilution 1:10). All immunohistochemical analyses were reviewed by LQ-M and MR.
Immunohistochemical staining was performed on an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) according to the company’s protocols, with slight modifications. Briefly, 5 μm thick paraffin sections were mounted on Fisherbrand/plus Superfrost Precleaned slides (Fisher Scientific, Pittsburgh, PA) and attached by overnight heating at 58°C. After deparaffinization and rehydration the slides were placed in a microwave pressure cooker in 0.01 mol/L citrate buffer (pH 6.0) containing 0.1% Tween 20 and heated in a microwave oven at maximum power (800 W) for 40 minutes. Sections for p27Kip1 staining were left in the hot buffer for 30 minutes. All other sections were immediately cooled in Tris-buffered saline. Thereafter, all sections were washed in Tris-buffered saline (pH 7.6) containing 5% fetal calf serum (Life Technologies, Inc., Grand Island, NY) for 30 minutes. p27Kip1, cyclin D1, and CD5 were incubated overnight at room temperature. The rest of the procedure (secondary antibody, avidin-biotin complex (ABC), color development, and counterstain) was performed on the Ventana immunostainer.
Positive controls for p53, p27Kip1, Ki67, and cyclin D1 were used to confirm the adequacy of the staining. The staining quality of cyclin D1 was considered evaluable when epithelial cells and histiocytes exhibited characteristic weak nuclear positivity. Tissues were scored as p53 positive if equal or greater than 20% of the tumor cells had nuclear staining and as p53 negative if less than 20% stained. To compare p27Kip1 staining between tissue samples, T cells were used as internal controls. The percentage of cells showing positive nuclear staining was assessed for p53, Ki67, and p27Kip1 in each case. A grid ocular objective was used to count 400 cells over three high-power fields (×40), and the percentage of positive cells was reported as 0 to 100%.
Double staining for p27Kip1 and L26 or CD3 was performed using two different detection systems. The primary antibody, p27Kip1, was incubated overnight, and the secondary antibody for 30 minutes. The reaction was developed using an ABC-alkaline phosphatase complex for 30 minutes (Vector Laboratories, Burlingame, CA), and Vector red substrate for 20 minutes (Vector Laboratories). The second primary antibody, L26 or CD3 was incubated for 1 hour, and the secondary antibody for 30 minutes. The reaction was then detected with the ABC complex and 3,3′-diaminobenzidine with nickel enhancement (Vector).
Southern Blot Analyses
Genomic DNA was extracted from frozen tissue in 25 cases using proteinase K/RNase treatment and phenol-chloroform extraction. Ten μg of DNA was digested with EcoRI or HindIII and BamHI, separated on 0.8% agarose gels, and transferred to GeneScreen Plus nylon membranes (NEN Research Products, Boston, MA). The membranes were hybridized with a 32P-labeled 1.5-kb EcoRI fragment containing the full-length p27Kip1 cDNA. 3 After washing at appropriate stringencies, bound probe was detected by autoradiography at −70°C using intensifying screens.
Statistical Analysis
The significance of the association of p53 overexpression between the two groups of MCLs and the significance of the association between p27Kip1 and p53 expression was assessed using Fisher’s exact test.
Results
p27Kip1 and Ki67 Protein Expression in Normal Lymphoid Tissue
In reactive tonsils and lymph nodes, p27Kip1 was strongly expressed in the nuclei of the mantle cells and in the interfollicular small lymphocytes, whereas germinal center cells were negative. Within the germinal centers there were scattered positive cells mainly in the light zone (Figure 1) ▶ . Double staining demonstrated that these cells corresponded mainly to reactive T cells; however, rare plasma cells with strong nuclear positivity were also identified. The pattern of expression was opposite to that seen with the proliferation-related antigen, Ki67.
Figure 1.

p27Kip1 protein expression in normal lymphoid tissue (tonsil). Germinal centers that contain a high percentage of proliferating cells show only scattered cells expressing p27Kip1, whereas most cells in the mantle zone and in the interfollicular T-cell area express high levels of p27Kip1. Immunoperoxidase; ×100.
p27Kip1, Ki67, and p53 Protein Expression in NHLs
A total of 66 cases of B-cell NHLs other than MCL were immunostained for p27Kip1, p53, and Ki67. The results are summarized in Table 1 ▶ . All cases of CLL (16 cases) and marginal zone lymphomas (9 cases) revealed strong p27Kip1 nuclear staining similar to the intensity seen in T lymphocytes (Figure 2A) ▶ . The occasional large cells in marginal zone lymphomas and the proliferation centers in CLLs were p27Kip1 negative. In contrast, Ki67 was positive in these cells. In the group of follicle center cell lymphomas (21 cases), examples of histological grades I, II, and III were included. In each case there was an inverse correlation between the percentage of cells in the neoplastic follicles that showed p27Kip1 staining and the cells that expressed the proliferation marker Ki67. In the DLBCL group (20 cases), p27Kip1 expression was completely negative in the large neoplastic cells in 10 cases (group 1) (Figure 2B) ▶ , and in 6 cases a small percentage of the neoplastic cells were positive (group 2). In all cases, intermingled reactive small lymphocytes with strong nuclear positivity for p27Kip1 were identified. In 4 cases (group 3), the neoplastic large cells, regardless of the high proliferative index, showed nuclear staining of p27Kip1. The morphology and the proliferation index of these tumors did not differ from those cases that showed lack of p27Kip1 expression.
Table 1.
Expression of p27Kip1, MIB1, and p53 in 66 NHLs
| NHL | Cases | p27Kip1+ cells (range) | MIB1+ cells (range) | p53+ no. of cases |
|---|---|---|---|---|
| Follicular lymphoma | 21 | 65% (0–98) | 41% (10–98) | 6* |
| Diffuse large B-cell lymphoma | 20† | 23% (0–98) | 74% (42–99) | 5 |
| Group 1 | 10 | 0 | 74% (42–99) | 3 |
| Group 2 | 6 | 28% (17–43) | 64% (42–80) | 1 |
| Group 3 | 4 | 80% (60–98) | 85% (66–98) | 1 |
| B-cell chronic lymphocytic leukemia | 16 | 85% (71–100) | 17% (6–30) | 0 |
| Marginal zone B-cell lymphoma | 8 | 90% (74–100) | 12% (1–38) | ND |
| Splenic marginal zone lymphoma | 1 | 66% | 40% | ND |
*p53-positive cases were all grade III with diffuse areas.
†Total group.
Figure 2.
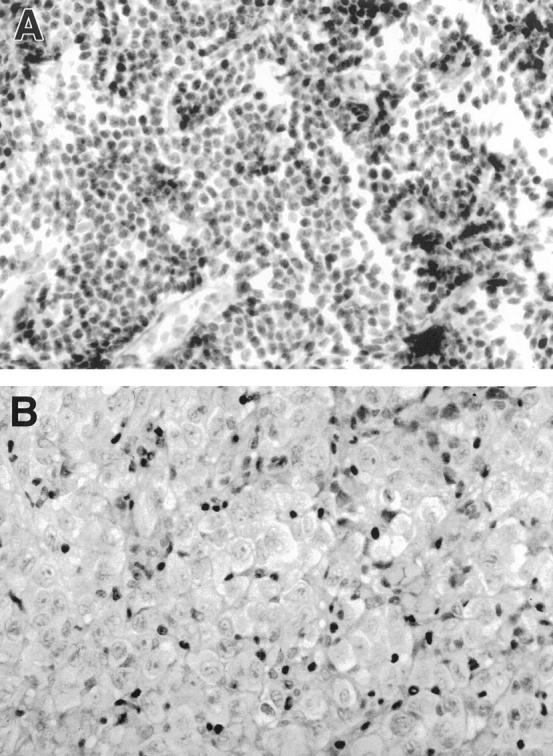
A: p27Kip1 protein expression in a case of CLL. The neoplastic cells show a diffuse strong nuclear positivity. Immunoperoxidase; ×400. B: p27Kip1 protein expression in a case of DLBCL. The large neoplastic cells are completely negative for p27Kip1, whereas the small reactive T lymphocytes are positive. Immunoperoxidase; ×400.
Expression of p53 was detected in 11 cases: 6 follicle center cell lymphomas and 5 DLBCLs. In the latter group there was no correlation between the expression of p27Kip1 and expression of p53. One of the 4 cases of the DLBCL that expressed p27Kip1 expressed p53.
p27Kip1, Ki67, and p53 Protein Expression in MCLs
A total of 50 cases of MCLs were immunostained for p27Kip1, p53, Ki67, and cyclin D1. The results are summarized in Table 2 ▶ . The cases included 40 typical MCLs (11 cases of multiple lymphomatous polyposis and 29 cases of typical MCL from lymph nodes and tonsils) and 10 cases of the blastic variant of MCLs. Cyclin D1 was expressed in all evaluable cases (Figure 3A) ▶ , as expected for these lymphomas. Nuclear staining was present in more than 80% of the tumor cells with variation in intensity among the cells. Five typical MCL cases were considered not evaluable for cyclin D1 due to the lack of staining of histiocytes and/or endothelial cells.
Table 2.
Expression of p27Kip1, MIB1, and p53 in 50 Cases of MCL
| Diagnosis | No. of cases | p27Kip1 (%) (range) | Ki-67 (%) (range) | p53+ # of cases |
|---|---|---|---|---|
| Typical MCL | ||||
| p27Kip1 negative | 35* | 0 (0–1) | 24 (2–90) | 7 |
| p27Kip1 positive | 5 | 81 (50–95) | 16 (2–31) | 1 |
| Blastic MCL | ||||
| p27Kip1 negative | 2 | 0 (0–0) | 82 (80–83) | 2 |
| p27Kip1 positive | 8 | 72 (30–95)† | 65 (32–100) | 5 |
*Includes all 11 cases of multiple lymphomatous polyposis.
†Most cases showed weak but definite staining for p27Kip1, at an intensity lower than the intermingled T cells.
Figure 3.
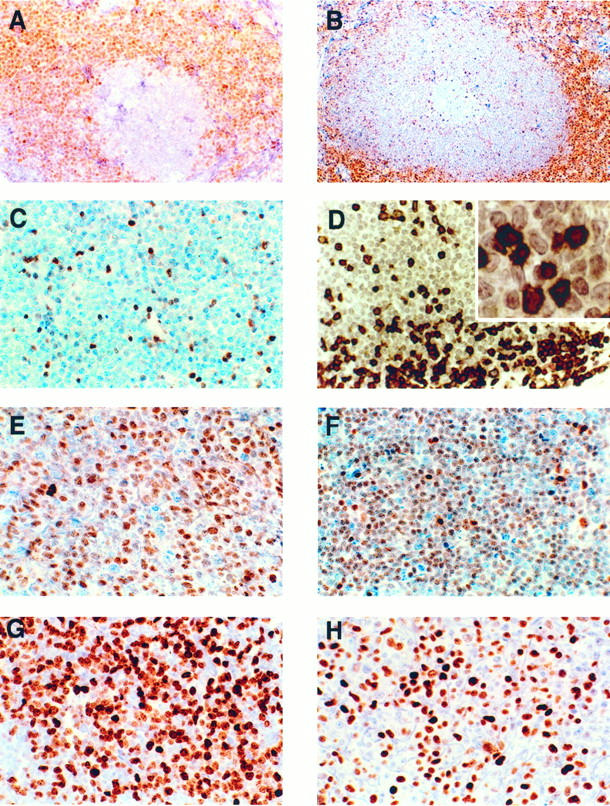
p27Kip1 and cyclin D1 expression in a case of typical MCL. A: MCL cells staining positively for cyclin D1. The residual germinal center shows a negative reaction. Immunoperoxidase; ×200. B: In contrast to cyclin D1, the negative p27Kip1 staining of the MCL cells accentuates the mantle zone (perifollicular) growth pattern. Note the negative staining in the reactive germinal center and the positive staining in the residual T-cell area. Immunoperoxidase; ×100). C: p27Kip1 in a typical case of MCL with a diffuse growth pattern. The tumor cells are negative, whereas the intermingled T lymphocytes are strongly positive. Immunoperoxidase; ×400. D: Double staining for p27Kip1 (red) and CD3 (black) in a case of MCL reveals that all of the p27Kip1-positive cells within the tumor co-express CD3. The lymphoma cells are negative for both p27Kip1 and CD3. Immunoperoxidase; ABC-alkaline phosphatase method (red) and ABC-3,3′-diaminobenzidine method (black); ×400; inset, ×1000. E Strong p27Kip expression in the majority of tumor cells in a case of a large-cell variant of MCL. Immunoperoxidase; ×400. F, G, and H: p27Kip, Ki67, and p53 expression in a case of blastic variant of MCL. F: p27Kip1 is weakly expressed by the majority of tumor cells despite the high proliferation rate. Note the stronger positivity of the intermingled T lymphocytes. Immunoperoxidase; ×400. G: Ki67 is expressed by the vast majority of the tumor cells. Immunoperoxidase; ×400. H: p53-positive staining in tumor cells. Immunoperoxidase; ×400.
p27Kip1 and Ki67 Expression in Typical MCL
p27Kip1 was undetectable by immunohistochemistry in the tumor cells in 35 of 40 cases (88%), regardless of the generally low proliferative index assessed with the proliferation marker Ki67 (median 15%; 2 to 90%) (Figure 3, B and C) ▶ . In all cases scattered small lymphocytes strongly positive for p27Kip1 were identified (Figure 3C) ▶ . Double staining for CD3 revealed that these p27Kip1-positive small lymphocytes were reactive T cells (Figure 3D) ▶ . In only five cases (12%) was p27Kip1 expressed by the tumor cells; however, in three of these cases the expression was uniformly weak and less intense than the scattered T lymphocytes. The morphology and the proliferation index of these tumors did not differ from those cases that lacked p27Kip1 expression.
p27Kip1 and Ki67 Expression in the Blastic Variant of MCL
In contrast to the group of typical MCL, 8 of 10 cases (80%) showed expression of p27Kip1 (Table 3) ▶ (Figure 3, E and F) ▶ ; 7 cases showed weak staining, less intense than the T lymphocytes, and 1 case was strongly positive in most of the neoplastic cells regardless of the high proliferation index (Ki67 > 50%) (Figure 3G) ▶ . Only 2 cases were completely negative for p27Kip1. In all cases intermingled p27Kip1 positive T-reactive lymphocytes were identified.
Table 3.
Expression of p27Kip1, MIB1, and p53 in 10 Cases of Blastic Variant of MCL
| Case | Diagnosis | p27* | MiB1 | p53 |
|---|---|---|---|---|
| 1 | MCL-blastic | − | 83% | 20% |
| 2 | MCL-blastic | − | 80% | 20% |
| 3 | MCL-blastic | + | 70% | >80% |
| 4 | MCL-blastic | ++ | 100% | 20% |
| 5 | MCL-blastic | + | 50% | >80% |
| 6 | MCL-blastic | + | 95% | <5% |
| 7 | MCL-blastic | + | 76% | 0%† |
| 8 | MCL-blastic | + | 32% | 50% |
| 9 | MCL-blastic | + | 50% | >80% |
| 10 | MCL-blastic | + | 48% | 0%‡ |
*+, most tumor cells faintly positive and less intense than T lymphocytes; ++, most tumor cells positive with equal intensity as T lymphocytes.
†In this case a homozygous deletion of p16 was detected by Southern blot.
‡In this case a hemizygous deletion of p16 was detected by cytogenetic analysis:del (9) (p12).
Correlation between p27Kip1 and p53 Expression in MCLs
Expression of p53 was detected in 15 of the 50 cases; 7 cases corresponded to the blastic variant group (70%) (Figure 3H) ▶ , and 8 cases to the typical MCL group (20%). The high association of p53 overexpression in the blastic variant as compared with the typical variant was statistically significant (P < 0.0045). When the expression of p53 was correlated with the staining for p27Kip1, cases expressing p53 were more likely to have detectable levels of p27Kip1 in the tumor cells (6 of 15 cases, 40%) than were the p53-negative cases (7 of 35 cases, 20%). However, this association was not statistically significant (P < 0.1704).
Southern Blot Analysis
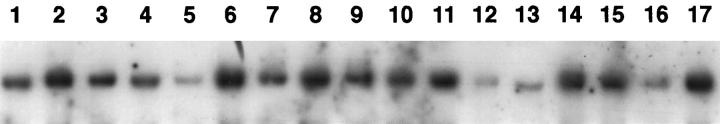
Southern blot analysis was performed on 25 cases of MCL to determine whether the lack of p27 expression in the typical MCL could be due to gross rearrangement or deletion of the gene. In all cases, hybridization with the p27Kip1probe revealed a germline configuration with identical signal intensity in comparison to the control hybridization (not shown), therefore showing no evidence of p27Kip1rearrangements or deletions (Figure 4) ▶ .
Figure 4.
Southern blot analysis of 16 cases of typical MCL (Lanes 1 to 16). BamHI-digested DNA separated by agarose gel electrophoresis and blotted into a nylon membrane was hybridized with a 32P-labeled p27Kipprobe. All cases show a germline configuration without evidence of rearrangement or deletion of p27Kip1gene. The varying signal intensities are due to unequal loading of DNA as confirmed by hybridization with a control probe (data not shown). Lane 17 corresponds to placental DNA.
Discussion
In this study we analyzed a large group of B-cell NHLs for p27Kip1 expression and correlated the results with the expression of Ki67 and p53. We found that p27Kip1 expression in normal lymphoid tissue and in lymphoid neoplasias is inversely proportional to the proliferation index as measured by Ki67. The normal mantle zone and the interfollicular lymphocytes, as well as B-cell CLL, marginal zone B-cell lymphomas, and follicular lymphomas grade I are mainly p27Kip1 positive, whereas the germinal centers in reactive tonsils and lymph nodes, as well as tumors characterized by a higher growth fraction, tend to lack p27Kip1 expression. In contrast, we found that most cases of typical MCLs do not express p27Kip1, regardless of their generally low proliferative rate. Paradoxically, MCLs with blastic morphology, which are characterized by a more aggressive clinical behavior and high proliferation rate, show expression of p27Kip1.
Our data are generally in agreement with a recent study by Sanchez-Beato et al, 31 who also found p27Kip1 expression to be inversely related to the proliferation rate as measured by Ki67 staining in normal lymphoid tissue and in B-cell NHL. However, there are some differences between the two studies. First, although we also identified scattered positive cells in the germinal centers, mainly in the light zone, our double staining studies revealed that these cells corresponded to reactive T cells and interfollicular plasma cells and not to centrocytes, as suggested by Sanchez-Beato et al. 31 Secondly, and of most interest, we found that the vast majority of typical MCLs (88%) do not express p27Kip1. However, that study included only two cases of MCL, limiting their assessment of this disease.
p27Kip1 is a CDK inhibitor that plays a critical role in the physiological process of cell cycle commitment. 32 It was initially discovered by Polyak et al, 7 who showed that p27Kip1 was responsible for the inhibition of cell growth due to transforming growth factor β, and by Toyoshima and Hunter, 15 who identified the protein through its binding with cyclin D1/CDK4 in a yeast interaction hybrid screen. The regulation of p27Kip1 protein levels in the cell appears to occur primarily at the translational and posttranslational level, as the amount of mRNA does not fluctuate during the cell cycle, despite the fact that p27Kip1 protein level changes during the cell cycle in response to external signals. 1,14
Through its interaction with G1 cyclin/CDK complexes, p27Kip1 appears to control entry into the cell cycle and progression through G1. p27Kip1 binds efficiently with CDK4 and D-type cyclins and less efficiently with CDK2 and cyclin E. 15,33 In contact-inhibited MyLu1 cells and in transforming growth factor β-arrested cells, p27Kip1 associates with and inhibits the activity of the cyclin E/CDK2 complex, thereby preventing cells from entering S phase. 7 By contrast, in lysates of growing Swiss 3T3 cells, only cyclin D1 and CDK4 coprecipitate with p27Kip1. 15 Furthermore, in transfected NIH-3T3 cells, overexpression of cyclin D1/CDK4 has been shown to override a p27Kip1-imposed G1 block and to promote S phase entry by sequestering p27Kip1 in active cyclin D1/CDK4 complexes. 12 This sequestration of p27Kip1 results in the release of its inhibitory effect on cyclin E/CDK2 complexes, allowing phosphorylation of the RB protein to be completed and progression into S phase to occur. 3,7 Like other CDK inhibitors, p27Kip1 has been proposed to act as a tumor suppressor gene. In addition to its inhibitory effect on cell cycle progression, the loss of its expression in p27Kip1knockout mice results in hyperplasias and pituitary tumors, again underscoring the central role of this gene in regulating cell growth and maintaining tissue homeostasis. 8-10
Of the various types of B-cell lymphomas analyzed in this study, only the MCLs did not show an inverse correlation of p27Kip1 expression and the proliferation rate. This is interesting because MCLs are characterized by the t(11;14) translocation, which leads to abnormally high levels of cyclin D1. Because cyclin D1 has little transforming activity, it has been of interest to identify factors that could potentiate its oncogenic activity. 34 The myc gene was first shown to cooperate with cyclin D1 in transfection studies 35,36 and to lead to acceleration of tumor formation in double transgenic mice. 37 Other candidates that may cooperate with cyclin D1 include the CDK inhibitors. 1,2,11 Although alterations in p16INK4aand p21Waf1 are rare in typical MCLs, the loss of expression of these genes, as well as deletions of p16INK4a, have been reported in aggressive variants of MCL. 26 The present report suggests that p27Kip1 may be yet another CDK inhibitor the loss of which can potentiate the oncogenic activity of cyclin D1.
The inability to detect p27Kip1 protein expression in typical MCLs cannot be explained by p27Kip1gene deletion, because none of the 25 MCL cases examined showed gross rearrangement or deletion of the gene. This finding is not surprising and is consistent with previous studies that have shown no homozygous deletions and only rare mutations of the p27Kip1gene in human tumors. 16-19
Although the relationship between the high cyclin D1 levels and the lack of p27Kip1 immunostaining in MCLs is uncertain, there are several possible explanations as to why these may be linked. One explanation is that in MCL the high level of cyclin D1 leads to the degradation of p27Kip1. We believe that this is unlikely because in experimental systems, high levels of cyclin D1 are reported to inhibit the degradation of p27Kip1. 12 A second possibility is that p27Kip1, when bound to cyclin D1/CDK complexes, is not immunologically detectable by the antibody used. The fact that this antibody reacts with the C terminus of p27Kip1, in the region of the cyclin D1 binding domain, is consistent with this explanation. Finally, it is possible that p27Kip1 expression is lost through a mechanism independent of the cyclin D1 abnormality in MCL.
Paradoxically, staining for p27Kip1 was observed in the majority of the cases of blastic variant of MCL (8 of 10 cases; 80%). In most of the cases, the intensity of staining was less than in the T lymphocytes, suggesting that even though there is some p27Kip1 protein in the tumor cells, its expression is still relatively low. The reason why blastic variants of MCL express some p27Kip1 despite their high proliferative rate is not clear. It is possible that these tumors have additional abnormalities that allow them to tolerate the high levels of p27Kip1 expression. Previous studies in MCLs have shown that the blastic variant of MCL, in contrast to the typical cases, have frequent occurrence of mutations and deletions in other negative cell cycle-regulatory genes (p53, p16INK4aand p21Waf1), which may contribute to the development of more aggressive disease with higher proliferative activity. 26,38 The loss of these and possibly other cell cycle-regulatory proteins may lead to a compensatory increase in the levels of p27Kip1. Accordingly, five of the eight cases with blastic morphology and p27Kip1 expression had immunohistochemical detection of p53; four of these cases showed p53 protein in a high number of cells (>50%). Although we did not study p53 gene alterations, previous studies have shown a good correlation between high expression of p53 protein expression and gene mutations in MCLs. 38 In addition, two of the three blastic cases with p27Kip1 positivity and no expression of p53 have been shown in a previous study to have deletions of the p16INK4a gene. 26
Interestingly, in human breast cancer, overexpression of cyclin D1 is accompanied by high expression of p27Kip1 protein. 23,39 Furthermore, overexpression of cyclin D1 in vitro has been shown to induce p27Kip1 expression in mouse mammary epithelial cells. 40 These data contrast with our findings in typical MCL, in which high levels of cyclin D1 are associated with low levels of p27Kip1. The reason for this difference is not clear; however, it may reflect tissue-specific differences in the cell cycle machinery. In contrast to epithelial cells, lymphoid cells do not normally express cyclin D1, and the molecular consequences of aberrant expression of cyclin D1 in these cells may be different.
In conclusion, we report the loss of detectable p27Kip1 protein expression in typical MCL and hypothesize that this loss of expression may be related to the high level of cyclin D1 expression in these tumors rather than to the presence of structural abnormalities that occur in other genes with tumor suppressor activity. We suggest that the loss of the immunologically detectable levels of p27Kip1 has functional consequences that are likely to play an important role in MCL tumorigenesis. Further studies are needed to elucidate the mechanism responsible for the lack of detection of p27Kip1 in MCL and its expression in the more highly proliferative blastic variants.
Acknowledgments
The authors thank Cynthia A. Harris and Sara E. Delay for their expert technical assistance and Ralph L. Isenberg for his photographic assistance.
Footnotes
Address reprint requests to Mark Raffeld, M.D., Senior Investigator, Hematopathology Section, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892. E-mail: mraff@box-m.nih.gov.
Supported in part by the Austrian Science Fund Charlotte-Bühler Habilitationstipendium (to LQ-M), an Erwin Schrödinger-Stipendium (to FF), and Grant SAF 96/61 from Comision Interministerial de Ciencia y Tecnologia (to EC).
References
- 1.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM: Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 1995, 9:1149-1163 [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J: Cloning of p27KIP-1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 4.Kato J, Matsuoka M, Polyak K, Massague J, Sherr CJ: Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27KIP1) of cyclin-dependent kinase 4 activation. Cell 1994, 79:487-496 [DOI] [PubMed] [Google Scholar]
- 5.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee M-H, Massague J, Crabtree GR, Roberts JM: Interleukin-2-mediated elimination of the p27KIP-1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 1994, 372:570-573 [DOI] [PubMed] [Google Scholar]
- 6.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E: Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science 1996, 271:499-502 [DOI] [PubMed] [Google Scholar]
- 7.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27KIP-1, a cyclin-dependent kinase inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 8.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A: Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27KIP-1. Cell 1996, 85:721-732 [DOI] [PubMed] [Google Scholar]
- 9.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishihdo N, Horii I, Loh DY, Nakayama K: Mice lacking p27KIP1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85:707-720 [DOI] [PubMed] [Google Scholar]
- 10.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai L-H, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM: A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27KIP1-deficient mice. Cell 1996, 85:733-744 [DOI] [PubMed] [Google Scholar]
- 11.Hirama T, Koeffler HP: Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood 1995, 86:841-854 [PubMed] [Google Scholar]
- 12.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE: Cyclin E-CDK2 is a regulator of p27 KIP-1. Genes Dev 1997, 11:1464-1478 [DOI] [PubMed] [Google Scholar]
- 13.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 14.Hengst L, Reed SI: Translational control of p27 KIP-1 accumulation during the cell cycle. Science 1996, 271:1861-1864 [DOI] [PubMed] [Google Scholar]
- 15.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 16.Bullrich F, Maclachlan TK, Sang N, Druck T, Veronese ML, Allen ST, Chiorazzi N, Koff A, Heubner K, Croce CM, Giordano A: Chromosomal mapping of members of the cdc2 family of protein kinases, cdk3,cdk6, PISSLRE, and PITALRE, and a cdk inhibitor, p27KIP-1, to regions involved in human cancer. Cancer Res 1995, 55:1199-1205 [PubMed] [Google Scholar]
- 17.Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilczynski S, Lee YY, Bartram CR, Koeffler HP: Molecular analysis of the cyclin-dependent kinase inhibitor p27/KIP-1 in human malignancies. Cancer Res 1995, 55:2266-2269 [PubMed] [Google Scholar]
- 18.Pietenpol JA, Bohlander SK, Sato Y, Papasopoulos N, Liu B, Friedman C, Trask BJ, Roberts JM, Kinzler KW, Rowley JD, Vogelstein B: Assignment of the human p27KIP-1 gene to 12p13 and its analysis in leukemias. Cancer Res 1995, 55:1206-1210 [PubMed] [Google Scholar]
- 19.Ponce-Castañeda MV, Lee M-H, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J, Cordon-Cardo C: p27KIP-1: chromosomal mapping to 12p12–12p13.1 and absence of mutations in human tumors. Cancer Res 1995, 55:1211-1214 [PubMed] [Google Scholar]
- 20.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 21.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27Kip-1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med 1997, 3:222-225 [DOI] [PubMed] [Google Scholar]
- 22.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M: The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 1997, 57:1259-1263 [PubMed] [Google Scholar]
- 23.Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D HP, Hanby AM, Barnes DM, Shousha S, O’Hare MJ, Lu X: High level expression of p27kip1 and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27Kip1 and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA 1997, 94:6380-6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Krenacs L, Otsuki T, Kumar D, Harris CA, Wellmann A, Jaffe ES, Raffeld M: bcl-1 rearrangement and cyclin D1 protein expression in multiple lymphomatous polyposis. Am J Clin Pathol 1996, 105:737-743 [DOI] [PubMed] [Google Scholar]
- 25.Wilson WH, Teruya-Feldstein J, Fest T, Harris C, Steinberg SM, Jaffe ES, Raffeld M: Relationship of p53, bcl-2, and tumor proliferation to clinical drug resistance in non-Hodgkin’s lymphomas. Blood 1997, 89:601-609 [PubMed] [Google Scholar]
- 26.Pinyol M, Hernandez L, Cazorla M, Balbin M, Jares P, Fernandez PL, Montserrat E, Cardesa A, Lopez-Otin C, Campo E: Deletions and loss of expression of p16INK4a and P21Waf1 genes are associated with aggressive variants of Mantle cell lymphomas. Blood 1997, 89:272-280 [PubMed] [Google Scholar]
- 27.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf Peters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Müller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 28.Lardelli P, Bookman MA, Sundeen J, Longo DL, Jaffe ES: Lymphocytic lymphoma of intermediate differentiation: morphologic and immunophenotypic spectrum and clinical correlations. Am J Surg Pathol 1990, 14:752-761 [DOI] [PubMed] [Google Scholar]
- 29.Ott G, Kalla J, Ott MM, Schryen B, Katzenberger T, Müller JG, Müller-Hermelink HK: Blastoid variant of Mantle cell lymphoma: frequent bcl-1 rearrangements at the major translocation cluster region and tetraploid chromosome clones. Blood 1997, 89:1421-1429 [PubMed] [Google Scholar]
- 30.Ott MM, Ott G, Porowski P, Gunzer U, Feller AC, Müller-Kermelink HK: The anaplastic variant of centrocytic lymphoma is marked by frequent rearrangements of the bcl-1 gene and high proliferation indices. Histopathology 1994, 25:329-336 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Beato M, Sáez AI, Martínez-Montero JC, Mateo MS, Sánchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin-dependent kinase inhibitor p27Kip1 in lymphoid tissue: p27Kip1 expression in inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 32.Coats S, Flanagan M, Nourse J, Roberts JM: Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272:877-880 [DOI] [PubMed] [Google Scholar]
- 33.Reynisdóttir I, Massagué J: The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev 1997, 11:492-503 [DOI] [PubMed] [Google Scholar]
- 34.Jiang W, Kahn SM, Zhou P, Zhang Y-J, Cacace AM, Infante AS, Doi S, Saantella RM, Weinstein IB: Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene 1993, 8:3447-3457 [PubMed] [Google Scholar]
- 35.Quelle DE, Ashmun RA, Shurleff SA, Kato J, Bar-Sagi D, Roussel M, Sherr CJ: Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev 1993, 7:1559-1571 [DOI] [PubMed] [Google Scholar]
- 36.Lovec H, Sewing A, Lucibello FC, Müller R, Möröy T: Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene 1994, 9:323-326 [PubMed] [Google Scholar]
- 37.Lovec H, Grzeschiczek A, Kowalski MB, Möröy T: Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J 1994, 13:3487-3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez L, Fest T, Cazorla M, Teruya-Feldstein J, Bosch F, Peinado MA, Piris MA, Montserrat E, Cardesa A, Jaffe ES, Campo E, Raffeld M: p53 gene mutations and protein overexpression are associated with aggressive variants of mantle cell lymphomas. Blood 1996, 87:3351-3359 [PubMed] [Google Scholar]
- 39.Sgambato A, Zhang Y-J, Arber N, Hibshoosh H, Doki Y, Ciaparrone M, Santella RM, Cittadini A, Weinstein IB: Deregulated expression of p27Kip1 in human breast cancers. Clin Cancer Res 1997, 3:1879-1887 [PubMed] [Google Scholar]
- 40.Han EK-H, Begemann M, Sagambato A, Soh JW, Doki Y, Xing WQ, Liu W, Weinstein B: Increased expression of cyclin D1 in a murine mammary epithelial cell line induces p27kip1, inhibits growth, and enhances apoptosis. Cell Growth Differ 1996, 7:699-710 [PubMed] [Google Scholar]