Abstract
Genetically marked hepatocytes from dipeptidyl peptidase (DPP) IV+ Fischer 344 rats were transplanted into the liver of DPPIV− mutant Fischer 344 rats after a combined treatment with retrorsine, a pyrrolizidine alkaloid that blocks the hepatocyte cell cycle, and two-thirds partial hepatectomy. In female rats, clusters of proliferated DPPIV+ hepatocytes containing 20 to 50 cells/cluster, mostly derived from single transplanted cells, were evident at 2 weeks, increasing in size to hundreds of cells per cluster at 1 month and 1000 to several thousand cells per cluster at 2 months, representing 40 to 60% of total hepatocyte mass. This level of hepatocyte replacement remained constant for up to 1 year, the duration of experiments conducted. In male rats, liver replacement occurred more rapidly and was more extensive, with transplanted hepatocytes representing 10 to 15% of hepatocyte mass at 2 weeks, 40 to 50% at 1 month, 90 to 95% at 2 months, 98% at 4 months, and 99% at 9 months. Transplanted hepatocytes were integrated into the parenchymal plates, exhibited unique hepatic biochemical functions, and fully reconstituted a normal hepatic lobular structure. The extensive proliferation of transplanted cells in this setting of persistent inhibition of resident hepatocytes represents a new general model to study basic aspects of liver repopulation with potential applications in chronic liver disease and ex vivo gene therapy.
Repopulation of a chronically diseased liver via hepatocyte transplantation would represent a valuable alternative to whole-organ transplantation, the method used currently to treat end-stage liver disease. 1,2 However, a major problem in most hepatocyte transplantation studies to date has been limited growth of transplanted cells in the recipient organ. 3-7 Efforts to solve this problem have generally used partial hepatectomy to remove a portion of the resident liver and thus provide a proliferative stimulus to the transplanted cells. 5-7 However, use of partial hepatectomy alone to augment cell proliferation has two major limitations: 1) only one or two rounds of cell division are needed to restore the liver mass to its original size, 8,9 and 2) both endogenous hepatocytes and transplanted cells can participate in the regenerative process. Other approaches have included injection of large numbers of cells into the spleen (up to 1 × 10 8 cells) with subsequent seeding of the liver, 10 multiple transplantations of cells, 11,12 or induction of hepatotoxicity before cell transplantation, 13 but these approaches have led to only modest liver repopulation.
Recently, two experimental models of extensive liver repopulation have been described: the urokinase-type plasminogen activator transgenic mouse 14-16 and the fumarylacetoacetate hydrolase (Fah)-null mouse, 17 the latter serving as a model for hereditary tyrosinemia type I. In the urokinase-type plasminogen activator model, endogenous hepatocytes that have lost expression of the transgene 14 and transplanted normal hepatocytes 15,16 proliferate extensively because of continuous proteolytic destruction of resident hepatocytes expressing the urokinase-type plasminogen activator transgene. 14 Similarly, in Fah-null mice, transplanted wild-type hepatocytes exhibit a growth advantage over enzyme-deficient cells in the recipient liver, because the latter accumulate toxic intermediates in the tyrosine metabolic pathway. 17 Both models have convincingly documented that transplanted hepatocytes have enormous proliferative potential. 15-17 In the Fah model, most recent studies report that transplanted wild-type hepatocytes can be serially transplanted through six or seven hosts with full repopulation from 10,000 donor cells in each recipient. 18 However, because both of these models rely on unique genetic backgrounds, their application as general models for hepatic cell transplantation is rather limited.
We reasoned that if we could develop an alternative method to provide a selective growth advantage to transplanted cells over endogenous hepatocytes, this could represent a generally useful approach to hepatocyte repopulation. The present study describes a new strategy for selective proliferation of transplanted cells by interfering with the proliferative capacity of resident hepatocytes, using the pyrrolizidine alkaloid retrorsine and then transplanting normal cells in conjunction with partial hepatectomy. The validity of this approach was tested by tracking genetically marked transplanted cells in an inbred strain of Fischer (F) 344 rats not expressing a specific marker gene, dipeptidyl peptidase IV (DPPIV), and by monitoring the ability of transplanted hepatocytes to replace hepatocyte mass. The proliferation, expansion, and integration of transplanted cells into the hepatic parenchymal structure was determined by morphological and histochemical analysis, and the biochemical function of transplanted hepatocytes was determined by analysis of liver tissue for expression of hepatocyte-specific proteins, albumin, and glucose-6-phosphatase, as well as glycogen synthesis and storage. Using these methods, we have observed replacement of 40 to 60% of hepatic mass in female rats for up to 1 year. Near-total replacement of hepatocyte mass was observed in male rats (98 to 99%) and persisted for 9 months after transplantation, the duration of experiments conducted.
Materials and Methods
Animals, Diets, and Cell Transplantation Protocols
DPPIV− F344 rats were provided by the Special Animal Core of the Liver Research Center at the Albert Einstein College of Medicine. Donor DPPIV+ F344 rats were purchased from Charles River Laboratories (Wilmington, MA). All animals were maintained on daily cycles of alternating 12-h light/darkness with food and water available ad libitum. Animals were given Purina Rodent Lab Chow diet (No. 5001; Dyets, Inc., Richmond, IN) throughout the experiments. F344 DPPIV− rats weighing 90 to 140 g were given two injections of retrorsine (Sigma Chemical Co., St. Louis, MO), 30 mg/kg each, intraperitoneally, 2 weeks apart. This protocol exerts a strong and persistent inhibition of hepatocyte cell division that lasts for at least several months (E. Laconi and P. Pani, unpublished observation). Four weeks after the last injection, two-thirds partial hepatectomy was performed, and each animal received 2 × 10 6 freshly isolated hepatocytes via portal vein infusion. All studies were conducted in accordance with U.S. federal guidelines (NIH Publication 86-23, revised 1985), under protocols approved by the Animal Care Use Committee of the Albert Einstein College of Medicine.
Hepatocyte Isolation
Hepatocytes were isolated from young adult DPPIV+ F344 donor rats according to a standard two-step collagenase perfusion technique. 19 The isolated cell fraction used for transplantation studies was judged to be ∼95% hepatocytes by morphological analysis. Cell viability was consistently between 85 and 95%, as determined by trypan blue dye exclusion. Rats were divided into four experimental groups: 1) animals receiving cell transplantation but no other treatment; 2) animals receiving retrorsine and cell transplantation but no partial hepatectomy; 3) animals receiving partial hepatectomy and cell transplantation but no retrorsine; and 4) animals receiving retrorsine, partial hepatectomy, and cell transplantation. Controls consisted of untreated rats and rats treated with retrorsine but neither partial hepatectomy nor cell transplantation. Rats from different experimental groups were killed at various time points postsurgery, starting from day 1 and up to 1 year. Livers were examined grossly, and separate samples from each lobe were either frozen or fixed in buffered formalin and processed by histological, histochemical, or immunohistochemical methods.
Detection of Liver-Specific Markers
DPPIV and ATPase histochemical staining was performed according to previously published techniques. 20 Quantitation of DPPIV-positive areas in the liver was performed by computer-assisted image analysis. This consisted of projecting images from DPPIV-stained slides onto a magnetic graphics tablet (NewSketch 1212 HR, Genius, KYE System Corp., Langenfeld, Germany) that converted the information into a computer-readable format from which the areas and percentages were determined by a simple computer program. Albumin and cytokeratin-19 were detected by immunohistochemical methods, also as previously reported, 20 using secondary antibodies conjugated to peroxidase and peroxidase enzyme activity detected with 3,3′-diaminobenzidine as substrate. Glycogen was detected by the method of Lillie. 21
Results
Identification and Proliferation of Transplanted Hepatocytes
Retrorsine treatment of DPPIV− F344 rats, hepatocyte isolation and transplantation, and two-thirds partial hepatectomy were conducted as noted in Materials and Methods. All procedures were well tolerated, and most animals (>95%) survived all protocols. After the acute effects of retrorsine subsided (these included dilatation and congestion of hepatic sinusoids, mild inflammation, and biliary epithelial cell proliferation), the hepatic parenchyma exhibited mild to moderate disruption of the parenchymal cord structure, residual biliary cell hyperplasia, and scattered hepatocyte megalocytosis (Figure 1A) ▶ . After partial hepatectomy (with or without cell transplantation), there was a marked increase in hepatic megalocytes (Figure 1B) ▶ . These cells have large nuclei, resulting from DNA synthesis in retrorsine-treated hepatocytes after a liver proliferative stimulus but with an inability of these cells to undergo mitosis ( Refs. 22-25 and E. Laconi and P. Pani, unpublished observations). Focal areas of small hepatocytes in clusters were also observed (Figure 1B) ▶ . There was no evidence of fibrosis (Figure 1, C and D) ▶ , chronic liver disease, or hepatic neoplasms, all of which occur with higher doses and/or repeated administration of pyrrolizidine alkaloids. 26,27 At the time of cell transplantation, there was no evidence of retrorsine toxicity in other organs, including lung, heart, brain, stomach, small intestine, colon, kidney, pancreas, adrenal gland, testes, and ovaries.
Figure 1.
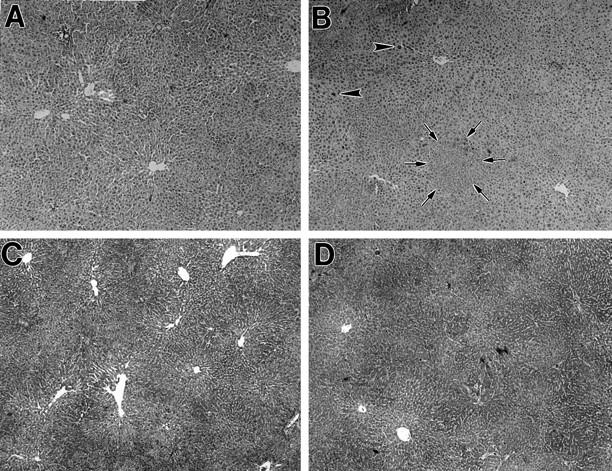
Liver histology of rats treated with retrorsine or retrorsine plus hepatocyte transplantation and partial hepatectomy. A: DPPIV− F344 rat 1 month after treatment with two doses of retrorsine. Note liver sinusoidal dilatation and moderate disorganization of hepatic lobular structure, but no evidence of acute or chronic inflammation (H&E stain). B: DPPIV− F344 rat treated with two doses of retrorsine as in A, followed by transplantation of 2 × 10 6 hepatocytes and two-thirds partial hepatectomy. The animal was sacrificed 2 months after cell transplantation, and histological analysis was performed. Note scattered foci of proliferating hepatocytes (one focus circumscribed by arrows). There is also marked variation in the size of hepatocytes, including some with very large nuclei (arrowheads). The lobular organization is improved compared to pretransplantation status, but this varies from animal to animal (H&E stain). C: Same as in A, using trichrome staining. Note moderate disruption of hepatic lobular structure, but no evidence of hepatic fibrosis. D: Same as in B, using trichrome stain. Note areas of focal proliferation of hepatocytes, but no evidence of margination between transplanted and endogenous hepatocytes, as well as absence of fibrotic changes. Original magnification: A through D, ×50.
To follow the fate of transplanted hepatocytes, we used the DPPIV-deficient (DPPIV−) F344 rat model. 28 DPPIV is a membrane exopeptidase expressed by many epithelial cell types; however, in hepatocytes, its expression pattern is unique in that it segregates to the apical domain of the plasma membrane. 29-31 DPPIV colocalizes with Mg2+/K+ ATPase, a classical marker of the bile canaliculus, 32 and both enzymes can be detected readily by histochemical analysis. 32,33
One day after transplantation of 2 × 10 6 hepatocytes into retrorsine and partial hepatectomy-treated female rats, DPPIV+ cells were difficult to find. They appeared mostly as single cells in the hepatic sinusoids in or near the portal triads (Figure 2A) ▶ . Initially, DPPIV histochemical staining was diffuse over the cell surface and not confined to the bile canaliculus, probably reflecting the recent isolation and transplantation of cells. By the 4th day posttransplantation, groups of 2 to 4 DPPIV+ cells with typical bile canalicular staining were discernible (Figure 2B) ▶ , and at 2 weeks, DPPIV+ hepatocytes appeared in clusters of 25 to 50 cells in a two-dimensional cross-sectional area (Figure 2C) ▶ . DPPIV+ hepatocyte clusters were much larger at 1 month posttransplantation (Figure 2D) ▶ , ranging from 100 to several hundred cells, and at 2 months posttransplantation, DPPIV+ clusters ranged up to 1000 or more cells, with many clusters becoming confluent (Figure 2E) ▶ . Using computer-based morphometric analysis in female rats at 2 weeks postsurgery, we found that about 3 to 5% of hepatocytic mass expressed DPPIV, increasing to 15 to 25% at 1 month and to 40 to 60% at 2 months. This level of hepatocyte replacement remained essentially constant for up to 1 year, the duration of experiments conducted. No significant growth of transplanted hepatocytes was seen in rats receiving partial hepatectomy but not treated with retrorsine (data not shown). In retrorsine-treated female rats receiving transplanted hepatocytes but not undergoing partial hepatectomy, there was also little proliferation of transplanted cells up to 4 months posttransplantation (Figure 2F) ▶ .
Figure 2.
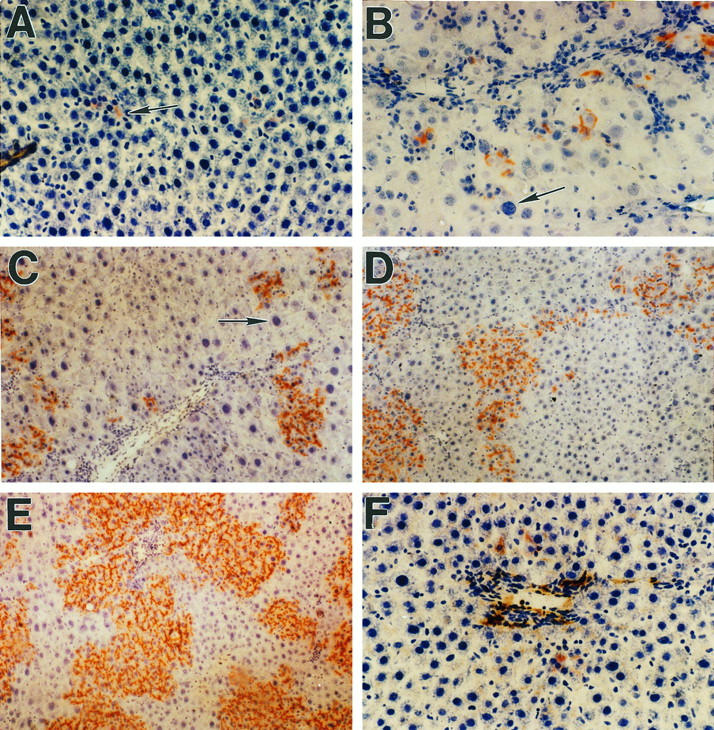
Proliferation of DPPIV+ hepatocytes in the liver of retrorsine-treated female DPPIV− mutant rats after partial hepatectomy. A: One day after transplantation of 2 × 10 6 hepatocytes. Scattered, isolated DPPIV+ cells are located in hepatic sinusoids near the portal triads (arrow). B: Four days posttransplantation. Transplanted cells are present in small clusters of two to four cells, suggesting several rounds of cell division. The arrow points to a megalocytic DPPIV− hepatocyte with a large hyperchromatic nucleus. C: Thirteen days posttransplantation. The size of DPPIV+ cell clusters is increasing, and clusters are now visible at a distance farther from the portal triads (mid-parenchyma). The arrow points to one of many megalocytic DPPIV− hepatocytes that become prominent after partial hepatectomy. D: One month posttransplantation. The average number of DPPIV+ cells in clusters continues to increase, and larger clusters are beginning to become confluent. Although there is variation from section to section and within sections, approximately 15 to 25% of total liver mass is now replaced by DPPIV+ cells. E: Two months posttransplantation. The size of DPPIV+ clusters continues to increase, and transplanted hepatocytes now constitute 40 to 60% of total hepatic mass. F: One month posttransplantation in the absence of partial hepatectomy. Under these conditions, there is very little proliferation of transplanted cells, which are visible as single cells or doublets primarily in the periportal region. Original magnifications: A, B, C, and F, ×200; D and E, ×100.
The distribution and growth pattern of transplanted DPPIV+ hepatocytes was also interesting. Hepatocytes transplanted via infusion into the portal vein were observed initially in the periportal region (Figure 2A) ▶ . As the number of DPPIV+ cells increased, they formed clusters that were also subsequently visible in the mid-parenchyma (Figure 2, C and D) ▶ . Whether this represented further seeding of the liver by cells migrating from primary clusters in the periportal regions, an increase in the size of clusters so that they were now more easily detected in other regions of the lobule that were initially sparsely populated by transplanted cells, or a combination of both processes is not clear. The average size of hepatocytes in DPPIV+ clusters was smaller than hepatocytes in the surrounding liver. As indicated previously, many of the endogenous hepatocytes (shown as DPPIV− in Figure 2 ▶ ) became megalocytic after partial hepatectomy with large hyperchromatic nuclei (Figure 2, B and C ▶ , arrows), reflecting the effect of the pyrrolizidine alkaloid. 22-25
Incorporation of Transplanted and Proliferating Hepatocytes into the Hepatic Parenchyma
To determine whether transplanted and newly proliferated hepatocytes become structurally integrated into the hepatic parenchyma, dual histochemical analysis was conducted with ATPase and DPPIV. In normal F344 rats, both stains were positive and superimposed (Figure 3A) ▶ . Under these circumstances, only DPPIV (rust/orange color) was visualized. In control DPPIV− F344 rats, ATPase was positive (brown color), but DPPIV was negative (Figure 3B) ▶ . Two months after DPPIV+ hepatocyte transplantation into DPPIV− rats, large clusters of DPPIV+ hepatocytes were noted (Figure 3C) ▶ . There was no marginal separation between transplanted and endogenous cells; the borders between transplanted and endogenous hepatocytes were irregular and transplanted cells appeared to be expanding into areas of surrounding liver. Transplanted cells were fully integrated into the hepatic parenchymal plates and formed hybrid canaliculi with adjacent, DPPIV−/ATPase+ endogenous hepatocytes (Figure 3D ▶ , arrows).
Figure 3.
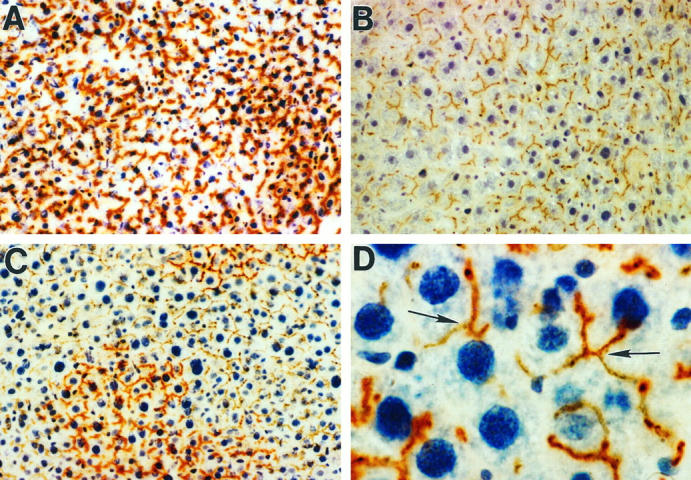
Integration of transplanted DPPIV+ hepatocytes into the parenchymal structure of the host liver. Histochemical staining for DPPIV (rust/orange color) and ATPase (brown color) of rat liver 2 months after transplantation of 2 × 10 6 DPPIV+ hepatocytes. A: Normal F344 rat with overlapping DPPIV and ATPase staining in a bile canalicular distribution pattern. Under these circumstances, only DPPIV (rust/orange color) is visualized. B: DPPIV− mutant F344 rat with canalicular expression of ATPase (brown color) but not DPPIV. C: DPPIV− rat transplanted with 2 × 10 6 DPPIV+ hepatocytes with two large clusters of DPPIV+ cells expanding into the hepatic parenchyma. There is no evidence of physical separation between DPPIV+ and DPPIV− cells or compression of host liver by transplanted cells. The margins between host hepatocytes and transplanted cells are irregular and indistinct, and transplanted cells appear to be expanding along the parenchymal plates. D: Higher magnification of C, showing physical contact between transplanted and endogenous hepatocytes with formation of hybrid bile canaliculi between transplanted and host hepatocytes (arrows). Original magnifications: A, B, and C, ×200; D, ×1000.
Near-Total Hepatocyte Replacement in Male Rats
In male F344 rats treated with the same protocol, the proliferative response of transplanted hepatocytes was even more dramatic with respect to both the rate and extent of liver replacement. One day after transplantation of 2 × 10 6 hepatocytes, once again isolated single cells were observed primarily in the periportal areas (usually not more than one or a few cells in one portal space per field, using either the ×4 or ×10 objective; Figure 4A ▶ ). Two weeks after cell transplantation, DPPIV+ hepatocytes were already observed in clusters of 100 or more cells in cross section (Figure 4B) ▶ , comparable to levels observed in female rats at 1 month. At 1 month post-cell transplantation in male rats, DPPIV+ hepatocytes represented approximately 50% of total hepatocyte mass (Figure 4C) ▶ . By 2 months, transplanted hepatocytes had replaced 90 to 95% of the parenchymal mass (Figure 4D) ▶ , and at 4 months, hepatocyte replacement was near total (98%; Figure 4E ▶ ). This high level of hepatocyte repopulation, determined by computer-assisted image analysis to be 99% at 9 months, persisted for the duration of experiments (Figure 4F) ▶ . Residual endogenous hepatocytes, usually with very large nuclei and negative for DPPIV enzyme activity, were observed at the margins of the lobules and in the area immediately adjacent to some central veins (Figure 4E ▶ , arrows). In the absence of partial hepatectomy, proliferation of transplanted hepatocytes in male rats was also quite limited, but in several animals it reached 3 to 5% within 1 month.
Figure 4.
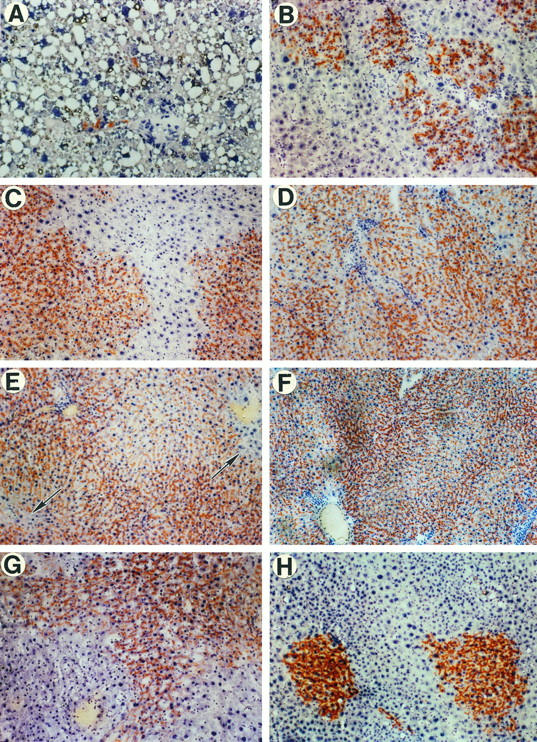
Proliferation of DPPIV+ hepatocytes in the liver of retrorsine-treated male DPPIV− mutant rats after partial hepatectomy. A: One day after transplantation of 2 × 10 6 hepatocytes. Several DPPIV+ cells are located near a portal triad, and one DPPIV+ cell is identified more deeply within the hepatic parenchyma. Extensive vacuolization is observed in hepatocytes, which occurs to a varying degree during the first 24 hours after hepatectomy. B: Two weeks posttransplantation. Transplanted hepatocytes show expansion into clusters containing up to 100 or more cells in a cross-sectional area. These clusters, which are more prominent in the periportal spaces, are also present in the midzone but are much smaller in size. Hepatocyte replacement ranged from 15 to 25% in different regions of the liver. C: One month after cell transplantation. Transplanted hepatocytes now form large confluent cell masses representing 50% or more of total hepatocytes. By computer analysis of multiple fields, total hepatocyte replacement was 49% in this animal. D: Two months posttransplantation. Extensive (94%) hepatocyte replacement was observed. Small groups of residual DPPIV− hepatocytes are identified. E: Four months posttransplantation. Near-total (98%) hepatocyte replacement is observed, with only a few remaining endogenous (DPPIV−) hepatocytes at the edges of the lobules and in the central vein region (arrows). F: Nine months posttransplantation. The liver parenchyma is essentially totally replaced by transplanted hepatocytes, which now represent 99% of the hepatocyte mass. Transplanted hepatocytes reform the parenchymal plates with a normal cord structure. G: Nine months posttransplantation in an animal receiving 10,000 cells. In this case, 30 to 50% hepatocyte replacement is observed. H: Six months posttransplantation of 100 DPPIV+ hepatocytes. Clusters containing 200 to 300 DPPIV+ hepatocytes are present in cross sections of the liver. These clusters appear totally integrated into the parenchymal structure. Original magnification: A through H, ×100.
To determine whether effective liver replacement could be obtained with fewer transplanted cells, dilution experiments were conducted. One month after cell transplantation, clusters of transplanted hepatocytes were noted in all animals except one, even in rats transplanted with 100 cells. At 9 months in rats transplanted with 10,000 hepatocytes, 30 to 50% hepatocyte replacement was observed (Figure 4G) ▶ . At 6 months in male rats transplanted with 100 cells, 2 to 6 clusters of DPPIV+ hepatocytes were observed in cross sections through the liver. In these animals, clusters of transplanted cells were of varying sizes on two-dimensional cross section, with larger clusters containing hundreds of hepatocytes (Figure 4H) ▶ .
The final appearance of the liver after long-term hepatocyte replacement at 9 months after hepatocyte transplantation in a male F344 rat compared to a normal rat liver is illustrated in Figure 5 ▶ . As shown by hematoxylin and eosin (H&E) staining at low magnification (Figure 5, A and B) ▶ , the lobular architecture and overall histological appearance were essentially normal. At higher magnification (Figure 5, C and D) ▶ , the morphology of hepatocytes, the hepatic plate structure, and the appearance of sinusoidal lining cells was normal compared to untreated liver. The only abnormality observed consistently was slight reduplication of the bile ducts, which occurred in all animals treated with retrorsine. As demonstrated by DPPIV histochemical staining before and after cell transplantation (Figure 5, E ▶ versus F), the extent of hepatocyte replacement was near total, with only a few remaining DPPIV− cells in small clusters. Figure 5F ▶ also shows restoration of a normal liver cord structure by transplanted hepatocytes.
Figure 5.
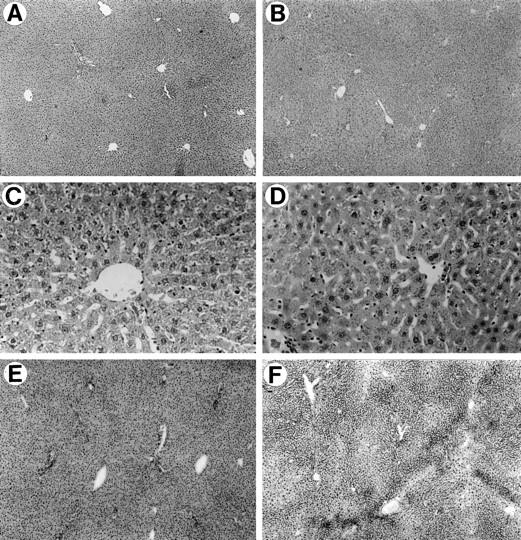
Histological and histochemical analysis of the liver 9 months after hepatocyte transplantation. A, C, and E: untreated DPPIV− rat liver. B, D, and F: retrorsine-treated DPPIV− rat liver 9 months after partial hepatectomy and transplantation of 1 × 10 6 DPPIV+ hepatocytes. A and B: H&E staining of formalin-fixed and paraffin-embedded tissue at ×50 magnification. C and D: H&E staining of formalin-fixed and paraffin-embedded tissue at ×200 magnification. E and F: DPPIV histochemical staining at ×50 magnification. Except for mild reduplication of bile ducts in B and D, the lobular structure and organization of the liver in the transplanted animal is essentially normal. E: Liver removed from this rat at the time of partial hepatectomy after retrorsine treatment but before cell transplantation. F: Liver from the same rat 9 months after transplantation of DPPIV+ hepatocytes.
Biochemical Function of Transplanted Hepatocytes
To study the ability of transplanted cells to perform unique hepatocyte biochemical functions, albumin synthesis, glucose metabolism, and gluconeogenesis were examined. As illustrated by serial sections in Figure 6, 1 ▶ month after cell transplantation, large clusters of hepatocytes that expressed DPPIV in a canalicular distribution (Figure 6A) ▶ also demonstrated synthesis of albumin (Figure 6B) ▶ and storage of glycogen (Figure 6C) ▶ . Similarly, there was high expression of glucose-6-phosphatase in clusters of transplanted (DPPIV+) hepatocytes (Figure 6, E and F) ▶ . Transplanted hepatocytes did not express cytokeratin-19 (Figure 6D) ▶ or γ-glutamyl transpeptidase (not shown), indicating that these cells are in the hepatocytic rather than the bile duct epithelial cell lineage. Transplanted cells were also negative for α-fetoprotein (data not shown).
Figure 6.
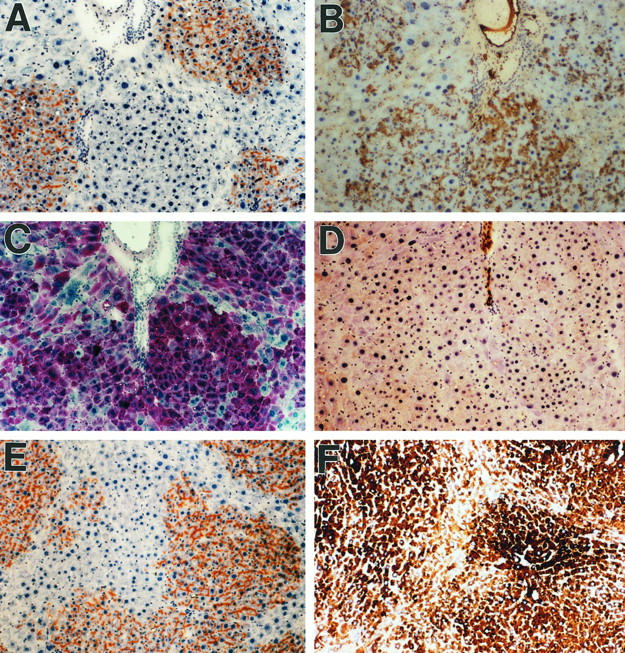
Hepatocytic biochemical function of DPPIV+-transplanted cells in DPPIV− mutant rat liver. A through D: Serial sections of liver 30 days after transplantation of 2 × 10 6 DPPIV+ hepatocytes, showing expression of (A) DPPIV, (B) albumin, (C) glycogen, and (D) cytokeratin-19. E and F: Separate serial sections showing (E) DPPIV and (F) glucose-6-phosphatase, another protein expressed uniquely in hepatocytes. Transplanted cells show expression of albumin, glycogen, and glucose-6-phosphatase at levels at least as high as in endogenous hepatocytes. At earlier time points, the expression of these proteins is substantially higher than in the surrounding liver. Original magnification: A through F, ×100.
Discussion
The present study demonstrates that normal hepatocytes transplanted into the rat liver can proliferate extensively, integrate into the hepatic parenchyma, repopulate the bulk of this organ, and exhibit normal differentiated hepatocyte function. This was achieved by treating the rats with retrorsine, a pyrrolizidine alkaloid, and subsequently performing hepatocyte transplantation in conjunction with partial hepatectomy. Pyrrolizidine alkaloids were studied originally because of their toxicity in animals, particularly sheep and cattle, where they cause both acute and chronic liver injury. 26,27 These agents are natural plant substances that are selectively taken up and metabolized by the liver to bioactive compounds that alkylate proteins and DNA. 27 They produce a block in the hepatocyte cell cycle with accumulation of cells in late S and/or G2 phase. 22-25 Although pyrrolizidine alkaloids are metabolized rapidly, their effect on hepatocyte proliferation lasts for weeks to months. 22-25,34,35 In this regard, we previously reported that transplantation of normal hepatocytes into the liver of rats treated with lasiocarpine, a related pyrrolizidine alkaloid, resulted in almost complete normalization of liver histology at 3 months posttransplantation. 35 This suggested that transplanted normal cells may have the potential to overcome chronic toxicity imposed by these agents. 35
Animals treated with this retrorsine/partial hepatectomy protocol have been maintained for up to 2 years without development of adenomas, hepatocellular carcinomas, or other hepatic malignancies. We have observed foci of basophilic hepatocytes that were negative for glutathione-S-transferase P and altered hepatic foci that are glutathione-S-transferase P positive, but the incidence (frequency) and number of these foci were not increased compared to untreated control F344 rats of the same age.
Transplanted cells were observed initially as single cells or doublets, and over a 1- to 2-month period, expanded until approximately 40 to 60% of the hepatocyte mass was replaced in female rats and virtually total replacement (99%) occurred in male rats. The cellular growth pattern appeared clonal, with individual cells forming clusters that increased progressively in size over a 1 to 2-month period. For clusters containing approximately 25 to 50 cells in two-dimensional cross section, this corresponds to 125 to 250 cells in three dimensions, assuming that each cluster represents a sphere and that the cross section is through the maximum diameter of the sphere. If we also assume that each cluster is derived from a single transplanted cell, the total proliferated mass in each cluster represents seven to eight cell divisions. This was observed at approximately 7 to 10 days in male rats and at 2 weeks in female rats. When the cross-sectional cluster size reached 1000 or more cells (1 month in male rats and 2 months in female rats), this represented ∼12 to 13 cell divisions. The expanding cells integrated into the parenchymal mass without compressing the surrounding structures. Initially, the proliferating, transplanted hepatocytes were smaller in size than endogenous hepatocytes, but within 3 to 4 days after transplantation, these cells exhibited canalicular expression of DPPIV. Hybrid canaliculi were also observed between transplanted and endogenous hepatocytes within several days, and there was no marginal separation between transplanted and host hepatocytes. After 2 to 4 months, transplanted hepatocytes began to reform normal liver plates with endothelial, Kupffer, and Ito cells in the sinusoids. By 9 months, the liver parenchymal structure was restored to normal, except for modest bile duct reduplication.
In retrorsine-treated rats, transplanted wild-type hepatocytes appear to undergo selective proliferation compared to endogenous host hepatocytes, which are mitoinhibited by the pyrrolizidine alkaloid. This proliferation is markedly enhanced by partial hepatectomy. This is in contrast to either the urokinase-type plasminogen activator transgenic or Fah-null mouse, in which cellular proliferation in the liver is a continuous process resulting from the toxic effects of genetic manipulation. In these models, both endogenous and transplanted hepatocytes can proliferate, but endogenous hepatocytes are removed by toxic injury, allowing the transplanted cells to become dominant. Although the specific mechanisms of hepatocyte replacement are different, it is interesting that in all three models, hepatocyte replacement is completed in approximately 2 months. How this process is controlled and the role of specific growth factors, hormones, and cytokines are interesting areas for future investigation.
Dilution experiments showed substantial liver repopulation after transplanting 10 4 cells and the presence of multiple clusters of transplanted hepatocyte in animals receiving as few as 100 cells. These findings are consistent with previous reports indicating that transplanted hepatocytes exhibit a high proliferative capacity under a variety of conditions in which there is a need to replace toxically injured hepatocytes. Overturf et al 17 recently showed in their Fah-null mouse model that both wild-type transplanted hepatocytes and mutant hepatocytes, genetically corrected in vivo by repeated intravenous injection of a retrovirus vector containing a normal Fah cDNA, were able to repopulate the liver of Fah-null mice and restore normal tyrosine metabolism. Thus, under appropriate circumstances, either cell transplantation or gene therapy approaches can be used to correct specific genetic deficiencies.
In our protocol, transplanted hepatocytes appeared morphologically, biochemically, and anatomically normal and, after two-thirds partial hepatectomy, expanded to restore hepatic mass. Functional activity of transplanted cells and their progeny was demonstrated by histochemical and immunohistochemical detection of hepatocyte-specific markers at levels comparable to those found in normal hepatocytes, including albumin, glucose-6-phosphatase, glycogen, and DPPIV. These results concerning differentiated hepatocyte function of transplanted cells are consistent with the findings of Overturf et al, in which glutamine synthetase activity was observed in transplanted hepatocytes in the central vein region of hepatic lobules reformed after wild-type hepatocytes were transplanted into Fah-null mice. 18
To our knowledge, this study represents the first example of extensive liver repopulation and function in the rat. In principle, the same strategy can be applied to virtually any mammalian species. Furthermore, this model is easily reproduced and offers a suitable system to investigate many aspects of basic liver biology, such as analysis of the lineage and proliferative potential of putative hepatocyte progenitor cells and cell lines. It can also be used to study the role of specific growth and differentiation factors in liver repopulation and to develop new animal models of liver disease using genetically engineered or modified cells. With appropriate modifications, the approach presented in this study could also bear relevance to the management of both acquired and genetically inherited human liver disease. One of the limitations in current studies of ex vivo gene therapy is the small number of transduced cells that ultimately reside in the host liver (estimates range from 0.1 to 1.0% of liver cell mass). 36 By coupling ex vivo gene therapy with the current cell transplantation strategy, it should be possible to eliminate this major technical barrier.
Acknowledgments
We thank Drs. J.A. Kessler, R. Kucherlapati, S. Gupta, and R. D. Burk for reviewing the manuscript; D.S.R. Sarma for helpful discussions and comments; Dr. D.C. Hixson for originally providing DPPIV− mutant F344 rats; and A. Caponigro and E. Bobe for secretarial assistance.
Footnotes
Address reprint requests to David A. Shafritz, M.D., Liver Research Center, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461. E-mail: shafritz@aecom.yu.edu.
Supported in part by research grants RO1 DK17609 and DK50636 and Digestive Diseases Core Center Grant P30 DK41296, all from the National Institute of Diabetes and Digestive and Kidney Diseases (to DAS), a research grant from Consiglio Nazionale delle Ricerche-Applicazioni Cliniche della Ricerca Oncologica, Rome, Italy (to PP), and the Gail I. Zuckerman Foundation for Research in Chronic Liver Diseases of Children. E.L. is the recipient of a research fellowship from the Italian Foundation for Cancer Research.
References
- 1.Gupta S, Roy Chowdhury J: Hepatocyte transplantation: back to the future. Hepatology 1992, 15:156–162 [DOI] [PubMed]
- 2.Wilson JM: Round two for liver gene therapy. Nat Genet 1996, 12:232-233 [DOI] [PubMed] [Google Scholar]
- 3.Roy Chowdhury J, Grossmann M, Gupta S, Roy Chowdhury N, Baker JR, Jr, Wilson JM: Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science 1991, 254:1802-1805 [DOI] [PubMed] [Google Scholar]
- 4.Grossmann M, Raper SE, Kozarsky K, Stein EA, Engelhardt JF, Muller D, Lupien PJ, Wilson JM: Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet 1994, 6:335-341 [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Miescher-Clemens E, Drugas G, Lee SM, Colombani P: Intrahepatic hepatocyte transplantation following subtotal hepatectomy in the recipient: a possible model in the treatment of hepatic enzyme deficiency. J Pediatr Surg 1992, 27:312-315 [DOI] [PubMed] [Google Scholar]
- 6.Kokudo N, Ohashi K, Takahashi S, Bandai Y, Sanjo K, Idezuki Y, Nozawa M: Effect of 70% hepatectomy on DNA synthesis in rat hepatocyte isograft into the spleen. Transplant Proc 1994, 26:3464-3465 [PubMed] [Google Scholar]
- 7.Borel Rinkes IH, Bijma A, Kazemier G, Sinaasappel M, Valerio D, Terpatra OT: Proliferative response of hepatocytes transplanted into spleen or solid support. J Surg Res 1994, 56:417-423 [DOI] [PubMed] [Google Scholar]
- 8.Bucher NLR, Swaffield MN: The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res 1964, 240:1611-1625 [PubMed] [Google Scholar]
- 9.Rabes HM, Wirshing R, Tuczek H-V, Iseler G: Analysis of cell cycle compartments of hepatocytes after partial hepatectomy. Cell Tissue Kinet 1976, 9:517-532 [DOI] [PubMed] [Google Scholar]
- 10.Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S: Studies of liver repopulation using the dipeptidyl peptidase IV-deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology 1996, 23:482-496 [DOI] [PubMed] [Google Scholar]
- 11.Rozga J, Holtzman M, Moscioni AD, Fujioka H, Morsiani E, Demetriou AA: Repeated intraportal hepatocyte transplantation in analbuminemic rats. Cell Transplant 1995, 4:237-243 [DOI] [PubMed] [Google Scholar]
- 12.Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S: Efficacy and safety of repeated hepatocyte transplantation for significant liver repopulation in rodents. Gastroenterology 1996, 111:1092-1102 [DOI] [PubMed] [Google Scholar]
- 13.Vranken Peeter M-JTD, Patijin GA, Lieber A, Perkins J, Kay MA: Expansion of donor hepatocytes after recombinant adenovirus-induced liver regeneration in mice. Hepatology 1997, 25:884-888 [DOI] [PubMed] [Google Scholar]
- 14.Sandgren EP, Palmiter RD, Heckel JL, Dougherty CC, Brinster RL, Degen JL: Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 1991, 66:245-256 [DOI] [PubMed] [Google Scholar]
- 15.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL: Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263:1149-1152 [DOI] [PubMed] [Google Scholar]
- 16.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL: Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci USA 1995, 92:4942-4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou C-N, Finegold M, Grompe M: Adenovirus-mediated gene therapy in a mouse model of hereditary tyrosinemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 18.Overturf K, Al-Dhalimy M, Ou C-N, Finegold M, Grompe M: Serial transplantation reveals stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997, 151:1273-1280 [PMC free article] [PubMed] [Google Scholar]
- 19.Seglen PO: Preparation of isolated rat liver cells. Methods Cell Biol 1976, 13:29-83 [DOI] [PubMed] [Google Scholar]
- 20.Dabeva MD, Hwang S-G, Vasa SRG, Hurston E, Novikoff PM, Hixson DC, Gupta S, Shafritz DA: Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci USA 1997, 94:7356-7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillie RD (Ed): Histopathologic Technic and Practical Histochemistry. New York, McGraw-Hill 1965, p 198
- 22.Peterson JE: Effects of the pyrrolizidine alkaloid lasiocarpine-N-oxide on nuclear and cell division in the liver of rats. J Pathol Bacteriol 1965, 89:153-171 [DOI] [PubMed] [Google Scholar]
- 23.Afzelius BA, Schoental RJ: The ultrastructure of the enlarged hepatocytes induced in rats with a single oral dose of retrorsine, a pyrrolizidine (Senecio) alkaloid. Ultrastruct Res 1967, 20:328-345 [DOI] [PubMed] [Google Scholar]
- 24.Jago MV: The development of the hepatic megalocytosis of chronic pyrrolizidine alkaloid poisoning. Am J Pathol 1969, 56:405-422 [PMC free article] [PubMed] [Google Scholar]
- 25.Samuel A, Jago MV: Localization in the cell cycle of the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine, and of its metabolite, dehydroheliotridine. Chem Biol Interact 1975, 10:185-197 [DOI] [PubMed] [Google Scholar]
- 26.McLean E: The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol Rev 1970, 22:429-483 [PubMed] [Google Scholar]
- 27.Mattocks AR: Chemistry and Toxicology of Pyrrolizidine Alkaloids. 1986. FL, Academic Press, Orlando
- 28.Thompson NL, Hixson DC, Callavan H, Panzica M, Flanagan D, Faris RA, Hong W, Hartel-Schenk S, Doyle D: A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein: use as a liver-cell transplantation model. Biochem J 1991, 273:497-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gossrau R: Peptidasen II: zur lokalization der dipeptidylpeptidase IV (DPPIV). Histochemistry 1979, 60:231-248 [DOI] [PubMed] [Google Scholar]
- 30.Hubbard AL, Bartles JR, Braiterman LT: Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol 1985, 100:1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walborg EF, Jr, Tsuchida S, Weeden DS, Thomas MW, Barrick M, McEntire KD, Allison JP, Hixson DC: Identification of dipeptidylpeptidase IV as a protein shared by the plasma membrane of hepatocytes and liver biomatrix. Exp Cell Res 1985, 158:509-518 [DOI] [PubMed] [Google Scholar]
- 32.Wachstein M, Meisel E: Histochemistry of hepatic phosphastases at a physiologic pH. Am J Clin Pathol 1955, 27:13-23 [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Rajvanshi P, Lee C-D: Integration of transplanted hepatocytes into host liver plates demonstrated with dipeptidylpeptidase IV-deficient rats. Proc Natl Acad Sci USA 1995, 92:5860-5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes MA, Roberts E, Farber E: Initiation and selection of resistant hepatocyte nodules in rats given the pyrrolizidine alkaloids lasiocarpine and senecionine. Cancer Res 1985, 45:3726-3734 [PubMed] [Google Scholar]
- 35.Laconi E, Sarma DSR, Pani P: Transplantation of normal hepatocytes modulates the development of chronic liver lesions induced by a pyrrolizidine alkaloid, lasiocarpine. Carcinogenesis 1995, 16:139-142 [DOI] [PubMed] [Google Scholar]
- 36.Kay MA, Woo SLC: Gene therapy for metabolic disorders. Trends Genet 1994, 10:253-257 [DOI] [PubMed] [Google Scholar]


