Abstract
Human tumors frequently express somatostatin receptors. However, none of the receptor subtype proteins have been individually visualized in normal or neoplastic human tissues. Here, the distribution of the sst2A receptor was investigated using immunohistochemistry with the specific anti-peptide antibody R2–88 in 47 human tumors. All tumors selected for their abundance of sst2 mRNA and/or strong binding of the sst2-preferring ligand 125I-labeled Tyr3-octreotide were specifically immunostained with R2–88. Conversely, all tumors without somatostatin binding or expressing predominantly other somatostatin receptor subtype mRNAs (sst1 or sst3) were not specifically immunostained by R2–88. Specificity was shown in immunoblots, demonstrating receptor migration as a 70-kd broad band. In immunohistochemical and immunoblotting experiments, the abolition of staining after antibody blockade with antigen peptide was demonstrated. Immunostaining was identified in cryostat and in formalin-fixed, paraffin-embedded sections. Heat-induced epitope retrieval was necessary to visualize sst2A receptors in formalin-fixed sections. Moreover, because of occasional high nonspecific staining, the demonstration of complete abolition of immunostaining by treatment with antigen peptide was a prerequisite for the correct identification of sst2A-positive tumors. The sst2A receptors were clearly located at the membrane of the tumor cells. These results provide the first localization of a somatostatin receptor subtype in human tissues at the cellular level. The sst2A receptor identification and visualization in tumors with simple immunohistochemical methods in formalin-fixed, paraffin-embedded material will open new diagnostic opportunities for pathologists.
It is well established that many human tumors can express receptors for the regulatory peptide somatostatin. 1 During the last decade, these receptors have been shown to represent the molecular basis for three different clinical applications of somatostatin analogues: a diagnostic one, namely, the in vivo visualization of somatostatin receptor-positive tumors and their metastases using 111In-labeled DTPA-octreotide (Octreoscan) scintigraphy2–4; a therapeutic one, namely, the symptomatic treatment with stable, unlabeled somatostatin analogues of somatostatin receptor-positive neuroendocrine tumors originating from the pituitary and gastroenteropancreatic systems5; and a radiotherapeutic one, involving the destruction of somatostatin receptor-positive tumors with high doses of radiolabeled somatostatin analogues. 6
Somatostatin receptors consist of a family of at least five different somatostatin receptor subtypes, 7,8 which are currently being characterized functionally. These somatostatin receptor subtypes are present in normal somatostatin target tissues 7 and are also found in various proportions in somatostatin-responsive human tumors. 9-11 One of the subtypes frequently expressed by human tumors is sst2, 10 as demonstrated by mRNA expression and ligand specificity. This observation is of clinical importance as sst2 is the human somatostatin receptor subtype with the highest affinity for commercially available, synthetic somatostatin analogues, such as octreotide. 12 The 111In-labeled DTPA-octreotide radioligand is therefore particularly efficient in localizing in vivo sst2-expressing tumors, 13,14 and octreotide therapy will be most efficient in sst2-expressing tumors. 5,14
The in vitro identification of sst2 receptors in human pathological tissues, such as neoplasms, is therefore particularly important clinically. Up to now, two in vitro methods have been used to detect these receptors: 1) binding studies on tissue homogenates 15 or tissue sections 16 (receptor autoradiography) using sst2-preferring ligands such as 125I-labeled Tyr3-octreotide and 2) sst2 mRNA analysis using either in situ hybridization methods on tissue sections 10,14 or reverse transcription polymerase chain reaction and RNAse protection assays on tissue homogenates. 11,17,18 These two methodological approaches, however, require a considerable specialized expertise, are time-consuming, frequently involve radioactive material (125I or 32P), do not always provide a high cellular resolution, and can in only one case (in situ hybridization) be performed in formalin-fixed material. An alternative specific and sensitive method to identify sst2 receptors in formalin-fixed human tissue is presently not available and would obviously be of great clinical relevance.
Recently, Schonbrunn and colleagues have developed a polyclonal somatostatin receptor antibody that, when tested in sst-transfected cells and in rat brain and pancreatic tissues, was shown to be highly specific for sst2A receptors. 19-21 The aim of the present study was therefore to evaluate this antibody immunohistochemically on tissue sections of human tumors, either formalin-fixed, frozen, or both, and to compare the results with those obtained using other available in vitro methods, namely, receptor autoradiography or in situ hybridization.
Materials and Methods
Selection of Material
Two types of tumor samples were selected for this study. 1) Frozen samples from 24 different tumors, which were characterized for their somatostatin receptor content by receptor autoradiography using the sst2-preferring 125I-labeled Tyr3-octreotide and the universal somatostatin receptor ligand 125I-labeled Leu8-DTrp22-Tyr25-somatostatin-28 (LTT-SS-28) 16 (these tumors were also tested for their sst mRNA using in situ hybridization 10 whenever possible). 2) Twenty-three other samples were divided into one piece frozen immediately after resection and another piece fixed in formalin for the routine histopathological diagnosis. The frozen piece of tissue was used as described in 1) above. As shown in Tables 2 and 3 ▶ ▶ , these tumors were divided into somatostatin receptor-positive and somatostatin receptor-negative types; somatostatin receptor-positive tumors were further divided into sst2-expressing and sst2-lacking specimens, according to selective ligand binding and in situ hybridization results.
Table 2.
Immunohistochemical Identification of sst2A Receptors in Cryostat Sections of Selected Tumors and Comparison with Receptor Autoradiography and in Situ Hybridization
| Tumor number | Tumor type | Immunohisto- chemistry R2-88 | Receptor autoradiography | In situ hybridization, sst mRNA | |
|---|---|---|---|---|---|
| OCT-R | SS-28-R | ||||
| SS-R positive (sst2) | |||||
| 1 | Intestinal carcinoid | + | + | + | sst2+ |
| 2 | Intestinal carcinoid | + | + | + | NT |
| 3 | Bronchial carcinoid | + | + | + | sst2+ |
| 4 | Ileal carcinoid | + | + | + | NT |
| 5 | GH-secreting pituitary adenoma | + | + | + | sst2+ |
| 6 | GH-secreting pituitary adenoma | + | + | + | NT |
| 7 | Invasive lobular breast carcinoma | + | + | + | sst2+ |
| 8 | Moderately differentiated invasive ductal breast carcinoma | + | + | NT | NT |
| 9 | Meningioma, meningothelial | + | + | + | sst2+ |
| 10 | Hemangiopericytoma, tight | + | + | + | sst2+ |
| 11 | Medulloblastoma | + | + | + | sst2+ |
| 12 | Paraganglioma | + | + | + | NT |
| 13 | Lymphoma, Hodgkin’s disease, mixed cellularity | + | + | NT | NT |
| SS-R positive (sst2 negative) | |||||
| 14 | Bronchial carcinoid | − | − | + | sst1+ |
| 15 | Leiomyosarcoma, small intestine | − | − | + | sst1+ |
| SS-R negative | |||||
| 16 | Intestinal carcinoid | − | − | − | NT |
| 17 | Bronchial carcinoid | − | − | − | NT |
| 18 | Bronchial carcinoid | − | − | − | NT |
| 19 | Intestinal carcinoid | − | − | − | sst2− |
| 20 | Nonfunctioning pituitary adenoma | − | − | − | NT |
| 21 | Moderately differentiated invasive comedo carcinoma of the breast | − | − | − | NT |
| 22 | Leiomyosarcoma, abdomen | − | − | − | sst2− |
| 23 | Moderately differentiated colon adenocarcinoma | − | − | − | NT |
| 24 | Moderately differentiated squamous cell carcinoma of the esophagus | − | − | − | NT |
OCT-R, receptor autoradiography using 125I-labeled Tyr3-octreotide; SS-28-R, receptor autoradiography using 125I-labeled LTT-SS-28; NT, not tested; SS-R, somatostatin receptors.
Table 3.
Immunohistochemical Identification of sst2A Receptors in Paraffin Sections and Cryostat Sections of Selected Tumors: Comparison with Receptor Autoradiography and in Situ Hybridization
| Tumor number | Tumor type | Immunohistochemistry (R2-88) | Receptor autoradiography | In situ hybridization, sst mRNA | |||
|---|---|---|---|---|---|---|---|
| Paraffin* | Cryostat sections | ||||||
| Cooker | Microwave | OCT-R | SS-28-R | ||||
| SS-R positive (sst2) | |||||||
| 1 | Gastrinoma | + | + | + | + | + | sst2+ |
| 2 | Bronchial carcinoid | + | + | + | + | + | NT |
| 3 | Nonsecreting neuroendocrine carcinoma, duodenum | + | + | + | + | + | NT |
| 4 | Intestinal carcinoid | + | + | + | + | + | NT |
| 5 | Glucagonoma | + | + | + | + | + | sst2+ |
| 6 | Insulinoma | + | + | + | + | + | NT |
| 7 | Meningioma, fibroblastic and meningothelial | + | + | + | + | + | NT |
| 8 | Meningioma, meningothelial | + | + | + | + | + | NT |
| 9 | Meningioma, meningothelial | + | + | + | + | + | NT |
| 10 | Meningioma, meningothelial | + | + | + | + | + | sst2+ |
| 11 | Meningioma, fibroblastic and meningothelial | + | + | + | + | + | NT |
| 12 | Meningioma, fibroblastic and meningothelial | + | + | + | + | + | NT |
| 13 | Pheochromocytoma, adrenal gland | + | + | + | + | + | NT |
| SS-R positive (sst2 negative) | |||||||
| 14 | Insulinoma | − | − | − | − | + | sst3+ |
| 15 | Prostatic adenocarcinoma, moderately differentiated | − | − | − | − | + | sst1+ |
| 16 | Prostatic adenocarcinoma, moderately differentiated | − | − | − | − | + | sst1+ |
| 17 | Prostatic adenocarcinoma, well differentiated | − | − | − | − | + | sst1+ |
| SS-R negative | |||||||
| 18 | Pancreatic carcinoma, poorly differentiated | − | − | − | − | − | NT† |
| 19 | Pancreatic ductal adenocarcinoma, well differentiated | − | − | − | − | − | NT† |
| 20 | Pancreatic ductal adenocarcinoma, moderately differentiated | − | − | − | − | − | NT† |
| 21 | Pancreatic adenocarcinoma, poorly differentiated | − | − | − | − | − | NT† |
| 22 | Pancreatic ductal adenocarcinoma, poorly differentiated | − | − | − | − | − | NT† |
| 23 | Pancreatic adenocarcinoma, moderately differentiated | − | − | − | − | − | NT |
*Formalin-fixed, paraffin-embedded section pretreated by boiling in pressure cooker or microwave oven. OCT-R, receptor autoradiography using 125I-labeled Tyr3-octreotide; SS-28-R, receptor autoradiography using 125I-labeled LTT-SS-28; NT, not tested; SS-R, somatostatin receptors.
†Shown by Buscail et al17 to lack sst2 mRNA by RT-PCR.
Receptor Autoradiography
Frozen sections were incubated for 2 hours at room temperature with a 125I-labeled tyrosine-3 analogue of the somatostatin octapeptide octreotide or with the somatostatin-28 analogue, 125I-labeled LTT-SS-28, as described previously. 16 After the sections were washed, they were apposed to 3H-Hyperfilms (Amersham, Little Chalfont, UK) and exposed for 1 week in x-ray cassettes. 16 Nonspecific binding was determined in parallel sections incubated with the same concentration of labeled peptide in the presence of 10−6 mol/L of the corresponding unlabeled peptide. The autoradiograms were quantified using a computer-assisted image-processing system, as previously described. 16
In Situ Hybridization
Cryostat sections (20 μm) were used for sst1, sst2, and sst3 mRNA detection by in situ hybridization. The protocol followed was essentially that described in detail previously, 9,10 using the same oligonucleotide probes as described earlier. 9,10 They were labeled at the 3′ end using [α-32P]dATP (>3000 Ci/mmol; Amersham, Aylesbury, UK) and terminal deoxynucleotidyltransferase (Boehringer Mannheim, Mannheim, Germany) to specific activities of 0.9 × 10 4 to 2.0 × 10 4 Ci/mmol. 10,22 All necessary controls were performed as reported previously. 10
Immunohistochemical Evaluation of the sst2A Antibody R2–88
The R2–88 rabbit polyclonal antibody was used as primary antibody. R2–88 was raised against a unique sequence in the carboxyl-terminal region of the sst2A receptor, corresponding to amino acids 339 to 359 in the rat protein. 20 The identical sequence is found in the human, rat, and mouse sst2A receptor proteins, and as a result, the antibody is expected to recognize the receptor from all three species. Previous studies showed positive reactivity with the rat receptor. 19-21 The antibody does not cross-react with any of the other sst receptor subtypes. 19,20
Frozen Tissues
Ten-micron-thick sections, adjacent to the sections used for in vitro receptor autoradiography and in situ hybridization, were cut on a cryostat (Leitz).
The following basic protocol was used. The sections were fixed for 10 minutes in acetone, post-fixed for 10 minutes in 4% paraformaldehyde (diluted in PBS), and incubated for 20 minutes in 5% normal goat serum diluted in Tris-buffered saline (TBS). The sections were then incubated with the R2–88 antibody against the sst2A receptor overnight at room temperature. The antibody R2–88 was used at a 1:6000 dilution in TBS containing 1% bovine serum albumin, 5% normal goat serum, and 0.1% NaN3. Sections were then incubated in a 1:200 dilution (same buffer as for primary antibody) of biotinylated goat anti-rabbit immunoglobulin antiserum (Dako, Glostrup, Denmark) and thereafter with avidin-biotin complex/horseradish peroxidase (1:120 in TBS; Dako). Finally, sections were developed in 0.05% 3,3′-diaminobenzidine (Fluka, Buchs, Switzerland) and 0.006% H2O2 (Merck, Darmstadt, Germany), weakly counterstained with hematoxylin, and mounted. A tumor was considered to be positive for R2–88 when the immunostaining was abolished after absorption of the antibody with the peptide antigen at 100 nmol/L concentration (30 minutes at room temperature, with agitation before application of the antibody to the tissue). The tumor was considered negative if the immunostaining was not suppressed in the presence of the antigen. In preliminary experiments, titrations with different concentrations of antigen were performed, and antibody reactivity both on Western blots and in ELISAs was tested; 100 nmol/L peptide completely blocked the staining of the receptor protein on a Western blot, and in ELISAs, the peptide was bound with an EC50 of 5 nmol/L. 20
As positive control for the immunohistochemistry protocol, an adjacent section of each of the tumors was stained with a mouse monoclonal antibody against factor-VIII-related antigen (clone F8/86; Dako), using the same protocol as above.
For optimization of R2–88 antibody dilution, serial R2–88 dilutions were performed to optimize signal-to-background ratio. The 1:6000 dilution had the highest immunohistochemical signal whereas the background remained low.
Formalin-Fixed, Paraffin-Embedded Tissues
As all tumors were primarily sent and processed for diagnostic purposes, it was usually not possible to standardize the fixation conditions and, therefore, to study in detail the effect of the fixation quality on the immunohistochemical signal, as fixation time and size of the specimen could vary from one case to another. In all tumors tested, the fixation time was, however, always on the order of 24 to 36 hours. In one case, the fresh tumor tissue was split into two parts, with one-half fixed in formalin for less than 24 hours and the other half fixed for 14 days. The fixed tissue was processed for conventional, 2- to 5-μm-thick paraffin (Paraplast) sections.
Effect of Pretreatment
Several different pretreatments were performed in a selected number of tumors to determine the optimal method for antigen retrieval in formalin-fixed tissue, according to the following protocols. Dewaxed and rehydrated tissue sections were 1) left untreated in TBS, 2) digested in 0.1% trypsin (Difco, Detroit, MI) in 50 mmol/L TBS, pH 8.0, with 10 mmol/L CaCl2 for 20 minutes at 37°C, 3) digested in 0.1% Pronase E (Sigma Chemical Co., St. Louis, MO) in 50 mmol/L TBS, pH 7.5, for 6 minutes at 37°C, 4) boiled in a total volume of 600 ml (3 × 200 ml) of 10 mmol/L citrate buffer, pH 6.0, in a microwave oven once for 8 minutes at 850 W and twice for 5 minutes at 410 W, followed by an additional period of 15 minutes in the hot buffer, and 5) immersed in 1.5 L of boiling 10 mmol/L citrate buffer, pH 6.0, in a pressure cooker that was then closed and slowly, over a period of 3 to 4 minutes, brought to 121°C. After a total time of 5 minutes, the pressure cooker was cooled under running tap water and opened, and the slides were transferred to H2O at room temperature for 5 minutes. After every pretreatment, slides were washed in TBS before the application of the primary antibody.
Signal Amplification
To enhance the immunohistochemical signal, the standard protocol, as described below, was followed by an additional amplification step: biotinylated tyramine was deposited onto the section through the activity of the bound peroxidase and subsequently served as a secondary target for another layer of avidin-biotin-peroxidase. 23 We used both a commercial kit (Renaissance TSA-Indirect, NEN Life Science Products, Boston, MA), according to the manufacturer’s directions, as well as an in-house system that had been developed according to Adams. 24 Briefly, after the application of the avidin-biotin complex/horseradish peroxidase, slides were washed in TBS, incubated with 30 μmol/L biotinylated tyramine and 0.01% H2O2 in TBS, pH 8.0, for 15 minutes at room temperature, washed again in TBS, and incubated for 30 minutes with avidin-biotin complex/horseradish peroxidase. Finally, slides were developed with 3,3′-diaminobenzidine as above.
Standard Protocol for All Paraffin-Embedded Sections
Formalin-fixed, paraffin-embedded sections were dewaxed, rehydrated, and boiled in 10 mmol/L citrate buffer, pH 6.0, in a pressure cooker as described above. Sections were then (and after all subsequent steps) washed in TBS and incubated with the R2–88 polyclonal antibody against sst2A receptors overnight at room temperature. In formalin-fixed material, the antibody R2–88 was used at a dilution of 1:2000. All subsequent steps, including absorption of the antibody with the peptide antigen, were performed exactly as in the protocol for frozen tissue, and the same criteria were applied to distinguish between positive and negative tumors.
Preparation of Tumor Membranes and Immunoblotting
Frozen tumor tissue was homogenized in 1 ml of cold homogenization buffer (10 mmol/L Tris/HCl, 5 mmol/L EDTA, 3 mmol/L EGTA, 250 mmol/L sucrose, pH 7.6) containing protease inhibitors (1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml soybean trypsin inhibitor, 10 μg/ml leupeptin, and 50 μg/ml bacitracin). After a low-speed centrifugation at 500 × g for 5 minutes, membranes were pelleted at 10,000 × g for 45 minutes. Membrane proteins from the sst2A-expressing rat growth hormone (GH)-producing pituitary cell line GH-R2 cells were prepared as previously described. 25 After solubilization in sample buffer (62.5 mmol/L Tris/HCl, 2% sodium dodecyl sulfate, 10% 2-mercaptoethanol (v/v), 6 mol/L urea, and 20% glycerol, pH 6.8) at 60°C for 15 minutes, proteins were subjected to SDS-polyacrylamide gel electrophoresis on 7.5% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. 25 Receptor expression was determined by immunoblotting with a 1:10,000 dilution of R2–88. 20
Results
Western Blot
Figure 1 ▶ demonstrates that the antibody R2–88 recognizes a single protein in each tumor sample and that reaction with this protein is blocked when the antiserum is incubated with the peptide. In each case, this specifically stained protein migrates as a diffuse band, consistent with glycosylation, and is similar in size to the sst2A receptor expressed in a GH rat pituitary tumor cell line (Figure 1) ▶ as well as in Chinese hamster ovary cells. 20 The observation that the receptor protein from different tumors migrates slightly differently indicates that glycosylation is variable. However, as ligand binding was observed with both of these tumors (see Table 2 ▶ ), glycosylation does not have a major effect on peptide recognition.
Figure 1.
Western blot analysis of sst2A receptor immunoreactivity in tumor tissue. Membrane proteins from sst2A-transfected GH-R2 cells (GH; 2.5 μg), a GH-producing pituitary adenoma (tumor 5 in Table 2 ▶ ) (Pit; 60 μg), and a meningioma (tumor 9 in Table 2 ▶ ) (Men; 90 μg) were separated by PAGE and electrophoretically transferred to PVDF membrane. The membrane was incubated with sst2A receptor antibody in the absence (left panel) or presence (right panel) of 100 nmol/L antigen peptide. Molecular size markers are shown on the left.
Methodological Developments
Figure 2 ▶ shows the results obtained with R2–88 using a standard immunohistochemical protocol for frozen specimens. It represents a bronchial carcinoid tumor with two different tumor parts: a central tumor region expressing only sst1 mRNA and lateral regions expressing both sst1 and sst2 mRNAs, as illustrated with in situ hybridization methods. 125I-labeled Tyr3-octreotide binding is exclusively seen in the lateral parts; the immunohistochemical staining for R2–88 is also detected in these lateral parts, and only in these. In this positively immunostained tumor, absorption with 100 nmol/L peptide antigen eliminates completely the immunostaining, as a further proof of specificity.
Figure 2.
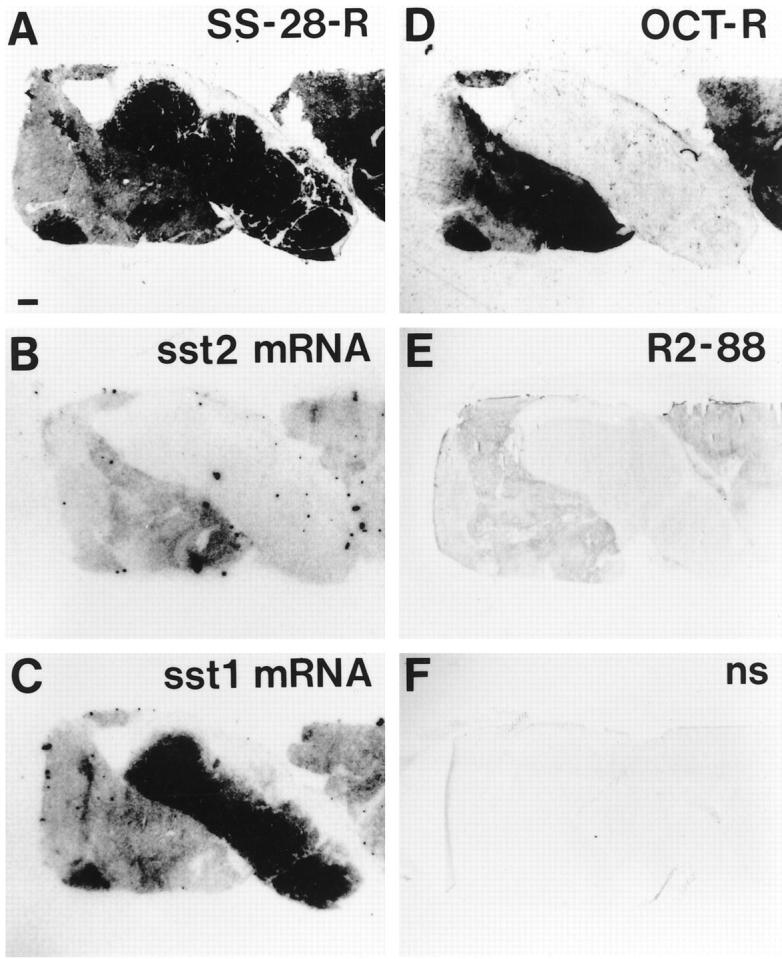
Immunohistochemical staining with R2–88 of a bronchial carcinoid tumor (frozen sample). Comparison with receptor autoradiography and in situ hybridization. A: Autoradiogram showing total binding of 125I-labeled LTT-SS-28 in the whole tumor, with particularly strong labeling of the central part. Bar, 1 mm. B: Autoradiogram showing sst2 mRNA located in the lateral parts of the tumor but not in the central part. C: Autoradiogram showing sst1 mRNA. It is abundant in the central part and detected in moderate amounts in the lateral parts. D: Autoradiogram showing total binding of 125I-labeled Tyr3-octreotide located in the lateral parts but not in the central part. E: Immunohistochemical staining with R2–88 located in the lateral parts but not in the central part. This cryostat section was not hematoxylin counterstained. F: Control, showing lack of R2–88 staining after absorption with 100 nmol/L peptide antigen (nonspecific staining); section not counterstained.
In paraffin-embedded specimens, an adequate immunohistochemical signal is detected only after specific pretreatment of the sections or, to a lesser extent, after tyramide amplification. Table 1 ▶ shows that heat-induced epitope retrieval methods, using either a pressure cooker or a microwave oven, give a strong signal with R2–88; however, in the absence of such a pretreatment as well as after Pronase or trypsin digestion of the sections, R2–88 does not reveal sst2A-positive tumors. Tyramide amplification gives a weak signal, which is, moreover, not completely eliminated by absorption with 100 nmol/L peptide antigen. This suggests that parts of the immunohistochemical staining with R2–88 in the presence of tyramide is nonspecific, whereas the immunohistochemical staining of the tumor (after boiling) appears highly specific. Figure 3 ▶ illustrates the various effects of pretreatment, and Figure 4 ▶ shows the effect of tyramide amplification on the R2–88 immunostaining of sst2A-expressing neuroendocrine tumors. The incomplete specificity of the tyramide amplification signal is clearly evident in this example.
Table 1.
Immunohistochemical Identification of sst2A Receptors: Effect of Various Pretreatments or Tyramide Amplification in Paraffin Sections
| Pretreatment* | No pretreatment | |||||
|---|---|---|---|---|---|---|
| Pronase | Trypsin | Boiling (microwave) | Boiling (cooker) | No tyramide amplification | Tyramide amplification | |
| Without peptide antigen | − | − | + | + | − | + |
| With peptide antigen (nonspecific binding) | − | − | − | − | − | (+) |
Results are representative from four different sst2-expressing tumors. −, no immunohistochemical signal in tumor; +, immunohistochemical signal in tumor; (+), immunohistochemical signal in tumor is only partly abolished in the presence of excess peptide antigen.
*Without tyramide amplification.
Figure 3.

Effects of various pretreatments on the immunohistochemical staining with R2–88 of a paraffin-embedded duodenal neuroendocrine carcinoma. A and B: Identification of 125I-labeled Tyr3-octreotide binding in cryostat sections of a frozen sample of the tumor. A: H&E-stained section. T, tumor. Bar, 1 mm. B: Autoradiogram of the total binding of 125I-labeled Tyr3-octreotide showing strong labeling of the tumor. C to H: Immunohistochemical R2–88 staining of paraffin-embedded samples adjacent to the above-mentioned frozen sample. T, tumor; m, mucosa. C: Pretreatment with boiling in a pressure cooker results in marked immunoreactivity of the tumor. D: Absorption with 100 nmol/L peptide antigen (nonspecific staining) of a section pretreated with boiling in a pressure cooker abolishes the immunoreactivity of the tumor. E: Pretreatment with boiling in a microwave oven results in a positive immunoreactivity comparable to that seen in C. F: Absorption with 100 nmol/L peptide antigen (nonspecific staining) of a section pretreated with boiling in a microwave oven abolishes the immunoreactivity. G: Pretreatment with Pronase does not yield a satisfactory immunoreactivity. H: Pretreatment with trypsin yields a negative result as in G. Only pretreatments with boiling in a pressure cooker or microwave oven give a strong and specific immunostaining.
Figure 4.

R2–88 immunohistochemistry of a gastrinoma: effect of tyramide amplification compared with boiling (microwave oven) pretreatment (paraffin sections). A: Boiling pretreatment showing the tumor R2–88 immunostaining. Bar, 1 mm. B: Boiling pretreatment including absorption with 100 nmol/L peptide antigen abolishes the reactivity of the tumor (nonspecific staining). C: Tyramide amplification of a non-pretreated, adjacent section of the tumor results in a moderate R2–88 reactivity of the tumor. D: Tyramide amplification, including absorption with 100 nmol/L peptide antigen, shows a marked nonspecific staining of the tumor. The tumor is less intensely immunostained with tyramide amplification than with boiling pretreatment. Moreover, the nonspecific staining is more intense with tyramide amplification.
The specificity control of the R2–88 immunostaining in paraffin-embedded sections is illustrated in Figure 5 ▶ with two exocrine pancreatic tumors that lack somatostatin receptor expression and that do not react with R2–88 specifically. It is important to note that one case (Figure 5 ▶ , right) shows a strong labeling of the tumor pancreatic ducts, which is not abolished by preincubation of the antibody with the peptide antigen. Therefore, some somatostatin receptor-negative tumors may react nonspecifically with R2–88 serum. Thus, preabsorption with the peptide antigen is a prerequisite for the specificity control of every single tumor. As positive control, this same section (Figure 5) ▶ shows sst2-expressing normal pancreatic islets, 26 which are specifically stained with R2–88. As positive controls, R2–88-immunoreactive sst2A receptors can also be identified in germinal centers of lymphatic follicles 27 located in the surroundings of other somatostatin receptor-negative tumors (data not shown).
Figure 5.

R2–88 immunohistochemistry in two somatostatin receptor-negative pancreatic tumors: a poorly differentiated pancreatic carcinoma (A to C, left vertical column) and a well differentiated ductal pancreatic adenocarcinoma (D to F, right vertical column). Paraffin sections. A and D: H&E-stained sections. Bar, 1 mm. Arrowheads indicate ductal carcinoma, and arrows indicate pancreatic islets. B and E: Immunohistochemistry with R2–88. No immunostaining is seen in B. E, however, shows stained islets (arrows) and a stained ductal carcinoma with tubulopapillary structure (arrowheads). C and F: Immunohistochemistry with R2–88 after absorption with 100 nmol/L peptide antigen. In F, the staining of the islets is completely abolished whereas the staining of the neoplastic ducts remains visible. The ductal staining is therefore not recognized by the antipeptide antibody R2–88 (nonspecific staining). There is no counterstain in E and F. Whereas both tumors are sst2A receptor negative, the staining of the islets is inhibited by the peptide, demonstrating that it is specific and representing an internal control.
Figure 6 ▶ illustrates the specificity of immunostaining in both frozen and paraffin-embedded sections. The left column is an example of a gastrointestinal carcinoid, with abundant sst2 mRNA as well as with a strong 125I-labeled Tyr3-octreotide and 125I-labeled LTT-SS-28 binding; this tumor yields a positive immunohistochemical signal with R2–88 in cryostat sections. Conversely, in the middle column, an sst1-expressing leiomyosarcoma with abundant sst1 mRNA as well as with strong 125I-labeled LTT-SS-28 binding, but lacking 125I-labeled Tyr3-octreotide binding as well as sst2 mRNA, does not react with R2–88. Finally, the right column shows an sst3-expressing insulinoma, with no 125I-labeled Tyr3-octreotide binding and no sst2 mRNA but strong 125I-labeled LTT-SS-28 binding and abundant sst3 mRNA; paraffin sections of this tumor are, as expected, not immunostained with R2–88.
Figure 6.
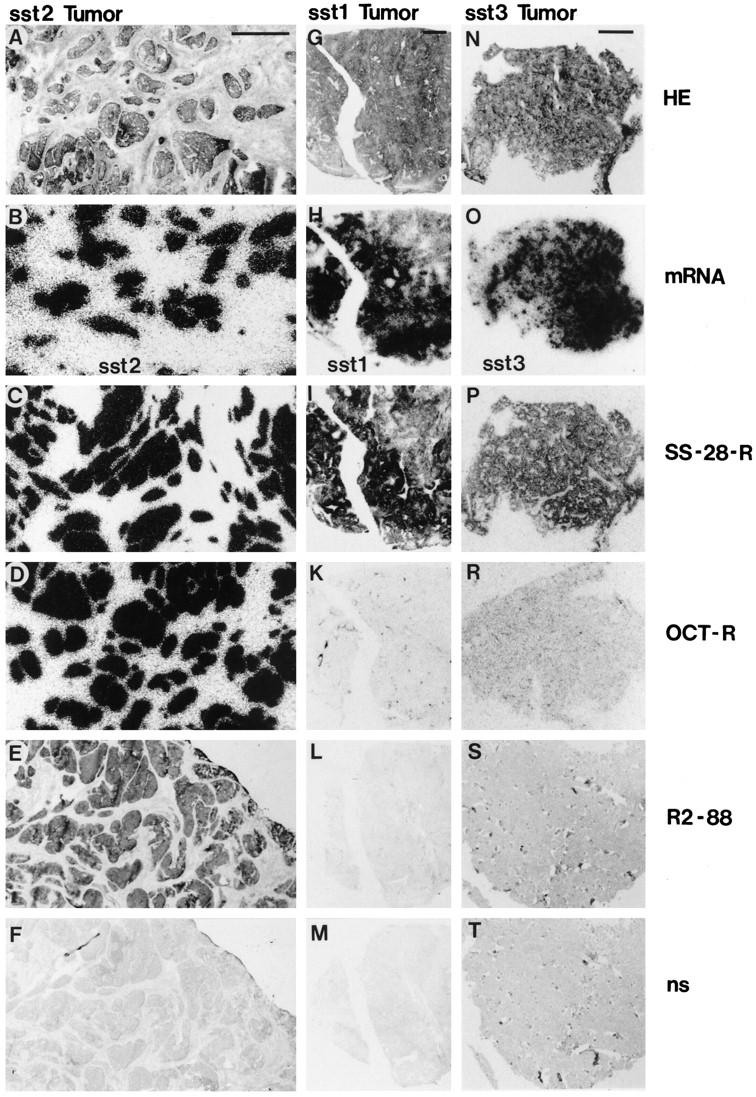
R2–88 immunohistochemical staining of tumors in relation to their respective somatostatin receptor subtype expression. The sst2A receptor immunostaining is compared with the receptor content measured by receptor autoradiography and to the mRNA content measured by in situ hybridization in an sst2-expressing gastrointestinal carcinoid (A to F, first vertical column), an sst1-expressing leiomyosarcoma (G to M, middle vertical column), and an sst3-expressing insulinoma (N to T, last vertical column). A, G, and N: H&E-stained sections. Bar, 1 mm. B, H, and O: Autoradiograms showing in situ hybridization for sst2 mRNA (B), for sst1 mRNA (H), and for sst3 mRNA (O). The sst1- and the sst3-expressing tumors do not express measurable amounts of sst2 receptors. C, I, and P: Autoradiograms showing total binding of the universal ligand 125I-labeled LTT-SS-28. All three tumors were labeled. D, K, and R: Autoradiograms showing total binding of the sst2-preferring 125I-labeled Tyr3-octreotide. Only the sst2-expressing tumor (D) was labeled. E, L, and S: Immunohistochemical R2–88 staining. Only the sst2-expressing tumor was stained (E). E and L were cut from frozen samples; S was from formalin-fixed, paraffin-embedded tissue. F, M, and T: Controls, showing lack of R2–88 immunohistochemical staining after absorption with 100 nmol/L peptide antigen (nonspecific staining). The tumor strands stained in E are no longer immunoreactive in F; only the weak hematoxylin counterstain remains visible.
Figure 7 ▶ documents that cryostat sections or paraffin-embedded sections give similar results with R2–88 in an example of an sst2-expressing glucagonoma. In addition, this figure shows that the quality and intensity of the immunostaining with R2–88 is relatively insensitive to the fixation time with formalin, as a 14-day-long fixation is able to produce a positive immunostaining with R2–88, comparable to a 12-hour fixation time.
Figure 7.

Comparison of the R2–88 immunohistochemistry in paraffin-embedded and in frozen samples of the same sst2-expressing glucagonoma. A: Autoradiogram showing total binding of 125I-labeled Tyr3-octreotide in this tumor. B: R2–88 immunohistochemistry in a frozen sample of the tumor described above. Section is adjacent to that used in A. The tumor is immunoreactive. C: R2–88 immunohistochemistry in a paraffin-embedded sample fixed for 12 hours in formalin. The tumor is immunoreactive. D: R2–88 immunohistochemistry in another paraffin-embedded sample of the same tumor fixed for 14 days in formalin. The tumor is also immunoreactive. In B to D, absorption with 100 nmol/L peptide antigen on adjacent sections prevented completely the immunostaining (not shown).
Figure 8 ▶ shows representative immunostaining with R2–88 of a gastrinoma, using the above-described standard protocol for paraffin-embedded sections pretreated with a pressure cooker. It shows a precise immunohistochemical staining of the tumor cells. The staining is completely abolished with antibody absorption by 100 nmol/L peptide antigen. The sst2A receptors are seen to be preferentially located on the cell membranes and expressed by the great majority of the tumor cells. Note the specificity of the labeling of tumor cells, as compared with adjacent ducts that are clearly unstained. No marked difference in the immunostaining quality at the cellular level is observed between boiling pretreatments using a microwave oven or a pressure cooker. In those cases with a weak immunostaining of the tumor cells, we have noticed that omitting the counterstaining of the sections usually allows a clearer identification of the membrane-bound receptor distribution, which is completely abolished by peptide absorption, as expected.
Figure 8.

R2–88 immunohistochemistry showing the membrane-bound localization of the sst2A receptors and its tissue selectivity in a gastrinoma at high magnification (paraffin sections). A: The brown immunoreactivity is predominantly located on the cell membrane of the tumor cells. Bar, 25 μm. B: The tumor cells but not the three pancreatic ducts (middle) are sst2A receptor positive. Bar, 50 μm. C: Membrane-bound sst2A receptors at high magnification. Bar, 16 μm. D: Adjacent section showing that absorption with 100 nmol/L peptide antigen abolishes the staining of the membrane. Weak residual, nonspecific brown staining is seen in the connective tissue. Bar, 16 μm. In C and D, cell bodies are stained in blue (hematoxylin).
Comparative Analysis of 47 Human Tumors
Tables 2 and 3 ▶ ▶ describe an extensive analysis of 47 tumors and compare the R2–88 immunohistochemistry with results of other methods, including receptor binding and in situ hybridization for sst mRNA. Table 2 ▶ shows that the antibody R2–88 detects immunohistochemically sst2A receptors expressed in cryostat sections of human tumors. A positive immunohistochemical signal is found in a variety of tumors, displaying a high-affinity binding for both 125I-labeled LTT-SS-28 and 125I-labeled Tyr3-octreotide. Some of these tumors had been shown by in situ hybridization to contain sst2 mRNA as the only or the predominant receptor subtype. Conversely, tumors expressing somatostatin receptor subtypes different from sst2 by in situ hybridization and having high-affinity binding only for the universal ligand 125I-labeled LTT-SS-28 but not for 125I-labeled Tyr3-octreotide (Table 2) ▶ do not react with R2–88. Finally, none of the somatostatin receptor-negative tumors, lacking both 125I-labeled LTT-SS-28 and 125I-labeled Tyr3-octreotide binding, react with R2–88 (Table 2) ▶ . Table 3 ▶ shows another series of human tumors, where sst2A somatostatin receptors are detected by R2–88 in formalin-fixed, paraffin-embedded sections. A positive immunohistochemical signal is found in all tumors having both 125I-labeled LTT-SS-28 and 125I-labeled Tyr3-octreotide binding and showing abundant sst2 mRNA. Conversely, tumors expressing somatostatin receptor subtypes different from sst2, ie, sst1 or sst3, and having high-affinity binding only for the universal ligand 125I-labeled LTT-SS-28, but not for 125I-labeled Tyr3-octreotide (Table 3) ▶ , do not react with R2–88. None of a series of somatostatin receptor-negative exocrine pancreatic tumors known to lack 125I-labeled Tyr3-octreotide binding 28 and to lack sst2 receptor mRNA 17 react with R2–88 (Table 3) ▶ . Furthermore, an exact correlation is observed between the immunohistochemical results in the paraffin-embedded sections and those in the cryostat sections of a given tumor (Table 3) ▶ .
Discussion
The present study represents the first immunohistochemical identification of sst2A receptors in human tissues, namely, in somatostatin receptor-expressing human tumors. Some of these tumors, as for instance neuroendocrine gastroenteropancreatic tumors or meningiomas, are optimally suited for such an evaluation as they usually have a very high somatostatin receptor density. 16,29 The tumors included in the study have been extensively characterized biochemically, pharmacologically, and molecularly for their somatostatin receptor content. Our study clearly shows that the R2–88 antiserum, which is known to be specific for sst2A and not to recognize any other sst receptor subtype, 19 can specifically label the sst2-expressing tumor tissue. In the present study, specificity of labeling can be seen at several levels: 1) only somatostatin receptor-expressing tumors can be labeled and 2) among them, only those tumors binding the sst2-preferring ligand 125I-labeled Tyr3-octreotide and having abundant sst2 mRNA, as determined by in situ hybridization, are labeled. The correlation between R2–88 immunostaining and 125I-labeled Tyr3-octreotide or sst2 mRNA measurements is excellent. Conversely, tumors showing 125I-labeled LTT-SS-28 binding but no 125I-labeled Tyr3-octreotide binding, and having an abundance of sst1 or sst3 mRNA rather than sst2 mRNA, are not labeled. 3) The immunohistochemical staining can be completely abolished by absorption of the antiserum with the antigen peptide. 4) In two sst2-expressing tumors, R2–88 antiserum recognizes a single protein by Western blot. Excess peptide blocks the antibody binding to this protein, demonstrating that the antipeptide antibody is the reactive moiety. The size and profile of the reactive protein on SDS-polyacrylamide gels is similar to that of sst2A expressed by transfection in pituitary tumor cells. Moreover, the antibody does not cross-react with other tissue proteins.
These results show for the first time that the sst2A receptor protein is expressed in human tumors, as previous methods of analysis did not differentiate between the sst2A and sst2B isoforms; both bind octreotide and both splice variants are detected by in situ hybridization experiments. The results show that all tumors that were previously shown to express sst2 mRNA express the unspliced sst2A receptor protein. These results are in agreement with a recent RT-PCR study by Panetta and Patel 11 showing that sst2A mRNA is frequently expressed in tumors. It is not yet clearly established whether sst2B protein is expressed in human tumors. 15 As the two splice sst2 variants have been suggested to vary in their signaling properties and regulation, 25,30,31 the expression of the sst2A form by human tumors is of functional significance.
To identify sst2A immunohistochemically, several technical requirements have to be considered. First, it should be stressed that the time of fixation in formalin does not seem to be crucial, as a comparable immunohistochemical signal is seen in the 12-hour and in the 14-day fixed tissue. However, it is essential to pretreat the formalin-fixed and paraffin-embedded sections for optimal antigen retrieval; without a specific pretreatment of the sections, no signal can be obtained. Only two pretreatments are efficient, namely, boiling treatments using a microwave oven or a pressure cooker; both give similarly good R2–88 immunostaining and the quality of the histopathological sections remains very good as well. Pronase and trypsin digestion treatment, however, do not allow detection of sst2A receptors with R2–88. Furthermore, the tyramide amplification method, which was successfully used in studies performed in intravitally perfused rat brain and pancreas, 19,21 gave a relatively weak immunohistochemical signal and a high nonspecific signal in tumor and surroundings; in tumor tissues, therefore, the tyramide amplification was less satisfactory than boiling pretreatments for the sst2A receptor visualization.
The immunohistochemical evaluation of sst2A can be performed in cryostat sections as well as in paraffin-embedded material. This second option is of considerable interest for the pathologist as it gives for the first time the possibility to evaluate somatostatin receptors in the routinely processed archival paraffin-embedded material of any diagnostic pathology center. 1 Although both methods are similar in terms of signal intensity, the paraffin-embedded sections have the advantage of a better histological quality and, therefore, better cellular resolution. With both methods, the membrane-bound localization of the sst2A receptors, as shown previously in rat brain with the same antibody, 21 is particularly well identified. This membrane-bound localization of the receptors can be even more clearly recognized when the hematoxylin counterstaining is weak or omitted.
It is well established that the immunohistochemical use of most antibodies can give specific as well as occasional nonspecific staining. This applies also to the R2–88 antibody. In contrast to most conventional immunohistochemical procedures in diagnostic histopathology, it is therefore mandatory with R2–88 to perform in every single case a control experiment involving absorption of the antibody with the antigen peptide; only those tissues with complete abolition of the staining by saturating peptide can be considered to be sst2A receptor positive. If the peptide does not block the staining, the tissue is considered to be nonspecifically labeled, ie, sst2A receptor-negative, as seen in some somatostatin receptor-negative exocrine pancreatic cancers, for instance.
Although one can expect that the immunohistochemical detection of sst2A will be less sensitive than receptor binding with 125I-labeled Tyr3-octreotide, the sensitivity of R2–88 under the conditions developed here, ie, with adequate pretreatment, appears sufficiently good as all sst2 receptor-positive tumors of the present study were found to be sst2A immunoreactive and as normal human somatostatin target tissues, such as pancreatic islets 26 or germinal centers of lymphatic follicles, 27 were also unequivocally identified with R2–88.
This study shows for the first time that several methods, including receptor binding, in situ hybridization, and immunohistochemistry, can be combined and correlated to identify sst2A receptors in human tumors. The immunohistochemical identification of sst2A is therefore an important confirmation of the adequacy of all other previously performed in situ methods, ie, receptor autoradiography and in situ hybridization, used for many years for somatostatin receptor and somatostatin receptor subtype evaluation in various human tissues. 1,9,16
The present investigation not only opens the gate for additional basic morphological investigations of sst2A receptors in human tumors and in normal human tissues, but it brings also, as an immediate consequence, a simple and rapid somatostatin receptor evaluation in the hand of the pathologist, with three major advantages. 1) This new method can analyze somatostatin receptors in paraffin-embedded tissues for the first time. 2) It requires only an immunopathological laboratory to perform the test and can be carried out without the complex and time-consuming receptor autoradiography or other techniques. 1 3) The entire immunohistochemical procedure requires less than 24 hours. This new method is likely to be useful in the following situations: 1) differential diagnosis of selected tumors, ie, sst2-expressing endocrine versus sst2-negative exocrine pancreatic tumors, 16,17 2) evaluation of the diagnostic potential of Octreoscan to visualize an individual tumor and its metastases, 3) evaluation of the potential clinical efficacy of octreotide and other stable sst2-preferring somatostatin analogues for the symptomatic therapy of gastroenteropancreatic and pituitary tumors, 4) evaluation of the potential for radiotherapy with radiolabeled octreotide analogues, and 5) evaluation of the prognosis of selected tumor types, in particular, neuroblastomas, which were shown previously to express somatostatin receptors of the sst2 type preferentially in those cases with favorable prognosis. 32,33
Footnotes
Address reprint requests to Dr. J. C. Reubi, Professor of Pathology, Division of Cell Biology and Experimental Cancer Research, Institute of Pathology, University of Berne, Murtenstrasse 31, CH-3010 Berne, Switzerland.
References
- 1.Reubi JC: Relevance of somatostatin receptors and other peptide receptors in pathology. Endocr Pathol 1997, 8:11-20 [DOI] [PubMed] [Google Scholar]
- 2.Krenning EP, Bakker WH, Breeman WAP, Koper JW, Kooij PPM, Ausema L, Lameris JS, Reubi JC, Lamberts SWJ: Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, I:242-244 [DOI] [PubMed] [Google Scholar]
- 3.Lamberts SWJ, Bakker WH, Reubi JC, Krenning EP: Somatostatin-receptor imaging in the localization of endocrine tumors. N Engl J Med 1990, 323:1246-1249 [DOI] [PubMed] [Google Scholar]
- 4.Reubi JC: Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 1995, 36:1825-1835 [PubMed] [Google Scholar]
- 5.Lamberts SWJ, Krenning EP, Reubi JC: The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev 1991, 12:450-482 [DOI] [PubMed] [Google Scholar]
- 6.Krenning EP, Valkema R, Kooij PPM, Breeman WAP, Bakker WH, Kwekkeboom DJ, de Jong M, Reubi JC, Pauwels S, Mäcke H: Radionuclide therapy with chelated octreotides. 44th Annual Meeting of Continuing Education Course Manual. 1997:pp 290-293 Society of Nuclear Medicine, Reston VA,
- 7.Reisine T, Bell GI: Molecular biology of somatostatin receptors. Endocr Rev 1995, 16:427-442 [DOI] [PubMed] [Google Scholar]
- 8.Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB: The somatostatin receptor family. Life Sci 1995, 57:1249-1265 [DOI] [PubMed] [Google Scholar]
- 9.Reubi JC, Schaer JC, Waser B, Mengod G: Expression and localization of somatostatin receptor SSTR1, SSTR2 and SSTR3 mRNAs in primary human tumors using in situ hybridization. Cancer Res 1994, 54:3455-3459 [PubMed] [Google Scholar]
- 10.Schaer JC, Waser B, Mengod G, Reubi JC: Somatostatin receptor subtypes sst1, sst2, sst3, and sst5 expression in human pituitary, gastroenteropancreatic and mammary tumors: comparison of mRNA analysis with receptor autoradiography. Int J Cancer 1997, 70:530-537 [DOI] [PubMed] [Google Scholar]
- 11.Panetta R, Patel YC: Expression of mRNA for all five human somatostatin receptors (hSSTR1–5) in pituitary tumors. Life Sci 1995, 56:333-342 [DOI] [PubMed] [Google Scholar]
- 12.Bruns C, Weckbecker G, Raulf F, Kaupmann K, Schoeffter P, Hoyer D, Lübbert H: Molecular pharmacology of somatostatin-receptor subtypes. Ann NY Acad Sci 1994, 733:138-146 [DOI] [PubMed] [Google Scholar]
- 13.John M, Meyerhof W, Richter D, Waser B, Schaer J, Scherübl H, Boese-Landgraf J, Neuhaus P, Ziske C, Mölling K, Riecken E, Reubi JC, Wiedenmann B: Positive somatostatin receptor scintigraphy correlates with the presence of somatostatin receptor subtype 2. Gut 1996, 38:33-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janson ET, Gobl A, Kälkner KM, Oeberg K: A comparison between the efficacy of somatostatin receptor scintigraphy and that of in situ hybridization for somatostatin receptor subtype 2 messenger RNA to predict therapeutic outcome in carcinoid patients. Cancer Res 1996, 56:2561-2565 [PubMed] [Google Scholar]
- 15.Taylor JE, Theveniau MA, Bashirzadeh R, Reisine T, Eden PA: Detection of somatostatin receptor subtype 2 (SSTR2) in established tumors and tumor cell lines: evidence for SSTR2 heterogeneity. Peptides 1994, 15:1229-1236 [DOI] [PubMed] [Google Scholar]
- 16.Reubi JC, Kvols LK, Waser B, Nagorney D, Heitz PU, Charboneau JW, Reading CC, Moertel C: Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res 1990, 50:5969-5977 [PubMed] [Google Scholar]
- 17.Buscail L, Saint-Laurent N, Chastre E, Vaillant J, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C: Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res 1996, 56:1823-1827 [PubMed] [Google Scholar]
- 18.Greenman Y, Melmed S: Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab 1994, 78:398-403 [DOI] [PubMed] [Google Scholar]
- 19.Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A: Localization of the somatostatin receptor sst2A in rat brain using a specific anti-peptide antibody. J Neurosci 1996, 16:4468-4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu WZ, Schonbrunn A: Coupling specificity between somatostatin receptor sst2A and G proteins: isolation of the receptor-G protein complex with a receptor antibody. Mol Endocrinol 1997, 11:527-537 [DOI] [PubMed] [Google Scholar]
- 21.Hunyady B, Hipkin RW, Schonbrunn A, Mezey E: Immunohistochemical localization of somatostatin receptor sst2A in the rat pancreas. Endocrinology 1997, 138:2632-2635 [PubMed] [Google Scholar]
- 22.Reubi JC, Waser B, Lamberts SWJ, Mengod G: Somatostatin (SRIH) messenger ribonucleic acid expression in human neuroendocrine and brain tumors using in situ hybridization histochemistry: comparison with SRIH receptor content. J Clin Endocrinol Metab 1993, 76:642-647 [DOI] [PubMed] [Google Scholar]
- 23.Bobrow MN, Litt GJ, Shaugnessy KJ, Mayer PC, Conlon J: The use of catalyzed reporter deposition as a means of signal amplification in a variety of formats. J Immunol Methods 1992, 150:145-149 [DOI] [PubMed] [Google Scholar]
- 24.Adams JC: Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 1992, 40:1457-1463 [DOI] [PubMed] [Google Scholar]
- 25.Hipkin RW, Friedman J, Clark RB, Eppler CM, Schonbrunn A: Agonist-induced desensitization, internalization and phosphorylation of the sst2A somatostatin receptor. J Biol Chem 1997, 272:13869-13876 [DOI] [PubMed] [Google Scholar]
- 26.Kubota A, Yamada Y, Kagimoto S, Shimatsu A, Imamura M, Tsuda K, Imura H, Seino S, Seino Y: Identification of somatostatin receptor subtypes and an implication of the efficacy of somatostatin analogue SMS 201–995 in treatment of human endocrine tumors. J Clin Invest 1994, 93:1321-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reubi JC, Waser B, Horisberger U, Krenning E, Lamberts SWJ, Gebbers JO, Gersbach P, Laissue JA: In vitro autoradiographic and in vivo scintigraphic localization of somatostatin receptors in human lymphatic tissue. Blood 1993, 82:2143-2151 [PubMed] [Google Scholar]
- 28.Reubi JC, Horisberger U, Essed CE, Jeekel J, Klijn JGH, Lamberts SWJ: Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology 1988, 95:760-763 [DOI] [PubMed] [Google Scholar]
- 29.Reubi JC, Maurer R, Klijn JGM, Stefanko SZ, Foekens JA, Blaauw G, Blankenstein MA, Lamberts SWJ: High incidence of somatostatin receptors in human meningiomas: biochemical characterization. J Clin Endocrinol Metab 1986, 63:433-438 [DOI] [PubMed] [Google Scholar]
- 30.Vanetti M, Vogt G, Höllt V: The two isoforms of the mouse somatostatin receptor (mSSTR2A and mSSTR2B) differ in coupling efficiency to adenylate cyclase and in agonist-induced receptor desensitization. FEBS Lett 1993, 331:260-266 [DOI] [PubMed] [Google Scholar]
- 31.Reisine T, Kong H, Raynor K, Yano H, Takeda J, Yasuda K, Bell GI: Splice variant of the somatostatin receptor 2 subtype, somatostatin receptor 2B, couples to adenylyl cyclase. Mol Pharmacol 1993, 44:1016-1020 [PubMed] [Google Scholar]
- 32.Moertel CL, Reubi JC, Scheithauer BS, Schaid DJ, Kvols LK: Expression of somatostatin receptors in childhood neuroblastoma. Am J Clin Pathol 1994, 102:752-756 [DOI] [PubMed] [Google Scholar]
- 33.Prévost G, Veber N, Viollet C, Roubert V, Roubert P, Bénard J, Eden P: Somatostatin-14 mainly binds the somatostatin receptor subtype 2 in human neuroblastoma tumors. Neuroendocrinology 1996, 63:188-197 [DOI] [PubMed] [Google Scholar]



