Abstract
Terminal differentiation in a variety of cell types has been associated with p53-independent up-regulation of p21WAF1. p21WAF1 mRNA and protein are expressed at low levels in normal human skin, but overexpression of p21WAF1 has been observed in differentiating keratinocytes in involved psoriatic epidermis and in human squamous cell carcinoma. In this study we investigated by immunohistochemistry and Western blotting whether calcium and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate, well characterized differentiation signals, induce p21WAF1 in cultured normal human keratinocytes and whether induction of p21WAF1 in this system depends on protein kinase C activation or functional p53. Phorbol ester induced p21WAF1 expression, which was maximal at 4 to 8 h with reduction back to baseline by 24 to 48 h. In contrast, increasing the extracellular Ca2+ concentration from 70 μmol/L to 1.5 mmol/L resulted in up-regulation of p21WAF1 expression with a slower time course, with peak induction at 18 to 24 h. No parallel increase in p53 expression was observed in normal human keratinocytes. Up-regulation of p21WAF1 was also observed in response to phorbol ester in HaCaT cells, which carry homozygous and inactivating mutations for p53. Induction of p21WAF1 by phorbol ester and Ca2+ was inhibited by the specific protein kinase C inhibitor Ro 31-8220. The results demonstrate a differential time course of p21WAF1 protein up-regulation in response to phorbol ester and Ca2+, signals that result in keratinocyte differentiation, and suggest that induction of p21WAF1 in differentiating human keratinocytes occurs through protein kinase C-dependent and p53-independent mechanisms.
Epidermis is a stratified squamous epithelium composed predominantly of keratinocytes. These cells divide in the lower basal layers and subsequently move upward through the epidermis and undergo a complex program of morphological and biochemical changes, including the sequential expression of differentiation-associated proteins. 1 These changes lead to the formation of terminally differentiated cornified cells within the stratum corneum that form the primary barrier of skin. Abnormalities in keratinocyte differentiation are observed in a number of skin diseases, including psoriasis 2 and nonmelanoma skin cancer. 3,4 Furthermore, it has been hypothesized that resistance of initiated cells to differentiation signals promotes a growth advantage during epidermal carcinogenesis. 5,6
Although there are some important differences between cell culture and human skin in vivo, cultures of keratinocytes have proved useful in investigating cell differentiation and identifying regulatory mechanisms involved in this process. Studies on cultured keratinocytes and in vivo have shown that the concentration of extracellular calcium ([Ca2+]o) and activation of the protein kinase C (PKC) signal transduction pathway are important regulators of keratinocyte differentiation. 7-13 Increased [Ca2+]o stimulates formation of inositol 1,4,5-trisphosphate and 1,2-diacylglycerol and activates PKC in mouse keratinocytes, 14,15 although whether activation of PKC is required for [Ca2+]o-induced human keratinocyte differentiation remains to be established. Indeed, mouse skin differs from human skin in several respects, including epidermal thickness, number of epidermal cell layers, and density of hair follicles, and murine and human keratinocytes show different responses to 12-O-tetradecanoylphorbol-13-acetate (TPA), a potent PKC agonist and calcium. 1 For example, induction of ornithine decarboxylase is observed in mouse skin in vivo and cultured mouse keratinocytes in response to TPA, whereas TPA suppresses ornithine decarboxylase expression and enzyme activity in cultured human keratinocytes. 16,17
Terminal differentiation is coupled to withdrawal from the cell cycle, and this process has been associated with increased expression of p21WAF1 in a variety of tissues in vivo, including muscle and gastrointestinal epithelia, 18-21 and in several well defined differentiating systems in vitro, including HL-60 cells. 22,23 p21WAF1 (also known as CIPI, SDII, MDA6, or CAP20) is transcriptionally regulated by p53 24 and is a downstream mediator of p53-induced growth arrest after DNA damage 24 through its interaction with proliferating cell nuclear antigen, cyclins, and cyclin-dependent kinases. 21,25-27 p21WAF1 may also be regulated through p53-independent pathways in response to diverse stimuli including growth factors, 28 and experiments in p53-null cells indicate that increased expression of p21WAF1 during terminal differentiation occurs through p53-independent pathways. 23 Although some agents such as TPA induce p21WAF1 and act as differentiation signals in several cell systems, including HL-60 cells 22,23 and keratinocytes, 29,30 cell type-specific mechanisms also appear to be operating. In myoblasts, for example, the myoD family of transcription factors regulates p21WAF1 promoter activity, whereas in mouse keratinocytes another protein, p300, is required. 29
We have previously reported on increased p21WAF1 mRNA and protein expression in suprabasal psoriatic epidermis, 31 with virtually absent expression in the basal layer. In the same study, we also observed up-regulation of p21WAF1 mRNA and protein expression in skin in vivo in response to agents that induce hyperproliferation, 31 although whether p21 is involved in regulating this hyperproliferative response is not clear. Overexpression of p21WAF1 has also been reported in the differentiating cells of human cutaneous squamous cell carcinomas. 32
In the present study we have investigated whether increased [Ca2+]o and TPA, two well characterized differentiation signals for human keratinocytes, induce p21WAF1 in cultured normal human keratinocytes and whether alteration in p21WAF1 expression is dependent on PKC activation and/or functional p53.
Materials and Methods
Cell Culture
Cultures of human keratinocytes were established from normal skin obtained from plastic and pediatric surgical procedures, essentially as described. 33 Epidermis was separated from dermis after overnight incubation at 4°C in Dispase II (neutral protease) (Boehringer Mannheim) and an epidermal cell suspension prepared after incubation with 0.05% trypsin and 0.02% ethylenediaminetetraacetic acid at 37°C. Keratinocytes were cultured in T75 flasks in MCDB-153 serum-free medium (Sigma-Aldrich) with a calcium concentration of 70 μmol/L, supplemented with ethanolamine (6.1 μl/L), phosphoethanolamine (14 μg/ml), hydrocortisone (0.18 μg/ml), insulin (5 μg/ml), transferrin (5 μg/ml), epidermal growth factor (10 ng/ml), penicillin G (5 IU/ml), streptomycin (5 μg/ml), and amino acids. 34 Keratinocytes were expanded by serial passage and used for experiments between passages 2 and 3. Spontaneously immortalized HaCaT cells, a kind gift from Dr. NE Fusenig (German Cancer Research Center, Heidelberg, Germany), were cultured in Dulbecco’s modified Eagle’s medium (Sigma Laboratories, Poole, UK) with 10% fetal calf serum.
Immunohistochemistry
Primary cultures of keratinocytes and HaCaT cells were passaged by trypsinization and were subsequently seeded into 12-well plates containing one round 16 mm-diameter coverslip/well at a density of 1 × 10 5 cells/well. After timed incubation with specified agents or with medium containing 1.5 mmol/L Ca2+, cells were fixed in 4% paraformaldehyde and immunostained with anti-p21WAF1 monoclonal antibody, diluted 1:70 (Oncogene Research Products, Cambridge, MA), using an avidin-biotin immunoperoxidase kit (Vector Laboratories, Burlingame, CA), with Ni2+ plus 3,3′-diaminobenzidine as the chromagen and counterstained with methyl green.
Western Analysis
Cells were treated with the specified agents and scraped into 2× sodium dodecyl sulfate lysis buffer (125 mmol/L Tris-HCl, pH 6.8, 0.05% bromphenol blue, 4% sodium dodecyl sulfate, 20% glycerol, and 10% β-mercaptoethanol). Protein concentrations were determined on samples, before addition of β-mercaptoethanol, using the bicinchoninic acid protein assay kit (Pierce Chemical Co., Rockford, IL), according to the manufacturer’s instructions. Equal protein quantities of samples were electrophoresed through 12.5% polyacrylamide gels, transferred onto nitrocellulose, blocked in blocking buffer (5% nonfat milk in Ca2+ and Mg2+-free PBS) for 1 hour at room temperature, and subsequently incubated overnight at 4°C in the appropriate dilution of primary mouse monoclonal antibody (anti-p21WAF1, 1:250; DO7, anti-p53; 1:1000; Novocastra Laboratories, Newcastle, UK) in blocking buffer. After incubation with rabbit anti-mouse peroxidase-conjugated secondary antibody and avidin-biotin complex, membranes were washed and protein bands were visualized using an enhanced chemiluminescent Western blotting detection system (Pierce). Band intensities were quantified by laser densitometry (LKB model 2222-020, LKB Prodakter, Bromma, Sweden) according to the manufacturer’s instructions.
Results
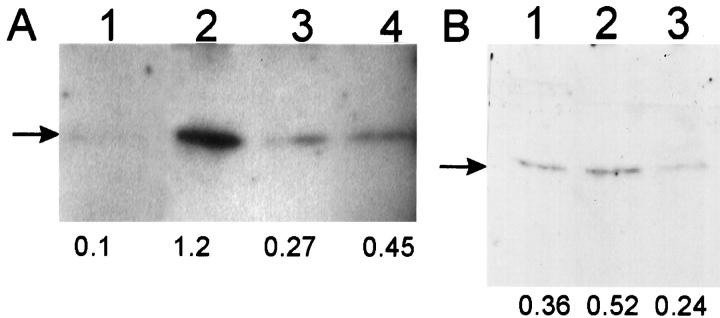
Human keratinocytes cultured as monolayers in low-calcium (70 μmol/L), serum-free MCDB-153 medium differentiate in response to TPA and increased extracellular calcium [Ca2+]o (1.5 mmol/L). 8,10,12,13 We initially investigated changes in p21WAF1 protein expression by immunohistochemistry in human keratinocytes after exposure to TPA and increased [Ca2+]o. Figure 1A ▶ shows that compared to control, TPA (50 nmol/L) induced p21WAF1 expression in cultured human keratinocytes with maximal induction at 4 to 8 h and reduction back toward baseline at 24 to 48 h. As expected, p21WAF1 expression was predominantly nuclear. In parallel experiments on keratinocytes derived from the same donor, increased p21WAF1 expression was also observed in response to increased [Ca2+]o but with a slower time course compared to TPA (Figure 1B) ▶ , and substantial expression persisted at 24 and 48 h. Induction of p21WAF1 expression after treatment of paired human keratinocyte cultures with TPA and increased [Ca2+]o was confirmed by Western analysis (Figure 2, A and B) ▶ . Western blotting experiments also confirmed a differential time course of p21WAF1 induction in response to TPA and increased [Ca2+]o (Figure 1, C and D) ▶ , with maximal induction at 4 to 8 h and 18 h for TPA and increased [Ca2+]o, respectively.
Figure 1.
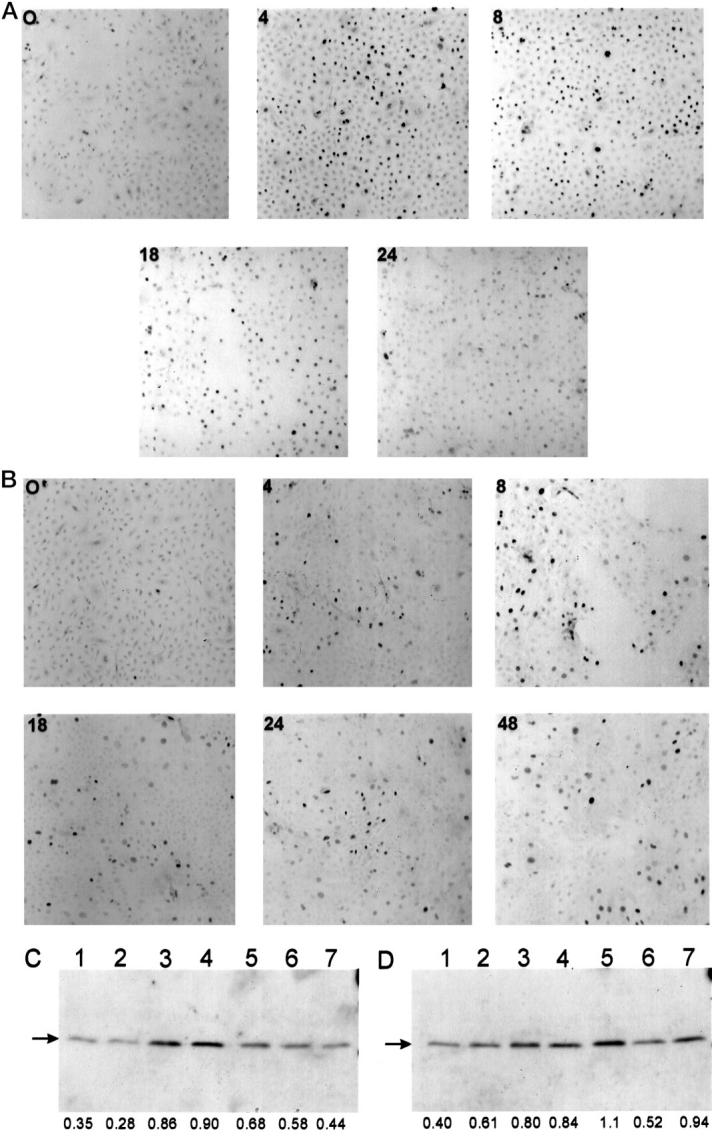
Kinetics of increased p21WAF1 expression in human keratinocytes in response to TPA and increased extracellular calcium. A and B: Cultured human keratinocytes derived from the same donor were treated with TPA (50 nmol/L) (A) or switched to medium containing 1.5 mmol/L [Ca2+]o (B) for various times (in hours) and immunostained with anti-p21WAF1 monoclonal antibody. Essentially identical results were obtained in at least three independent experiments from three different donors. C and D: Cultured human keratinocytes derived from the same donor were treated for the specified times with TPA (50 nmol/L) (C) or switched to medium containing 1.5 mmol/L [Ca2+]o (D), and cell lysates were prepared, fractionated in sodium dodecyl sulfate/12.5% polyacrylamide gels, transferred to nitrocellulose membrane, and immunoblotted with anti-p21WAF1 monoclonal antibody, as described in Materials and Methods. Lane 1, vehicle; lane 2, 2 h; lane 3, 4 h; lane 4, 8 h; lane 5, 18 h; lane 6, 24 h; lane 7, 48 h. Relative densitometric values (arbitrary units) are indicated below the lanes.
Figure 2.
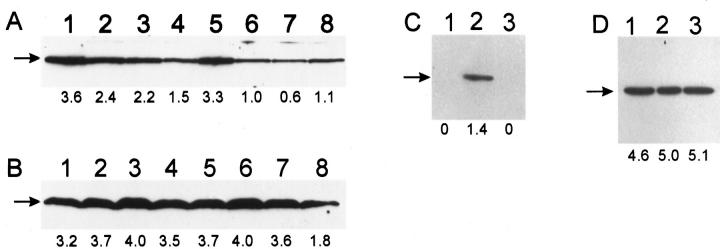
Ro 31-8220 partially inhibits up-regulation of p21WAF1 by TPA and increased [Ca2+]o. Cell lysates were prepared from cultured human keratinocytes treated with the specified agents, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotted with anti-p21WAF1 monoclonal antibody, as described in Materials and Methods. A: Lane 1, vehicle; lane 2, TPA (50 nmol/L) for 4 h; lane 3, TPA (50 nmol/L) plus Ro 31-8220 (100 nmol/L) for 4 h; lane 4, Ro 31-8220 (100 nmol/L). B: Lane 1, vehicle; lane 2, 1.5 mmol/L [Ca2+] for 8 h; lane 3, 1.5 mmol/L [Ca2+] plus Ro 31-8220 (100 nmol/L) for 8 h. Relative densitometric values (arbitrary units) are indicated below the lanes. Results are representative of at least three independent experiments that yielded similar results.
Because PKC agonists induce keratinocyte differentiation and because PKC-mediated cell cycle arrest involves induction of p21WAF1 in a number of cell types, including HL-60 cells and intestinal epithelial cells, 23,35 we investigated the effect of the relatively specific PKC inhibitor Ro 31-8220 36 on p21WAF1 expression in differentiating human keratinocytes. Western analysis showed that Ro 31-8220 (100 nmol/L) substantially abrogated TPA-induced up-regulation of p21WAF1 at 4 h and 1.5 mmol/L [Ca2+]o-induced up-regulation of p21WAF1 at 8 h (Figure 1, C and D) ▶ . Similar results were obtained using immunohistochemical analysis (data not shown). No significant induction of p21WAF1 was observed after exposure of keratinocytes to Ro 31-8220 alone (Figure 2A) ▶ . These results provide evidence that early up-regulation of p21WAF1 in human keratinocytes in response to both TPA and increased [Ca2+]o occurs through a PKC-dependent pathway.
p53-null mouse keratinocytes retain their ability to respond to differentiation signals and to up-regulate p21WAF1 in response to increased [Ca2+]o. 29,30,37 In our experiments in which TPA and increased [Ca2+]o resulted in up-regulation of p21 in normal human keratinocytes, we observed a progressive reduction in p53 expression after treatment with TPA (Figure 3A) ▶ but no significant change in p53 expression in response to increased [Ca2+]o (Figure 3B) ▶ . Interestingly, TPA-induced down-regulation of p53 was substantially reduced by Ro 31-8220 (Figure 3A) ▶ . As p53-null human keratinocytes are not currently available for study, we used HaCaT cells (a spontaneously immortalized human keratinocyte cell line) containing two mutant alleles of p53, 38 which are unable to transactivate p21, 39 to examine whether p53 is required for p21WAF1 induction in human keratinocytes. Induction of p21WAF1 in HaCaT cells was observed by Western analysis (Figure 3C) ▶ and immunohistochemistry (data not shown), indicating that p53 is not required for this response. No reduction in basal p53 protein expression was observed in HaCaT cells in response to TPA (Figure 3D) ▶ .
Figure 3.
Induction of p21WAF1 in human keratinocytes is independent of p53. A and B: Cultured human keratinocytes derived from the same donor were treated with the specified agents, and Western analysis of cell lysates was performed using an anti-p53 monoclonal antibody. A: Lane 1, vehicle; lane 2, TPA (50 nmol/L) for 2 h; lane 3, TPA (50 nmol/L) for 4 h; lane 4, TPA (50 nmol/L) for 8 h; lane 5, TPA (50 nmol/L) plus Ro 31-8220 (100 nmol/L) for 8 h; lane 6, TPA (50 nmol/L) for 18 h; lane 7, TPA (50 nmol/L) for 24 h; lane 8, TPA (50 nmol/L) for 48 h. B: Lane 1, 70 μmol/L [Ca2+]o; lane 2, 1.5 mmol/L [Ca2+]o for 2 h; lane 3, 1.5 mmol/L [Ca2+]o for 4 h; lane 4, 1.5 mmol/L [Ca2+]o for 8 h; lane 5, 1.5 mmol/L [Ca2+]o plus Ro 31-8220 (100 nmol/L) for 8 h; lane 6, 1.5 mmol/L [Ca2+]o for 18 h; lane 7, 1.5 mmol/L [Ca2+]o for 24 h; lane 8, 1.5 mmol/L [Ca2+]o for 48 h. C and D: Cultured HaCaT cells containing functionally inactive homozygous mutations of p53 were treated with TPA (50 nmol/L) for 4 h (lane 2) or 24 h (lane 3) or treated with vehicle (lane 1), and Western analysis of cell lysates was carried out using anti-p21WAF1 (C) and anti-p53 monoclonal antibodies (D). Relative densitometric values (arbitrary units) are indicated below the lanes.
Discussion
In this study we have examined the relationship between two well characterized keratinocyte differentiation signals, increased [Ca2+]o and TPA, and p21WAF1 expression in cultured normal human keratinocytes. We have used a specific PKC inhibitor and HaCaT cells to determine whether induction of p21WAF1 by increased [Ca2+]o and TPA requires PKC activation or functional p53. Our results demonstrate a low level of p21WAF1 protein expression in proliferating cultured human keratinocytes consistent with virtually absent p21WAF1 mRNA and protein expression in normal human skin. 31 Calcium-induced differentiation in mouse keratinocytes has previously been associated with up-regulation of p21WAF1. 29,30 We observed up-regulation of p21WAF1 in human keratinocytes in response to TPA and increased [Ca2+]o. Both TPA and increased [Ca2+]o induce growth arrest and terminal differentiation in human keratinocytes. 7,8,10,12 However, TPA induces more rapid and profound differentiation than calcium in these cells. 40 Our experiments show a differential time course of p21WAF1 up-regulation, with TPA inducing p21WAF1 protein more swiftly than increased [Ca2+]o. These results further support an association between induction of p21WAF1 and terminal differentiation in human keratinocytes. p21WAF1 expression appears low in normal skin as detected by immunohistochemistry and in situ hybridization, and it is therefore difficult to comment about the relative expression of p21WAF1 in basal and suprabasal differentiating keratinocytes. 31 However, increased p21WAF1 mRNA and protein was observed in suprabasal differentiating keratinocytes in involved psoriatic epidermis, 31 which displays abnormal keratinocyte differentiation. 2 Similarly, in cutaneous human squamous cell carcinomas, which show alterations in keratin expression compared to normal epidermis, 3,4 p21WAF1 is overexpressed in superficial differentiating keratinocytes. 32 Further studies are required to determine whether these observations reflect a role for p21WAF1 in induction and maintenance of normal keratinocyte differentiation in vivo or whether p21WAF1 accumulates because of an abnormal differentiation program.
The bisindoylmaleimide Ro 31-8220 is a relatively specific PKC inhibitor 36 that inhibits TPA-induced growth arrest and differentiation in human keratinocytes. 41 Our studies with Ro 31-8220 provide important evidence that induction of p21WAF1 protein by both TPA and increased [Ca2+]o in human keratinocytes is dependent in part on PKC activation. In human keratinocytes, increased [Ca2+]o results in inositol 1,4,5-trisphosphate formation 42 and increased intracellular Ca2+ ([Ca2+]i), 43 but whether increased [Ca2+]o activates PKC in human keratinocytes remains to be formally established. We are currently performing experiments to address this issue. Up-regulation of p21WAF1 by PKC activation in human keratinocytes may involve the Sp family of transcription factors, as TPA significantly increased Sp1 protein levels and Sp1 binding in K562 cells, 44 and Sp1 and Sp3 both activate the p21WAF1 promoter, although overexpression studies suggest that only Sp3 is directly involved in promoter induction during mouse keratinocyte differentiation. 45
Although up-regulation of p21WAF1 is associated with cellular differentiation, p21WAF1-null mice showed normal organ and skin development with no morphological defects in cell differentiation, indicating that p21WAF1 is not essential. 46,47 However, primary keratinocytes derived from p21WAF1-null mice showed a significantly increased proliferative potential and an alteration of the keratinocyte differentiation program with a reduction of late differentiation markers. 47 In addition, the absence of p21WAF1 resulted in aggressive keratinocyte-derived tumors after transformation by ras. 47 These results and our previous work 31 suggest that p21WAF1 may be involved in the regulation of keratinocyte differentiation and that dysregulation of p21WAF1 expression in human skin may contribute to abnormal keratinocyte differentiation observed in psoriasis and cutaneous squamous cell carcinoma.
Up-regulation of p21WAF1 in HaCaT cells by transforming growth factor β, which induces cell cycle arrest but not cell differentiation, indicates that induction of p21WAF1 is not sufficient for keratinocyte differentiation. 39 Datto et al. also showed that HaCaT cells contain two mutant p53 alleles that are unable to transactivate the p21WAF1 promoter when overexpressed, indicating that induction of p21WAF1 by transforming growth factor β occurs through a p53-independent mechanism. 39 Within lesional psoriatic epidermis in which p21WAF1 was overexpressed in suprabasal layers, p53 protein expression was only marginally elevated within the low epidermal layers, suggesting that induction of p21WAF1 in psoriatic epidermis may be independent of p53. 31 Our observations in human keratinocytes and HaCaT cells support this hypothesis. A decrease in p53 protein expression was observed in response to TPA (Figure 3A) ▶ consistent with experiments in mouse keratinocytes. 30 Interestingly, this response was almost completely blocked by Ro 31-8220 (Figure 3A) ▶ . However, our findings of stable p53 protein expression in human keratinocytes after treatment with increased [Ca2+]o (Figure 3B) ▶ contrasts with reduced p53 synthesis in mouse keratinocytes. 30 Although the absence of increased p53 protein expression in response to TPA and increased extracellular Ca2+ (Figure 3, A and B) ▶ does not exclude a p53-mediated response, 30 induction of p21WAF1 by TPA in HaCaT cells provides evidence for a p53-independent mechanism of action in human keratinocytes, consistent with observations in p53-null mouse keratinocytes. 29
Three main conclusions can be drawn from the results presented. TPA and increased [Ca2+]o induced up-regulation of p21WAF1 in human keratinocytes with different time courses. Up-regulation of p21WAF1 by both TPA and increased [Ca2+]o in human keratinocytes is dependent in part on PKC activation. Finally, up-regulation of p21WAF1 by TPA occurs through a p53-independent mechanism.
Acknowledgments
We thank Dr Eugene Healy and Professor Jonathan Rees for critical review of the manuscript and helpful suggestions.
Footnotes
Address reprint requests to Dr. Nicholas J. Reynolds, Department of Dermatology, Medical School, University of Newcastle upon Tyne, Newcastle upon Tyne NE2 4HH, UK. E-mail: n.j.reynolds@ncl.ac.uk.
Supported in part by British Skin Foundation Grant 148/97 (to NJR).
References
- 1.Fuchs E: Epidermal differentiation: the bare essentials. J Cell Biol 1990, 111:1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard BA, Asselineau D, Schaffar-Deshayes L, Darmon MY: Abnormal sequence of expression of differentiation markers in psoriatic epidermis: inversion of two steps in the differentiation program? J Invest Dermatol 1988, 90:801-805 [DOI] [PubMed] [Google Scholar]
- 3.Weiss RA, Guillet GY, Freedberg IM, Farmer ER, Small EA, Weiss MM, Sun TT: The use of monoclonal antibody to keratin in human epidermal disease: alterations in immunohistochemical staining pattern. J Invest Dermatol 1983, 81:224-230 [DOI] [PubMed] [Google Scholar]
- 4.Markey AC, Lane EB, Churchill LJ, MacDonald DM, Leigh IM: Expression of simple epithelial keratins 8 and 18 in epidermal neoplasia. J Invest Dermatol 1991, 97:763-770 [DOI] [PubMed] [Google Scholar]
- 5.Parkinson EK: Defective responses of transformed keratinocytes to terminal differentiation stimuli: their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br J Cancer 1983, 52:479-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuspa SH: The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis–thirty-third G. H. A. Clowes Memorial Award Lecture. Cancer Res 1994, 54:1178-1189 [PubMed] [Google Scholar]
- 7.Hawley-Nelson P, Stanley JR, Schmidt J, Gullino M, Yuspa SH: The tumor promoter 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp Cell Res 1982, 137:155-167 [DOI] [PubMed] [Google Scholar]
- 8.Boyce ST, Ham RG: Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol 1983, 81:33S-40S [DOI] [PubMed] [Google Scholar]
- 9.Menon GK, Elias PM: Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol 1991, 127:57-63 [PubMed] [Google Scholar]
- 10.Pillai S, Bikle DD, Mancianti ML, Cline P, Hincenbergs M: Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol 1990, 143:294-302 [DOI] [PubMed] [Google Scholar]
- 11.Dlugosz AA, Yuspa SH: Coordinate changes in gene expression which mark the spinous to granular cell transition in epidermis are regulated by protein kinase C. J Cell Biol 1993, 120:217-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders NA, Bernacki SH, Vollberg TM, Jetten AM: Regulation of transglutaminase type I expression in squamous differentiating rabbit tracheal epithelial cells and human epidermal keratinocytes: effects of retinoic acid and phorbol esters. Mol Endocrinol 1993, 7:387-398 [DOI] [PubMed] [Google Scholar]
- 13.Reynolds NJ, Baldassare JJ, Henderson PA, Shuler JL, Ballas LM, Burns DJ, Moomaw CR, Fisher GJ: Translocation and downregulation of protein kinase C isoenzymes α and ε by phorbol ester and bryostatin-1 in human keratinocytes and fibroblasts. J Invest Dermatol 1994, 103:364-369 [DOI] [PubMed] [Google Scholar]
- 14.Isseroff RR, Stephens LE, Gross JL: Subcellular distribution of protein kinase C/phorbol ester receptors in differentiating mouse keratinocytes. J Cell Physiol 1989, 141:235-242 [DOI] [PubMed] [Google Scholar]
- 15.Lee E, Yuspa SH: Changes in inositol phosphate metabolism are associated with terminal differentiation and neoplasia in mouse keratinocytes. Carcinogenesis 1991, 12:1651-1658 [DOI] [PubMed] [Google Scholar]
- 16.Ruhl KK, Pomidor MM, Rhim JS, Tuan RS, Hickok NJ: Post-transcriptional suppression of human ornithine decarboxylase gene expression by phorbol esters in human keratinocytes. J Invest Dermatol 1994, 103:687-692 [DOI] [PubMed] [Google Scholar]
- 17.Fischer SM, Lee ML, Maldve RE, Morris RJ, Trono D, Burow DL, Butler AP, Pavone A, Warren B: Association of protein kinase C activation with induction of ornithine decarboxylase in murine but not human keratinocyte cultures. Mol Carcinog 1993, 7:228-237 [DOI] [PubMed] [Google Scholar]
- 18.Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB: Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 1995, 267:1018-1021 [DOI] [PubMed] [Google Scholar]
- 19.Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, Olson EN, Harper JW, Elledge SJ: p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 1995, 267:1024-1027 [DOI] [PubMed] [Google Scholar]
- 20.Skapek SX, Rhee J, Spicer DB, Lassar AB: Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 1995, 267:1022-1024 [DOI] [PubMed] [Google Scholar]
- 21.El-Deiry WS, Tokino T, Waldman T, Oliner JD, Burell M, Hill DE, Rees JL, Hamilton SR, Kinzler KW, Vogelstein B: Topological control of p21WAF1 expression in normal and neoplastic tissues. Cancer Res 1995, 55:2910-2919 [PubMed] [Google Scholar]
- 22.Jiang H, Lin J, Su ZZ, Collart FR, Huberman E, Fisher PB: Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene 1994, 9:3397-3406 [PubMed] [Google Scholar]
- 23.Steinman RA, Hoffman B, Iro A, Guillouf C, Liebermann DA, el-Houseini ME: Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene 1994, 9:3389-3396 [PubMed] [Google Scholar]
- 24.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 25.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75:805-816 [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y, Zhang H, Beach D: D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 1992, 71:505-514 [DOI] [PubMed] [Google Scholar]
- 27.El-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, Wiman KG, Mercer WE, Kastan MB, Kohn KW, Elledge SJ, Kinzler KW, Vogelstein B: WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994, 54:1169-1174 [PubMed] [Google Scholar]
- 28.Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D: Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res 1994, 54:3391-3395 [PubMed] [Google Scholar]
- 29.Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP: Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA 1995, 92:5451-5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg WC, Azzoli CG, Chapman K, Levine AJ, Yuspa SH: p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene 1995, 10:2271-2279 [PubMed] [Google Scholar]
- 31.Healy E, Reynolds NJ, Smith MD, Harrison D, Doherty E, Campbell C, Rees JL: Up-regulation of p21WAF1/CIP1 in psoriasis and after the application of irritants and tape stripping. J Invest Dermatol 1995, 105:274-279 [DOI] [PubMed] [Google Scholar]
- 32.Tron VA, Tang L, Yong WP, Trotter MJ: Differentiation-associated overexpression of the cyclin-dependent kinase inhibitor p21waf-1 in human cutaneous squamous cell carcinoma. Am J Pathol 1996, 149:1139-1146 [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpe GR, Fisher C, Gillespie JI, Greenwell JR: Growth and differentiation stimuli induce different and distinct increases in intracellular free calcium in human keratinocytes. Arch Dermatol Res 1993, 284:445-450 [DOI] [PubMed] [Google Scholar]
- 34.Pittelkow MR, Scott RE: New techniques for the in vitro culture of human skin keratinocytes and perspectives on their use for grafting of patients with extensive burns. Mayo Clin Proc 1986, 61:771-777 [DOI] [PubMed] [Google Scholar]
- 35.Frey MR, Saxon ML, Zhao X, Rollins A, Evans SS, Black JD: Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem 1997, 272:9424-9435 [DOI] [PubMed] [Google Scholar]
- 36.Harris W, Hill CH, Lewis EJ, Nixon JS, Wilkinson SE: Protein kinase C inhibitors. Drug Future 1993, 18:727-735 [Google Scholar]
- 37.Calautti E, Missero C, Stein PL, Ezzell RM, Dotto GP: fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Dev 1995, 9:2279-2291 [DOI] [PubMed] [Google Scholar]
- 38.Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA, Metcalf RA, Stampfer MR, Fusenig N, Rogan EM, Harris CC: p53 mutations in human immortalized epithelial cell lines. Carcinogenesis 1993, 14:833-839 [DOI] [PubMed] [Google Scholar]
- 39.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF: Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA 1995, 92:5545-5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younus J, Gilchrest BA: Modulation of mRNA levels during human keratinocyte differentiation. J Cell Physiol 1992, 152:232-239 [DOI] [PubMed] [Google Scholar]
- 41.Jones KT, Sharpe GR: Staurosporine, a non-specific PKC inhibitor, induces keratinocyte differentiation and raises intracellular calcium, but Ro31-8220, a specific inhibitor, does not. J Cell Physiol 1994, 159:324-330 [DOI] [PubMed] [Google Scholar]
- 42.Talwar HS, Fisher GJ, Harris VA, Voorhees JJ: Agonist-induced hydrolysis of phosphoinositides and formation of 1,2-diacylglycerol in adult human keratinocytes. J Invest Dermatol 1989, 93:241-245 [DOI] [PubMed] [Google Scholar]
- 43.Sharpe GR, Gillespie JL, Greenwell GR: An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Lett 1989, 254:25-28 [DOI] [PubMed] [Google Scholar]
- 44.D’Angelo DD, Oliver BG, Davis MG, McCluskey TS, Dorn GW: Novel role for Sp1 in phorbol ester enhancement of human platelet thromboxane receptor gene expression. J Biol Chem 1996, 271:19696-19704 [DOI] [PubMed] [Google Scholar]
- 45.Prowse DM, Bolgan L, Molnar A, Dotto GP: Involvement of the Sp3 transcription factor in induction of p21Cip1/WAF1 in keratinocyte differentiation. J Biol Chem 1997, 272:1308-1314 [DOI] [PubMed] [Google Scholar]
- 46.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82:675-684 [DOI] [PubMed] [Google Scholar]
- 47.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP: The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev 1996, 10:3065-3075 [DOI] [PubMed] [Google Scholar]