Abstract
Because hepatocyte growth factor (HGF) is a potent mitogen for normal human exocrine pancreas cells (NPCs) in vitro, we have analyzed the expression of HGF and its receptor, Met, in NPC and pancreas cancer cells and studied its effects in vitro. Using immunohistochemistry, Northern blotting, and reverse transcription-polymerase chain reaction, we examined the expression of HGF and Met in normal pancreas and pancreas cancer. Scatter assays, wound-healing assays, and migration through transwell filters were used to study HGF-stimulated motility of IMIM-PC-2 cancer cells. In tumors, HGF is mainly detected in stromal cells, whereas Met is overexpressed in cancer cells with an unpolarized distribution. In vitro, HGF stimulates motogenesis but not proliferation in cancer cells. Cell motility is accompanied by a rapid decrease in the cytoskeleton-bound E-cadherin, an acceleration of cellular adhesion to the substrate, an up-regulation of urokinase plasminogen activator (u-PA) RNA and protein, and a change in the solubility and proteolysis of the u-PA receptor. Cell motility is significantly reduced by inhibitors of u-PA proteolytic activity such as antibodies neutralizing u-PA activity, plasminogen activator inhibitor 1, and amiloride. These results show that a paracrine loop of HGF activation may participate in the development or progression of pancreas cancer. In vitro, the HGF-stimulated motogenesis of pancreas cancer cells involves the activation of the u-PA/u-PA receptor proteolytic system, suggesting its role in the invasive stages of tumor progression.
Hepatocyte growth factor (HGF), identified also as scatter factor, 1 is a potent mitogen for primary hepatocyte cultures and a variety of other epithelial cell types, including normal exocrine pancreas, an inducer of tubular networks and a scatter factor for epithelial cells. 2-7 Therefore, HGF has mitogenic, morphogenetic, and motogenic activity. The receptor for HGF, Met, is a heterodimeric membrane tyrosine kinase synthesized as a 170-kd precursor that is proteolytically cleaved to 50-kd and 145-kd subunits, and it was initially identified because of its transforming activity as part of a translocation in HOS cells treated with N-methyl-N-nitro-N-nitrosoguanidine. 8
Experimental evidence has led to the proposal that HGF and Met are involved in development, tissue regeneration, and tumor progression through autocrine, paracrine, and endocrine mechanisms. First, HGF is mainly produced by mesenchymal cells, whereas Met is mainly expressed in epithelial cells. 4,8 During mouse development, HGF is detected in mesenchymal cells in the vicinity of Met-expressing epithelia. 9 In addition, mice in which the HGF gene has been inactivated by homologous recombination fail to develop a normal liver and placenta. 10,11 Second, after partial hepatectomy, HGF production is induced in sinusoidal endothelial cells in the remnant liver as well as in other organs. 5 Third, numerous reports have described the overexpression of Met in a variety of tumor types. 12,13 Transfection of NIH 3T3 cells with the murine met cDNA leads to increased invasiveness in vitro and metastatic capacity in vivo14; similarly, the establishment of an autocrine HGF/Met loop by transfection of HGF cDNA in human leiomyosarcoma cells increases tumorigenesis and confers metastatic capacity. 15 In addition, high HGF levels have been associated with more aggressive bladder cancers. 16 Altogether, these findings suggest a role for HGF/Met in tumor progression.
The mechanisms through which HGF exerts its pleiotropic effects have been only partially elucidated. The HGF-induced scatter response is dependent on phosphatidylinositol-3-OH kinase and Rac activation, 17,18 the mitogenic effect requires stimulation of the Ras-mitogen-activated protein kinase cascade, 19 while tubulogenesis is dependent on stimulation of the signal transducers and activators of transcription pathway. 20 Downstream of these effects, a role for the plasmin proteolytic system has been proposed. In vitro, HGF increases the expression of urokinase plasminogen activator (u-PA) and its receptor (u-PAR), 15,21,22 and pro-HGF, secreted as a single-chain peptide, can be activated by u-PA 23 and by tissue plasminogen activator (t-PA). 24
We have described that HGF is a potent mitogen for normal human exocrine pancreas cells (NPCs). 6 Because a strong desmoplastic reaction is a hallmark of pancreas cancer, 25 we and others have proposed that the HGF/Met autocrine/paracrine loop might be involved in pancreas cancer development or progression. 6,26-28 The availability of cultures of normal and neoplastic pancreatic epithelial cells provides a unique opportunity to study in a systematic way the differential effects of HGF on the proliferation, migration, and cell-cell interactions of both normal and neoplastic epithelia.
In this work, we have first compared the expression and distribution of Met and HGF in normal and neoplastic pancreas and show that the receptor is overexpressed in tumor cells and HGF is present mainly in mesenchymal cells. Second, we have examined the effects of HGF in vitro on proliferation and motility of NPCs and pancreas cancer cells. NPCs display mainly a proliferative response, whereas pancreas cancers respond predominantly with scattering. Because the plasmin proteolytic system, activated by t-PA and u-PA, is involved in normal and pathological forms of cell invasiveness, 29,30 we investigated its role in the HGF-stimulated motility of IMIM-PC-2 using a variety of motility assays and found that u-PA plays a major role in migration. In addition, we have found that the HGF-stimulated motility is accompanied by changes in cell-cell and cell-substrate interactions.
Materials and Methods
Cell Culture and Reagents
The phenotypical properties of NPC and IMIM-PC-1, IMIM-PC-2, SK-PC-1, and SK-PC-3 pancreas cancer cell lines have been reported. 31,32 AsPC-1, RWP 1, and RWP 2 pancreas cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). MZ-PC-2, MZ-PC-3, and MZ-PC-4 were obtained from A. Knuth (Nordwest Krankenhaus, Frankfurt, Germany). MKN-45, a gastric carcinoma cell line in which met is amplified, was provided by J. Sakamoto (Aichi Cancer Center, Nagoya, Japan). Tumor cell cultures were maintained in 10% Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (FBS), as described. 32 Collagen type IV and laminin were obtained from Collaborative Biomedical Products (Bedford, MA). Recombinant human HGF was purified from transformed Chinese hamster ovary cells. The HGF preparation used was greater than 90% pure, and its biological activity was determined in a hepatocyte proliferation assay. Unless stated otherwise, HGF was used at 10 ng/ml. Amiloride and ε-amino caproic acid (EACA) were purchased from Sigma Chemical Co. (St. Louis, MO). Plasminogen activator inhibitor 1 (PAI-1) was kindly provided by Dr. N. Booth (University of Aberdeen, Aberdeen, UK). Plasminogen was purchased from Boehringer-Mannheim (Mannheim, Germany).
Antibodies
Mouse monoclonal antibody (mAb) 19S, raised against the bacterially expressed p50 form of human Met, and C28 rabbit polyclonal serum, raised against a 28-amino acid synthetic peptide corresponding to the C-terminal domain of human Met, were kindly provided by Dr. G. F. Vande Woude (National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, MD). met-3, met-6, and met-7 polyclonal antisera were generated by immunizing rabbits with synthetic peptides encompassing residues 468 to 485, 1449 to 1467, and 1308 to 1324 of human Met deduced sequence, respectively. 6 Mouse mAb 10C11, detecting human HGF, was kindly provided by Dr. E. M. Rosen (Long Island Jewish Medical Center, New York, NY), 33 and anti-E-cadherin antibody was obtained from Dr. A Cano (Instituto de Investigaciones Biomédicas, Madrid, Spain). Polyclonal rabbit anti-cytokeratin antiserum was a kind gift of Dr. S. Vilaró (Universitat de Barcelona, Barcelona, Spain). Neutralizing goat antibodies to u-PA (reference 398) and to t-PA (reference 387) and or rabbit anti u-PA antibodies (reference 389) were purchased from American Diagnostica (Greenwich, CT). Anti-u-PAR antibody was a kind gift of Dr. D Talarico (Ospedale San Raffaele, Milano, Italy). Peroxidase-coupled anti-rabbit immunoglobulin was purchased from Dakopatts (Glostrup, Denmark). Preimmune sera from rabbits immunized with C28 and met-7 and isotype-matched irrelevant mAbs were used as negative controls.
Cell Adhesion Assays
Assays were performed by plating 35S-labeled cells on collagen (10 μg/ml), laminin (10 μg/ml), or bovine serum albumin as described. 34
Scatter, Wound-Healing, and Cell Migration Assays
IMIM-PC-2 cells were seeded at approximately 2.5 × 103/cm 2 in complete medium and cultured for 24 to 48 h. Cells were washed twice with serum-free medium and serum starved for 24 h, and HGF (10 ng/ml) was added in medium without FBS. Scattering was evaluated 24 h later.
Confluent monolayers were serum starved for 24 h and washed with phosphate-buffered saline, and wounds were made with a pipette tip. After washing to remove cell debris, cultures were incubated in DMEM alone or in the presence of HGF. Healing was evaluated 24 h later.
In some experiments, cells cultured for four passages in medium containing plasminogen-depleted FBS were used. FBS was depleted of plasminogen by two consecutive passages on lysine-Sepharose columns and elution with 50 mmol/L benzamidine. Soluble u-PA and t-PA proteins were subsequently removed by chromatography on immobilized p-aminobenzamidine (Pierce, oud-Beijerland, the Netherlands).
Cell migration was assessed using 8 μm-pore Transwell culture chambers (Costar, Cambridge, MA). Quantitative determinations were obtained using overnight [3H]thymidine-labeled cells (1 μCi/2.5 × 10 5 cells/ml) seeded in medium with or without HGF placed in the bottom chamber. Forty-eight hours later, cells in the upper part of the filters were removed with a cotton swab, and filters were extensively washed with phosphate-buffered saline and cut, and radioactivity was quantitated in a β-scintillation counter. All experiments were performed in triplicate and repeated twice.
[3H]Thymidine Uptake Assays
Cells (2 × 104) were seeded in 24-well plates (Nunc) in complete medium supplemented with 1% FBS. Forty-eight hours later, HGF was added (10 ng/ml) for 12 h, and cultures were then labeled with 1 μCi/ml [3H]thymidine for an additional 18 hours. Cells were processed as described by Hiraki et al. 35 All measurements were carried out in triplicate, and all experiments were performed at least twice independently.
Western Blotting
Membrane fractions were prepared from preconfluent and 10-day postconfluent cells. Briefly, cells were sonicated in scraping buffer (0.1 mol/L sodium phosphate, pH 7.4, 5 mmol/L EDTA, 0.25 mol/L sucrose, 50 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride, aprotinin (10 μg/ml), and leupeptin (50 μg/ml)) and centrifuged at 650 × g for 10 minutes. Membranes were isolated by ultracentrifugation at 100,000 × g for 1 h at 4°C and resuspended in lysis buffer (5 mmol/L sodium phosphate, pH 7.4, 1 mmol/L EDTA, and 1% Triton X-100) supplemented with phenylmethylsulfonyl fluoride and aprotinin. Membrane protein samples (50 μg) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose filters. An even loading of all lanes was demonstrated by Western blotting with biotinylated wheat germ agglutinin (Vector Laboratories, Burlingame, CA) (10 μg/ml).
Triton X-100-soluble and -insoluble cell protein fractionation and immunoblotting were performed as described elsewhere. 36 Reacting antigens were visualized using a peroxidase-labeled secondary antibody and enhanced chemoluminiscence detection reagents (Amersham). Rabbit sera were used at a 1/200 dilution.
Zymography
The conditioned medium of cells cultured in the absence of FBS was centrifuged at 13,000 × g for 15 minutes at 4°C. Sample volumes were adjusted on the basis of protein concentration in the cell lysates, and proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis in plasminogen- and gelatin-containing gels, as described elsewhere. 37 The proteolytic activity was evidenced by incubating the gels in 2.5% Triton X-100 and transferring them to 0.1 mol/L glycine, pH 8.3, overnight at 37°C. After fixation, gels were stained with 0.1% amido black.
Domain-Selective Biotinylation Assays
Selective biotinylation of IMIM-PC-2 and SK-PC-1 cells was performed as described. 38
Northern Blotting
Total RNA was isolated using guanidine thiocyanate as described elsewhere. 39 Samples containing 15 μg of total cellular RNA were denatured, size fractionated using 1% formaldehyde agarose gel electrophoresis, transferred to nitrocellulose filters, and hybridized with the pMT2 human met cDNA probe (provided by Dr. PM Comoglio, University of Torino, Torino, Italy) labeled by the random priming method. 40
Reverse Transcription-Polymerase Chain Reaction
To detect HGF transcripts, RNA was isolated as described above. The following oligonucleotides were used for amplification of cDNA: forward, ATCAGACACCA CACCGGCACAAAT; reverse, GAAATAGGGCAATAATC CCAAGGAA. A total of 35 cycles of amplification were performed: 1 minute at 94°C, 30 seconds at 55°C, and 1.5 minutes at 72°C. The reverse transcription-polymerase chain reaction products were analyzed by ethidium bromide staining after electrophoresis in 1% agarose gels.
Immunohistochemical and Immunocytochemical Methods
Normal pancreas tissue was obtained from organ donors, and tumor samples were obtained from surgical specimens. The indirect immunoperoxidase method was used, as described elsewhere. 31 Reactions were developed with 3,3′-diaminobenzidine and nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate, respectively. Reactions were scored as “+++” when reactions were clearly seen at the ×100 magnification, “++” when they were clearly seen at the ×200 magnification, and “+” when definite reactions could only be seen at the ×400 magnification. In all assays, control preimmune antiserum or irrelevant mouse mAb was used and found to be unreactive. Inhibition assays with synthetic peptides or with HGF were performed to establish the specificity of reactions of anti-Met rabbit sera and anti-HGF mAb 10C11, respectively.
Confocal immunofluorescence analysis was performed on cells cultured on sterile SonicSeal plastic slides (Nunc). Cells were fixed with 4% paraformaldehyde for 20 minutes at 4°C, incubated with anti E-cadherin antibody, washed, and incubated with rhodamine-coupled rabbit anti-mouse immunoglobulin (Pierce). Reactions were visualized using a Leica TCS 4D confocal microscope.
Results
Met and HGF Expression in Pancreas Tumor Tissues
As previously described, 6 antibodies detecting Met were weakly reactive with the apical membrane of ductal cells in normal pancreas (Figure 1, A and B) ▶ . In contrast, stronger reactivity with the cytoplasm of neoplastic cells in 20 of 20 pancreas adenocarcinomas was observed (Figure 1, C and D) ▶ , although there were slight differences in the proportion and intensity of reactive cells depending on the antiserum used. Met expression was similar in primary and metastatic tumors.
Figure 1.
Met expression in normal pancreas and pancreas cancer tissues. In normal pancreas ducts, Met is apically distributed (A and B). In pancreas cancers, Met is mainly distributed in the cytoplasm and is overexpressed (C and D). A and C: Preimmune rabbit serum; B and D: met-7 serum. Original magnification: ×400.
HGF expression was examined using mAb 10C11. 33 In normal pancreas, HGF was detected in mesenchymal or mesenchyme-derived cells (Figure 2, A and B) ▶ . In 11 of 13 tumors, immunoreactive HGF was detected in nonneoplastic cells with a mesenchymal morphology surrounding tumor glands (Figure 2, C and D) ▶ . Double immunostaining for HGF and cytokeratins showed that in most tumors HGF-producing cells lacked cytokeratin expression (not shown). In two cases, co-expression of HGF and cytokeratins in the same cells suggests that tumor cells produce HGF. Expression of HGF in connective tissue cells was observed both in areas of tumor and in tumor-associated obstructive pancreatitis. The specificity of the reactions obtained with this antibody was determined by preincubation with HGF or nerve growth factor. HGF completely blocked antibody binding, whereas nerve growth factor had no effect.
Figure 2.

HGF expression in normal pancreas and pancreas cancer tissues. In normal pancreas, few HGF-expressing cells are present in the mesenchyme surrounding the ducts (A and B). In pancreas cancer tissues, strong HGF expression is detected in stromal areas (C and D). A and C: Anti-HGF mouse mAb 10C11 preincubated with HGF (100 μg/ml); A through D: mAb 10C11. Original magnification: ×200.
Thus, a high Met expression in tumor cells and the detection of HGF in the peritumoral mesenchyme suggest the establishment of a paracrine, and perhaps autocrine, loop of Met activation in pancreatic tumors.
Met and HGF Expression in Normal and Neoplastic Exocrine Pancreas Cultures
To study the effects of HGF, we used a series of well-characterized normal and neoplastic cells and examined Met expression in vitro. When the normal exocrine fraction of the pancreas is cultured in vitro, NPCs rapidly lose acinar features and acquire phenotypic and functional properties characteristic of ductal cells. 31 The pancreas cancer cell lines selected for this study display a wide spectrum of ductal differentiated properties: postconfluent IMIM-PC-2 and SK-PC-1 cells form a polarized monolayer with domes and develop transmonolayer resistance; in contrast, IMIM-PC-1, SK-PC-3, and AsPC-1 cells are not polarized and display a less differentiated phenotype. 32
Using Northern blotting with total RNA, met transcripts were undetectable in normal pancreas tissue and in the exocrine fraction before culture (Figure 3A) ▶ . In contrast, a 7-kb transcript was detected in samples from NPCs and cultured tumor cells. Met protein levels were comparatively higher in four of five pancreas cancer cell lines than in normal cultures, but they were lower than in MKN45 gastric cancer cells, which harbor c-met gene amplification 41 (Figure 3B) ▶ . The predominant component detected in normal pancreas was the 170-kd precursor, whereas in tumor cells the 145-kd β-chain was relatively more abundant. In IMIM-PC-2 and SK-PC-1 cells, the two lines growing as a polarized monolayer, met RNA and protein levels were lower in postconfluence than in exponentially growing cells (Figure 3) ▶ . These results indicate that met RNA and protein are down-regulated in quiescent, differentiated cells.
Figure 3.
Met expression in pancreas cancer cell lines. A: met transcript levels in normal pancreatic tissue (NPT), NPCs, and pancreas cancer cell lines. In IMIM-PC-2 cells, expression was analyzed in preconfluent (p), 2 days postconfluent (2), and 10 days postconfluent cultures (10). Photographs of 28 S ribosomal RNA in ethidium bromide-stained gels are shown below each autoradiograph to normalize RNA deposits. B: Western blotting analysis of met using membrane fractions from preconfluent (p) and 10 days postconfluent (10) pancreas cancer cells. MKN45 and AsPC-1 cell membranes were obtained from preconfluent cultures. Equal amounts of protein were loaded in each lane (50 μg), and met was detected with mAb 19S. Two bands corresponding to 145 kd and 170 kd are detected. Normalization of protein deposits was performed by Ponceau red staining and wheat germ agglutinin lectin blotting (not shown).
In polarized IMIM-PC-2 and SK-PC-1 cells, Met protein was localized in both the apical and basolateral membrane compartments, as determined by domain-selective biotinylation (not shown).
None of these cell lines produced HGF RNA or protein, as determined by reverse transcription-polymerase chain reaction and by testing concentrated supernatants for scatter activity on IMIM-PC-2 cells, respectively (results not shown).
Mitogenic and Scattering Effects of HGF on Cultured Exocrine Pancreatic Cells
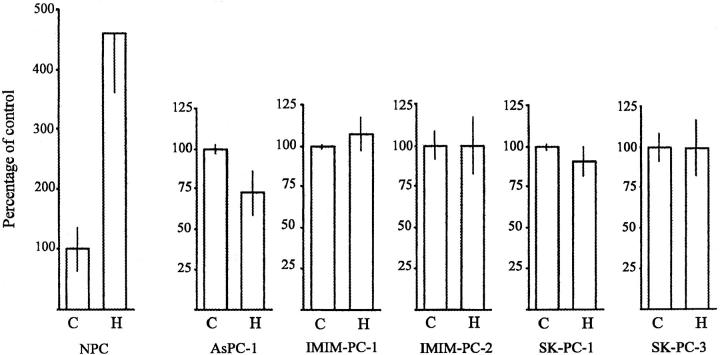
The effect of HGF on the proliferation of normal and tumor cells in vitro was examined under a variety of experimental conditions using [3H]thymidine uptake assays (Figure 4) ▶ or total protein determination (not shown). HGF strongly stimulated NPC proliferation 6 but did not significantly affect [3H]thymidine uptake in any of the five tumor cell lines studied. Similar results were obtained when HGF was added to cells cultured in medium supplemented with 10% or 1% serum (not shown). There was no association between the growth pattern of cells on plastic and in collagen gels, nor between the HGF-induced scatter effects on plastic and the tubulogenic effect in collagen.
Figure 4.
Effect of HGF on proliferation of NPC and pancreas cancer cells. Cells were plated in the absence (C) or in the presence (H) of HGF (10 ng/ml), and [3H]thymidine incorporation was determined as described in Materials and Methods. Results are expressed as the mean ± SD of triplicate cultures. Data are from one representative experiment of several performed.
HGF induced scattering of IMIM-PC-2 (Figure 5A) ▶ and AR-42J rat pancreas cancer cells but not of the other cells studied (data not shown). Similarly, HGF stimulated healing in wounded monolayers of IMIM-PC-2 cells (Figure 5B) ▶ and cell migration through polycarbonate porous filters (Figure 5C) ▶ .
Figure 5.

HGF stimulates IMIM-PC-2 pancreas tumor cell motility through activation of u-PA. Motogenesis was evaluated by scattering assays (A), wound-healing assays (B), and migration through polycarbonate transwell filters (C), performed as described in Materials and Methods. Cells grown in DMEM supplemented with normal FBS (a, b, and c) or in DMEM containing plasminogen-depleted FBS (d, e, and f) were serum starved for 24 h and incubated with HGF (20 ng/ml) (HGF+) overnight (A and B) or for 48 h (C). Control cells were incubated in DMEM alone (HGF−) or DMEM supplemented with other compounds (Plg+; plasminogen was added at 2 μg/ml). Inhibition of the HGF-stimulated motility was evaluated by incubating cells with neutralizing rabbit anti-u-PA antibodies (150 μg/ml) (a u-PA+), PAI-1 (50 μg/ml) (PAI-1+), amiloride (0.2 mmol/L) (Am.), and EACA (50 mmol/L). A and B show an experiment representative of several performed with similar results; C, data from one of two independent experiments (in triplicate samples). Original magnifications: A, frames a through c, ×200; A, frames d through f, and B, ×100.
Mechanisms Involved in HGF-Induced Cell Motility
One characteristic of tumor cells is their ability to move and invade tissues. For this reason, we examined in more detail the molecular mechanisms involved in HGF-stimulated motogenesis of IMIM-PC-2 pancreas tumor cells in vitro. Antibodies neutralizing u-PA activity (150 μg/ml), as well as the physiological inhibitor of plasminogen activators, PAI-1 (50 μg/ml), significantly inhibited the HGF-stimulated motility of IMIM-PC-2 cells (P < 0.049) (Figure 5C) ▶ , whereas anti t-PA antibodies had no effect. In addition, migration was prevented by EACA (50 mmol/L) and amiloride (0.2 mmol/L), a drug that inhibits u-PA but does not affect t-PA. 42 At the concentrations used, these agents did not have toxic effects on the cells, as demonstrated by measuring lactate dehydrogenase released to the medium. These results indicate that u-PA is likely the serine protease involved.
To analyze the role of plasmin in the HGF-induced scatter, cells cultured for four passages in plasminogen-depleted serum were used. Using both scatter and wound-healing assays, a reduced motility response of IMIM-PC-2 cells to HGF was observed in the absence of plasminogen (Figure 5, A and B ▶ ; compare b with e). Similar results were obtained in three independent assays. The addition of plasminogen (2 μg/ml) to the cells in the course of these assays restored full motility response to HGF (Figure 5A, f) ▶ . As control, addition of plasminogen (Figure 5A, d) ▶ or plasmin (not shown) in the absence of HGF failed to induce motility, suggesting that the protease by itself is not able to trigger motility in these cells. These results support that both active u-PA and plasmin participate in the motogenic response to HGF.
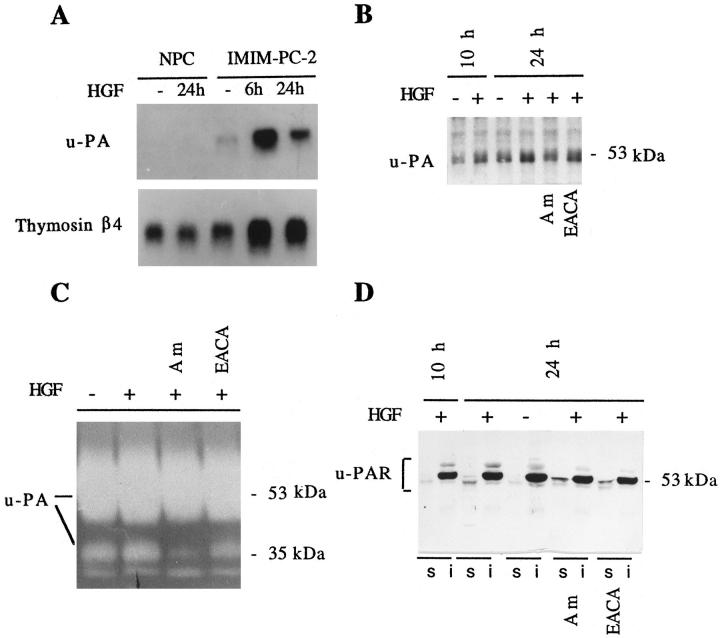
To confirm the involvement of u-PA in HGF-stimulated motility of IMIM-PC-2 cells, we analyzed the expression of u-PA, u-PAR and t-PA by Northern blotting, Western blotting, and gelatin zymography (Figure 6) ▶ . Although these cells constitutively express u-PA, HGF selectively induced a 5-fold increase in u-PA transcript levels at 6 hours of treatment, which decreased to 1.7-fold after 24 h. However, in NPCs, the levels of u-PA transcripts did not change on HGF treatment; in these experiments, levels of thymosin β4 mRNA were examined for comparison, because they are not affected by proliferation in these cells. 43
Figure 6.
HGF affects the levels of expression of u-PA and the localization of u-PAR in pancreas cells. Cells were serum starved for 24 h and treated with HGF (20 ng/ml) for the time periods shown, in the absence or in the presence of amiloride (Am) (0.2 mmol/L) or EACA (50 mmol/L), conditions not permissive for scatter. A: Northern blotting with total RNA (15 μg) from NPCs and IMIM-PC-2 pancreas cancer cells was hybridized to u-PA cDNA probe and subsequently to a thymosin β4 cDNA probe to normalize for RNA deposits. Filters were exposed for 48 h to autoradiography. B: Equal amounts of protein (50 μg) from cell lysates were analyzed by Western blotting with anti-u-PA antibody. C: Twenty-four hour conditioned medium (20 μl) from the same experiment shown in B was analyzed by gelatin zymography in the presence of plasminogen as a substrate. D: Equal amounts of 1% Triton X-100-soluble proteins (s) (50 μg) and equivalent amounts of the insoluble fractions (i) were analyzed by Western blotting using anti-u-PAR antibody. Mobility markers correspond to ovalbumin (53 kd) and carbonic anhydrase (35 kd).
u-PA protein in cell lysates from IMIM-PC-2 cells also showed a slight increase (1.6-fold) on HGF treatment (Figure 6B) ▶ . The lower induction of u-PA at the protein level may result from its rapid secretion. The levels of other proteases such as t-PA, gelatinases A and B, and stromelysin in the conditioned medium from HGF-treated cells were unaffected, as examined by gelatin zymography (not shown). However, a broad band of approximately 54 kd corresponding to u-PA and a minor band of 35 kd, corresponding to the low molecular weight form of active u-PA (LMW u-PA) generated on pro-u-PA activation, were observed in plasminogen/gelatin substrates (Figure 6C) ▶ . Cells treated with HGF and amiloride, conditions not permissive for scatter, showed a decrease in the level of the LMW u-PA, suggesting that inhibition of u-PA prevented plasmin generation and further proteolytic activation of pro-u-PA. However, in cells treated with HGF and EACA, the LMW u-PA is produced, although scatter is still inhibited. This was expected, because EACA acts by competing for plasminogen/plasmin-binding sites at the cell membrane but does not inhibit plasmin proteolytic activation of soluble pro-u-PA. These results indirectly suggest that it is the presence of active u-PA and/or plasmin at the cell membrane that is important for the HGF-induced scattering.
Because u-PAR is essential to focus u-PA activity at the cell surface, we analyzed HGF effects on this protein. This receptor is anchored to the membrane through a glycosylphosphatidylinositol (GPI) bond, and it has been localized in caveolae. 44 Because GPI-linked proteins in caveolae distribute to the 1% Triton X-100-insoluble phase, 45 we used Triton X-100 fractionation to examine receptor levels and distribution. Both in untreated and in HGF-treated IMIM-PC-2 cells, u-PAR was predominantly detected in the insoluble fraction (Figure 6D) ▶ . However, HGF induced an increase of u-PAR in the soluble phase, which was unaffected by the presence of amiloride or EACA. In this fraction, u-PAR was detected as a doublet: in the presence of plasminogen activator inhibitors, the lower mobility form was predominant; in contrast, in the absence of inhibitors, the higher mobility form was more abundant (Figure 6D) ▶ , indicating that the inhibition of u-PA activity affects the electrophoretic mobility of u-PAR. The two forms of u-PAR observed may correspond to the conformational receptor variants resulting from proteolysis already described. 46,47
These results suggest that, in pancreatic tumor cells, an active u-PA/u-PAR complex is necessary, although not sufficient, to mediate the effects of HGF on cellular motility.
The Scatter Effect Induced by HGF Is Associated with Changes in Cell-Cell Contacts and Cell-Substrate Adhesion
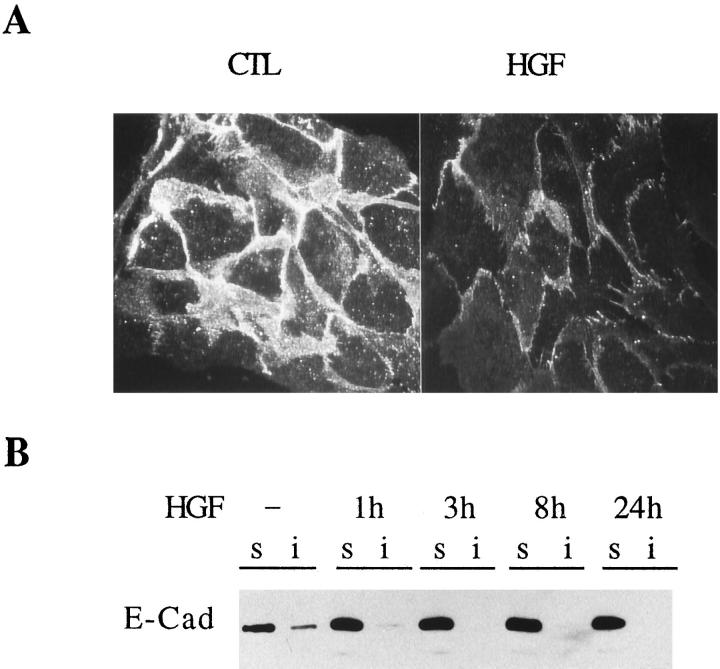
Because E-cadherin is involved in homotypic interactions in epithelial cells and HGF induces a loss of cell-cell contacts, the effect of HGF on the levels and distribution of E-cadherin in IMIM-PC-2 cells was examined using confocal microscopy and Western blotting (Figure 7) ▶ . In serum-starved IMIM-PC-2 cells, E-cadherin was membrane associated and distributed to both the Triton X-100-soluble and -insoluble fractions. HGF induced a decrease in the levels of membrane-associated E-cadherin, as indicated by immunofluorescence assays using nonpermeabilized cells and by a redistribution from the insoluble to the soluble fraction. Although the total levels of the protein were unchanged, E-cadherin was no longer detected in the insoluble fraction after 3 hours of treatment with HGF (Figure 7B) ▶ . In contrast, no changes in the pattern of expression of E-cadherin were detected on HGF treatment of NPC (not shown). These results indicate that HGF-induced scatter is associated with a rapid dissociation of E-cadherin from the cytoskeleton, which has been shown to correlate with a loss of functionality of the protein. 48
Figure 7.
HGF affects the subcellular distribution of E-cadherin. Twenty-four hour serum-starved IMIM-PC-2 cells were treated with HGF (20 ng/ml) for different time periods. A: Unpermeabilized, 4% paraformaldehyde-fixed cells were incubated with anti-E-cadherin antibody. Rhodamine-coupled secondary antibody was used to reveal immunoreactivity, and photographs were taken by confocal microscopy. CTL, control cells; HGF, cells treated with HGF for 24 hours. B: Equal amounts of 1% Triton X-100-soluble (s) (50 μg) proteins and equivalent amounts of the insoluble (i) fractions from untreated or HGF-treated cells for the indicated time points were analyzed by Western blotting using anti-E-cadherin antibody.
In cells undergoing scatter, one of the early events observed is the switch to a flattened morphology with increased attachment to the substrate. 17,34 To determine whether the effects induced by HGF are also mediated by changes in cell-substrate adhesion, NPCs and IMIM-PC-2 cells were treated with HGF, labeled, and seeded on plastic plates coated with collagen type IV, laminin, or bovine serum albumin. Adhesion of NPCs to substrate was unaffected by HGF treatment, whereas pretreatment of IMIM-PC-2 cells with HGF resulted in a 25% increase in adhesion to plates coated with bovine serum albumin 30 minutes after seeding, but not at later time points (results not shown). Thus, IMIM-PC-2 cell scattering is preceded by a faster adhesion to the substrate.
Discussion
A detailed comparison of the effects of HGF on cultured normal cells and tumor cells displaying a similar differentiation phenotype has seldom been reported; our results indicate that normal and neoplastic pancreatic cells respond to HGF differently in terms of both motility and proliferation. In pancreas cancer tissues, all of the components necessary for a paracrine Met/HGF activation loop can be detected and, in pancreatic tumor cells, HGF induces motogenesis in vitro first by down-regulating the functional E-cadherin and later by activating the plasmin proteolytic system.
In agreement with prior studies, 26-28 we find that Met is overexpressed in ductal-type adenocarcinomas of the pancreas; however, we also observe that its subcellular distribution in normal ducts and in tumor cells is different. In the former, Met is mainly detected in the apical membrane using a panel of antibodies raised against three different synthetic peptides of the Met sequence (this work and Ref. 6 ). Similarly, Tsarfaty et al. have reported apical expression in the fetal gastrointestinal epithelium in mice. 49 In contrast, in pancreatic tumor tissues, Met protein is mainly found in the cytoplasm of cancer cells and, in polarized cultured tumor cells, it is present both in the apical and basolateral membranes. A basolateral distribution has been reported for Met in T84 and MDCK cells. 50,51 The abnormal subcellular distribution of Met in tumor with respect to normal pancreatic cells may increase the availability of mesenchyme-derived HGF to tumor cells. Clearly, more work is necessary to establish the mechanisms leading to the variable membrane distribution of Met as well as the functional implications derived from it.
HGF was detected in mesenchymal cells of pancreas cancer tissues and, in a few cases, in tumor cells as well, although not in cultured tumor cell lines. Recently, the production of HGF by some neoplastic epithelial cells has been reported. 52 Because HGF and c-met mRNA expression can be regulated by inflammatory cytokines (ie, interleukin 1 and tumor necrosis factor), 53,54 production of HGF by fibroblasts, macrophages, or neoplastic cells in the tumor may require complex molecular interactions that are lost on selective culture of pancreas cancer cells.
In vitro, HGF induced different effects on NPC and pancreas cancer cells; while the former respond with proliferation, the latter respond mainly with increased motility. The distinct mitogenic and motogenic responses observed in normal and neoplastic cells may be a reflection of the constitutive K-ras activation resulting from codon 12 mutations present in the pancreas cancer cells. 32 This observation may support previous findings indicating that when Ras is constitutively activated, further stimulation of mitogenic signal transduction pathways is ineffective. 19,55 In HPAF cells, a modest increase of cell proliferation induced by HGF has been reported. 27
We have shown that the full motogenic response of IMIM-PC-2 cells to HGF is dependent on the activation of the u-PA/u-PAR system and on plasmin generation. Cell migration was reduced in the absence of plasminogen and significantly decreased by specific inhibitors of u-PA proteolytic activity, including neutralizing anti-u-PA antibodies, PAI-1, and amiloride. In agreement with these findings, plasminogen-deficient mice show severe impairment of keratinocyte migration in wounded areas. 56 Similarly, HGF-induced tubulogenesis of MDCK cells in fibrin gels is reduced in the presence of inhibitors of serine proteases, suggesting a role for plasminogen activators in the matrix remodeling required for tubulogenesis. 57 However, to our knowledge the present study is the first description of the inhibition of the HGF-stimulated cell scatter by inhibitors of urokinase activity. These observations suggest that protease activation is not only important for extracellular matrix degradation but also for the degradation of other cellular components, eg, cell surface adhesion molecules. Recently, the proteolytic degradation of the ectodomain of membrane molecules has been implicated in the response to phorbol esters and growth factors. 58
Relevant to the effect of HGF on motogenesis may also be the redistribution and cleavage of the u-PAR. HGF increased the levels of u-PAR present in the Triton X-100-soluble fraction, whereas no major changes were observed in the insoluble phase enriched in GPI-linked proteins. Caveolin and GPI-linked proteins are particularly abundant in caveolae, membrane invaginations implicated in potocytosis and transcytosis of macromolecules, 45 and u-PAR has been localized in caveolae. 44 Thus, in IMIM-PC-2 cells u-PAR is in the fraction of proteins corresponding to caveolae, whereas, in HGF-stimulated cells, it also localizes in another compartment, soluble in Triton X-100. This situation is reminiscent of the receptor for the protease tissue factor, which is active at the cell surface in a compartment different from caveolae. Redistribution to caveolae is concomitant with the down-regulation of its function by formation of a complex with the inhibitor. 59 HGF treatment of IMIM-PC-2 cells resulted in a decrease in the electrophoretic mobility of the u-PAR, and this change was inhibited by amiloride and EACA, supporting the role of u-PA in the proteolytic processing of the receptor. 60 Recently, it has been shown that only the proteolysed soluble form of u-PAR is active in signal transduction during the chemotactic response. 61 Therefore, we propose that the active u-PAR complexed to u-PA that participates in the motility response is represented by the proteolysed Triton X-100-soluble form of the receptor.
In agreement with recent reports, 62,63 the inhibition of the HGF-mediated IMIM-PC-2 cell motility by PAI-1 would point to a role for PAI-1 in blocking cell-substrate adhesion and migration mediated by u-PAR and/or integrin complex. In those reports, it is proposed that u-PA releases this inhibition by physically sequestering PAI-1, thus uncovering vitronectin attachment sites that become available for interaction with integrin/u-PAR complex on the cell membrane. Our data support those findings and, in addition, indicate that proteolytic activity of u-PA is also determinant for cell movement, conceivably affecting the conformation of the u-PAR, which increases its affinity for the substrate (vitronectin). 63
HGF-induced scattering was also accompanied by a decrease in the Triton X-100-insoluble, cytoskeleton-associated E-cadherin, which has been defined as the functional form of the protein. This novel finding would be in agreement with the observation that epithelial cells that display a disperse growth pattern do not express functional E-cadherin, unlike epithelial cells growing as compact colonies, 36,64 and with more recent observations showing that E-cadherin-mediated cell-cell adhesion depends on Tiam and Rac signaling. 65 Ectopic expression of these proteins increases cell adhesion mediated by E-cadherin and blocks the HGF-induced scatter of MDCK cells. Thus, HGF acts by reducing the functionality of E-cadherin, although expression levels are not changed, as also described by other investigators. 50,64,66 The change in solubilization of E-cadherin is paralleled by a modest but rapid and transient increase in cell adhesion to the substrate. In addition, we observed that the chronic treatment of IMIM-PC-2 cells with HGF is accompanied by a flattened, scattered morphology and increased cell-substrate adhesion (unpublished data). These observations are common to other scatter-inducing factors: scattering induced by TPA on HT-29 M6 cells is accompanied by a decreased homotypic aggregation, enhanced attachment to the substrate, and changes in the functionality of E-cadherin. 34 In contrast to the effects observed in pancreas tumor cells, HGF stimulates mitogenesis but does not affect cell motility, E-cadherin functionality, and the induction of u-PA in NPCs. These observations support the notion that proliferative and motogenic signals go through different pathways that, as mentioned above, may be mutually exclusive.
The redistribution of E-cadherin to a nonfunctional compartment, the activation of the u-PA proteolytic system, 21,67 and the increased expression of u-PA and u-PAR in invasive cells 15 are mechanisms through which HGF is able to confer cells with properties that lead to tumor progression and invasion.
Acknowledgments
We thank M. Garrido and M. C. Torns for excellent technical assistance; the investigators mentioned in the text for providing reagents; and D. Andreu, A. García de Herreros, A. Skoudy, and T. Thomson for valuable contributions.
Footnotes
Address reprint requests to Francisco X. Real, Institut Municipal d’Investigació Mèdica, Dr. Aiguader, 80, 08003-Barcelona, Spain. E-mail: preal@imim.es.
RP and MRV contributed equally to this paper.
Supported in part by grants from Comisión Interministerial de Ciencia y Tecnologia (SAF94-0971 and SAF97-0084), Comissió Interdepartamental de Recerca i Innovació Tecnològica (CIRIT) (Generalitat de Catalunya) (GRQ93-9301), and Marató-TV3. MRV is a recipient of a Beca de Formación del Personal Investigador (Ministerio de Educación, Madrid). TA is a recipient of a Beca Predoctoral from CIRIT.
References
- 1.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W: Scatter factor: molecular characteristics and effect on the invasiveness. J Cell Biol 1990, 111:2097-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gherardi E, Stoker M: Hepatocytes and scatter factor. Nature 1991, 346:228. [DOI] [PubMed] [Google Scholar]
- 3.Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, Birchmeier W: Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci USA 1991, 88:7002-7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen EM, Nigam SK, Goldberg ID: Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol 1994, 127:1783-1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarnegar R, Michalopoulos GK: The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol 1995, 129:1177-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilá MR, Nakamura T, Real FX: Hepatocyte growth factor is a potent mitogen for normal human pancreas cells in vitro. Lab Invest 1995, 73:409-418 [PubMed] [Google Scholar]
- 7.Montesano R, Matsumoto K, Nakamura T, Orci L: Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 67:901-908 [DOI] [PubMed] [Google Scholar]
- 8.Cooper CS: The met oncogene: from detection by transfection to transmembrane receptor for hepatocyte growth factor. Oncogene 1992, 7:3-7 [PubMed] [Google Scholar]
- 9.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C: Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 1993, 123:223-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschlesche W, Sharpe M, Gherardi E, Birchmeier C: Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373:699-702 [DOI] [PubMed] [Google Scholar]
- 11.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N: Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373:702-705 [DOI] [PubMed] [Google Scholar]
- 12.Rong S, Jeffers M, Resau JH, Tsarfaty I, Oskarsson M, Vande Woude GF: Met expression and sarcoma tumorigenicity. Cancer Res 1993, 53:5355–5360 [PubMed]
- 13.Ferracini R, Di Renzo MF, Scotland K, Baldini N, Olivero M, Lollini PL, Cremona O, Campanacci M, Comoglio PM: The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene 1995, 10:739-749 [PubMed] [Google Scholar]
- 14.Rong S, Segal S, Anver M, Resau JH, Vande Woude GF: Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA 1994, 91:4731–4735 [DOI] [PMC free article] [PubMed]
- 15.Jeffers M, Rong S, Vande Woude GF: Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol Cell Biol 1996, 16:1115–1125 [DOI] [PMC free article] [PubMed]
- 16.Joseph A, Weiss GH, Jin L, Fuchs A, Chowdhury S, O’Shaughnessy P, Goldberg ID, Rosen EM: Expression of scatter factor in human bladder carcinoma. J Natl Cancer Inst 1995, 87:372-376 [DOI] [PubMed] [Google Scholar]
- 17.Ridley AJ, Comoglio PM, Hall A: Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. J Cell Biol 1995, 15:1110-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royal I, Park M: Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem 1995, 270:27780-27787 [DOI] [PubMed] [Google Scholar]
- 19.Ponzetto C, Zhen Z, Audero E, Maina F, Bardelli A, Basile ML, Giordano S, Narsimhan R, Comoglio P: Specific uncoupling of GRB2 from the Met receptor. J Biol Chem 1996, 271:14119-14123 [DOI] [PubMed] [Google Scholar]
- 20.Boccaccio C, Andò M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM: Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 1998, 391:285-288 [DOI] [PubMed] [Google Scholar]
- 21.Pepper MS, Matsumoto K, Nakamura T, Orci L, Montesano R: Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and u-PA receptor expression in Madin-Darby canine kidney epithelial cells. J Biol Chem 1992, 267:20493-20496 [PubMed] [Google Scholar]
- 22.Boccaccio C, Gaudino G, Gambarotta G, Galimi F, Comoglio PM: Hepatocyte growth factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGF. J Biol Chem 1994, 269:12846-12851 [PubMed] [Google Scholar]
- 23.Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM: Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J 1992, 11:4825-4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mars WM, Zarnegar R, Michalopoulos GK: Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol 1993, 143:949-958 [PMC free article] [PubMed] [Google Scholar]
- 25.Kern HF, Elsässer H-P: Fine structure of human pancreatic adenocarcinoma. Go VLW DiMagno EP Gardner JD Lebenthal E Reber HA Scheele GA eds. The Pancreas: Biology, Pathobiology, and Disease. 1993, :pp 857-869 Raven Press, New York [Google Scholar]
- 26.Ebert M, Yokoyama M, Friess H, Büchler MW, Korc M: Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 1994, 54:5775-5778 [PubMed] [Google Scholar]
- 27.Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR: Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 1995, 55:1129-1138 [PubMed] [Google Scholar]
- 28.Furukawa T, Duguid WP, Kobari M, Matsuno S, Tsao M-T: Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol 1995, 147:889-895 [PMC free article] [PubMed] [Google Scholar]
- 29.Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS, Skriver L: Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 1985, 44:139-266 [DOI] [PubMed] [Google Scholar]
- 30.Kwaan HC: The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev 1992, 11:291-311 [DOI] [PubMed] [Google Scholar]
- 31.Vilá MR, Lloreta J, Real FX: Normal human pancreas cultures display functional ductal characteristics. Lab Invest 1994, 71:423-431 [PubMed] [Google Scholar]
- 32.Vilá MR, Lloreta J, Schüssler MH, Berrozpe G, Welt S, Real FX: New pancreas cancer cell lines which represent distinct stages of ductal differentiation. Lab Invest 1995, 72:395-404 [PubMed] [Google Scholar]
- 33.Bhargava M, Joseph A, Knesel J, Halaban R, Li Y, Pang S, Goldberg I, Setter E, Donovan MA, Zarnegar R, Michalopoulos GA, Nakamura T, Faletto D, Rosen EM: Scatter factor and hepatocyte growth factor: activities, properties, and mechanisms. Cell Growth Differ 1992, 3:11-20 [PubMed] [Google Scholar]
- 34.Fabre M, García de Herreros A: Phorbol ester-induced scattering of HT-29 human intestinal cancer cells is associated with down-modulation of E-cadherin. J Cell Sci 1993, 106:513–522 [DOI] [PubMed]
- 35.Hiraki Y, Rosen OM, Birnbaum MJ: Growth factors rapidly induce expression of the glucose transporter gene. J Biol Chem 1988, 263:13655-13662 [PubMed] [Google Scholar]
- 36.Skoudy A, Llosas MM, García de Herreros A: Intestinal HT-29 cells with dysfunction of E-cadherin show increased pp60src activity, and tyrosine phosphorylation of p120-catenin. Biochem J 1996, 317:279–284 [DOI] [PMC free article] [PubMed]
- 37.Heussen C, Dowdle EB: Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrate. Anal Biochem 1990, 102:196-202 [DOI] [PubMed] [Google Scholar]
- 38.Lisanti MP, Le Bivic A, Saltiel AR, Rodríguez-Boulan E: Preferred apical distribution of glycosyl phosphatidylinositol (GPI)-anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J Membr Biol 1990, 113:155-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chomczynski P, Sacchi N: Single-step method for RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 40.Feinberg AP, Volgelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 1983, 132:6-13 [DOI] [PubMed] [Google Scholar]
- 41.Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM: Tyrosine kinase receptor indistinguishable from the c-Met protein. Nature 1989, 339:155-156 [DOI] [PubMed] [Google Scholar]
- 42.Vassalli JD, Belin D: Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett 1987, 214:187-191 [DOI] [PubMed] [Google Scholar]
- 43.Paciucci R, Berrozpe G, Torà M, Navarro E, García de Herreros A, Real FX: Isolation of tissue-type plasminogen activator, cathepsin H, and non-specific cross-reacting antigen from SK-PC-1 pancreas cancer cells using subtractive hybridization. FEBS Lett 1996, 385:72–76 [DOI] [PubMed]
- 44.Stahl A, Mueller BM: The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol 1995, 129:335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisanti MP, Scherer PE, Vidgiriene J, Tang ZL, Hermanowski-Vosatka A, Tu Y-H, Cook RF, Sargiacomo M: Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 1994, 126:111-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behrendt N, Ronne E, Ploug M, Petri T, Løbert D, Nielsen LS, Schleuning W-D, Blasi F, Apella E, Dano K: The human receptor for urokinase plasminogen activator: NH2-terminal amino acid sequence and glycosylation variants. J Biol Chem 1990, 265:6453-6460 [PubMed] [Google Scholar]
- 47.Ploug M, Ellis V, Dano K: Ligand interaction between urokinase-type plasminogen activator and its receptor probed with 8-anilino-1-naphthalenesulfonate: evidence for a hydrophobic binding site exposed on the intact receptor. Biochemistry 1994, 33:8991-8997 [DOI] [PubMed] [Google Scholar]
- 48.Hinck L, Nelson WJ, Papkoff J: Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol 1994, 124:729-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsarfaty I, Resau JH, Rulong S, Keydar I, Faletto DL, Vande Woude GF: The met proto-oncogene receptor, and lumen formation. Science 1992, 257:1258–1261 [DOI] [PubMed]
- 50.Nusrat A, Parkos CA, Bacarra AE, Godowski PJ, Delp-Archer C, Rosen EM, Madara JL: Hepatocyte growth factor/scatter factor effects on epithelia: regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest 1994, 93:2056-2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crepaldi T, Pollack AL, Prat M, Zborek A, Mostov K, Comoglio PM: Targeting of the SF/HGF receptor to the basolateral domain of polarized epithelial cells. J Cell Biol 1994, 125:313-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin L, Fuchs A, Schnitt SJ, Yao Y, Joseph A, Lamszus K, Park M, Goldberg ID, Rosen EM: Expression of scatter and c-met receptor in benign and malignant breast tissue. Cancer 1996, 79:749-760 [DOI] [PubMed] [Google Scholar]
- 53.Tamura M, Arakaki N, Tsubouchi H, Takada H, Daikuhara Y: Enhancement of human hepatocyte growth factor production by interleukin-1α and 1β and tumor necrosis factor-α by fibroblasts in culture. J Biol Chem 1993, 268:8140-8145 [PubMed] [Google Scholar]
- 54.Moghul A, Lin L, Beedle A, Kanbour-Shakir A, DeFrances MC, Liu Y, Zarnegar R: Modulation of c-met proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-met transcript. Oncogene 1994, 9:2045-2052 [PubMed] [Google Scholar]
- 55.Thomson TM, Green SH, Trotta RJ, Burstein DE, Pellicer A: Oncogene N-ras mediates selective inhibition of c-fos induction by nerve growth factor, and basic fibroblast growth factor in a PC12 cell line. Mol Cell Biol 1990, 10:1556-1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, Dano K: Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med 1996, 2:287-292 [DOI] [PubMed] [Google Scholar]
- 57.Montesano R, Orci L: Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell 1985, 42:469-477 [DOI] [PubMed] [Google Scholar]
- 58.Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rose-John S, Massagué J: Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem 1996, 271:11376-11382 [DOI] [PubMed] [Google Scholar]
- 59.Sevinsky JR, Rao LVM, Ruf W: Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol 1996, 133:293-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoyer-Hansen G, Rone E, Solberg H, Behrendt N, Ploug M, Lund LR, Ellis V, Dano K: Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem 1992, 267:18224-18229 [PubMed] [Google Scholar]
- 61.Resnati M, Guttinger M, Valcamonica F, Sidenius N, Blasi F, Fazioli F: Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J 1996, 15:1572-1582 [PMC free article] [PubMed] [Google Scholar]
- 62.Stefansson S, Lawrence DA: The serpin PAI-1 inhibits cell migration by blocking integrin αVβ3 binding to vitronectin. Nature 1996, 383:441-443 [DOI] [PubMed] [Google Scholar]
- 63.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ: Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol 1996, 134:1563-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birchmeier W, Weidner KM, Behrens J: Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci 1993, 17:159-164 [DOI] [PubMed] [Google Scholar]
- 65.Hordijk P, Ten Klooster JP, Van der Kammen RA, Michiels F, Oomen L, Collar JG: Inhibition of invasion of epithelial cells by Tiam 1-Rac signaling. Science 1997, 278:1464-1466 [DOI] [PubMed] [Google Scholar]
- 66.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F: Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun 1994, 1:295-305 [DOI] [PubMed] [Google Scholar]
- 67.Paciucci R, Torà M, Díaz VM, Real FX: The plasminogen activator system in pancreas cancer: role of t-PA in the invasive potential in vitro. Oncogene 1998, 16:625-634 [DOI] [PubMed] [Google Scholar]