Abstract
Mononuclear phagocytes play an important role in atherosclerosis and its sequela plaque rupture in part by their secretion of matrix metalloproteinases (MMPs), including MMP-9. Peroxisomal proliferator-activated receptor γ (PPARγ), a transcription factor in the nuclear receptor superfamily, regulates gene expression in response to various activators, including 15-deoxy-Δ12,14-prostaglandin J2 and the antidiabetic agent troglitazone. The role of PPARγ in human atherosclerosis is unexplored. We report here that monocytes/macrophages in human atherosclerotic lesions (n = 12) express immunostainable PPARγ. Normal artery specimens (n = 6) reveal minimal immunoreactive PPARγ. Human monocytes and monocyte-derived macrophages cultured for 6 days in 5% human serum expressed PPARγ mRNA and protein by reverse transcription-polymerase chain reaction and Western blotting, respectively. In addition, PPARγ mRNA expression in U937 cells increased during phorbol 12-myristate 13 acetate-induced differentiation. Stimulation of PPARγ with troglitazone or 15-deoxy-Δ12,14-prostaglandin J2 in human monocyte-derived macrophages inhibited MMP-9 gelatinolytic activity in a concentration-dependent fashion as revealed by zymography. This inhibition correlates with decreased MMP-9 secretion as determined by Western blotting. Thus, PPARγ is present in macrophages in human atherosclerotic lesions and may regulate expression and activity of MMP-9, an enzyme implicated in plaque rupture. PPARγ is likely to be an important regulator of monocyte/macrophage function with relevance for human atherosclerotic disease.
Macrophages influence many aspects of atherosclerosis, including the vulnerability of plaques to undergo disruption and thrombosis. 1,2 Pathological studies have shown abundant macrophages in ruptured atheroma.3 In vitro biomechanical studies have shown that the fibrous cap of macrophage-rich plaques has reduced tensile strength. 4 The role of macrophages in plaque rupture may involve secretion of matrix metalloproteinases (MMPs), enzymes that participate in extracellular matrix degradation. 5,6 MMP-9, also referred to as gelatinase B, is the predominant MMP secreted by monocytes/macrophages in vitro. 6-8 A number of inflammatory cytokines found in atheroma can augment MMP-9 expression by mononuclear phagocytes, including interleukin 1, tumor necrosis factor α, 9 and CD40 ligand. 10 Nonetheless, the majority of atheroma are stable. This suggests that inhibitors of MMP-9 expression must be at work, opposing the effects of proinflammatory mediators in the plaque.
We therefore undertook an effort to identify endogenous inhibitors of MMP-9 expression. Work from other groups has established that activation of various nuclear hormone receptors can inhibit MMP expression through a variety of mechanisms. 11 Interest is growing regarding the role of peroxisomal proliferation activator receptors (PPARs), a subgroup of the nuclear receptor superfamily, as transcriptional mediators. 12,13 One of these, PPARγ, has been implicated as a “master regulator” of lipid metabolism and adipogenesis; ectopic overexpression of PPARγ in fibroblasts redirects these cells into an adipogenic program. 14,15 Like other nuclear receptors, PPARγ contains a ligand binding domain and a central DNA binding domain, which interacts with PPAR response elements in the promoter of target genes. 16 Specific activators identified thus far include both the naturally occurring prostaglandin D metabolite 15-deoxy-Δ12,14-prostaglandin J2 (15 d-PGJ2) 17-19 and the synthetic antidiabetic agent troglitazone. 20-22 The role of PPARγ in nonadipocytes has received little attention, although expression had been previously noted in hematopoietic cell lines. 23 Recent work suggests PPARγ stimulation can inhibit both cytokine-induced activation of macrophages 24 and in vitro expression of transfected promoter constructs of genes implicated in atherogenesis, including MMP-9. 25
The present study tested the hypotheses 1) that macrophages in human atheroma express PPARγ, 2) that this novel nuclear receptor is regulated during differentiation of monocytes into macrophages, and 3) that PPARγ activation can limit MMP-9 expression and enzymatic activity by these cells.
Materials and Methods
Immunohistochemistry
Surgical specimens of human carotid atherosclerotic lesions were obtained by protocols approved by the Human Investigation Review Committee at Brigham and Women’s Hospital. Serial cryostat sections (5 mm) were cut, air dried onto microscopic slides, and fixed in acetone at −20°C for 5 minutes. Staining for PPARγ was performed with a polyclonal rabbit anti-human PPARγ peptide antibody 19 (a generous gift from Dr. Mitchell Lazar, University of Pennsylvania School of Medicine, Philadelphia). Macrophages were identified by staining with anti-CD68 antibody (DAKO, Carpinteria, CA). Sections were preincubated with PBS containing 0.3% hydrogen peroxidase activity and stained for 1 hour with primary antibody diluted in PBS supplemented with 5% appropriate serum. Negative control was performed by preabsorbing the anti-PPARγ antibodies with the peptide from which the antibody was derived and subsequently using these “peptide-blocked PPARγ antibodies” at concentrations similar to those of experimental conditions. Finally, sections were incubated with the respective biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) followed by avidin-biotin-peroxidase complex (Vectastain ABC kit, Vector Laboratories). Antibody binding was visualized with 3-amino-9-ethyl carbazole (Vector Laboratories) or with True Blue Peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Sections were counterstained with Gill’s Hematoxylin or Contrast Red (Kirkegaard & Perry Laboratories). Computer-assisted image analysis was used to quantify staining on sections using Optimas 5.2 software. Percentage area of positive staining for PPARγ or CD68 in the shoulders of the plaques, defined as the intimal regions flanking the lipid core, was compared with the percentage area of positive staining in other zones of the sections.
Cell Culture
Human monocytes were isolated from peripheral blood of healthy volunteers by sequential gradient centrifugation with Lymphocyte Separation Medium (Organon Technika, Durham, NC) and One Step Monocytes (Accurate Chemical and Scientific Co., Westbury, NY). Monocytes were plated at a concentration of 3 × 10 9 cells/L in serum-free M199 medium (BioWhittaker, Walkersville, MD) and isolated by adherence to plastic dishes at 37°C. Nonadherent cells were washed three times with Hanks’ buffer (Life Technologies, Gaithersburg, MD), and the remaining adherent cells were cultured in M199 medium with 5% human serum at 37°C/5%CO2. Medium was changed every 2 days for 6 days, and resulting cells were used as “monocyte-derived macrophages.” In some experiments, monocytes were cultured for 6 days with 5% human serum in the absence or presence of PPARγ activators troglitazone (provided by Parke Davis (Morris Plains, NJ) and dissolved according to manufacturer’s instructions) or 15 d-PGJ2 (CalbioChem, La Jolla, CA) at concentrations indicated. After 6 days, cells were changed to serum-free conditions, and supernatants were collected after 24 hours for further analysis. The human monocyte-like cell line U937, obtained from American Type Culture Collection (Manassas, VA), was cultured in RPMI 1640 medium (BioWhittaker) with 1% glutamine (Sigma Chemical Co., St. Louis, MO), 1% penicillin-streptomycin (Sigma) and 10% fetal calf serum.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
Total RNA from 10 7 cells was isolated by the single-step guanidium thiocyanate-phenol-chloroform method using RNAzol from Tel-Test (Friendswood, TX). Two micrograms of total RNA was reverse-transcribed into cDNA with 1 U/ml reverse transcriptase (Superscript, Life Technologies) at 37°C for 1 hour in standard buffer. For the amplification of PPARγ cDNA, two oligonucleotide primers were designed from nucleotides +235 to 708 (a 473-bp fragment): sense primer, 5′-TCTCTCCGTAATGGAAGACC-3′; anti-sense primer, 5′-CCCCTACAGAGTATTACG-3′. The polymerase chain reaction reaction was carried out in a standard buffer (Life Technologies) with 200 ng of each primer (IDT, Coralville, CA), 33 mmol/L MgCl2, and 0.5 U Taq polymerase (Life Technologies) for 30 cycles. Polymerase chain reaction products (10 μl/25μl reaction) were analyzed on a 2% agarose gel.
Northern Blot Analysis
Five micrograms of total RNA from undifferentiated and phorbol 12-myristate 13-acetate (PMA)-differentiated U937 cells was subjected to electrophoresis on a 1.2% agarose gel and transferred using traditional Northern blotting techniques. The membranes were ultraviolet-crosslinked, prehybridized at 42°C (50% formamide, 5× Denhardt’s solution, 5× standard saline citrate, 0.5% sodium dodecyl sulfate, and 20 mmol/L salmon sperm DNA), and hybridized in the same buffer with a random primed radiolabeled ([α-P32]dCTP) Sal-1 fragment of pCMX-PPARγ (generously provided by Dr. Bruce Spiegelman, Dana-Farber Cancer Institute, Boston, MA). 14 The membranes were washed at 60°C in 1% sodium dodecyl sulfate/2× standard saline citrate and exposed (1–3 days, −70°C) to Kodak X-OMAT film with an intensifying screen.
Preparation of Nuclear and Cytosolic Extracts and Western Blot Analysis
For Western blot analysis, a positive control was generated by transiently transfecting a PPARγ expression construct, pCMX-PPARγ, 14 into human skin fibroblasts using lipofectamine (Life Technologies) according to the manufacturer’s protocol. Nuclear and cytosolic extracts of 10 7 cells were prepared separately. Cells were lysed in 10 mmol/L Hepes (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, and 0.5% Nonidet P-40. Nuclei were pelleted at 13,000 × g for 5 minutes, and the resulting supernatant was used as the cytosolic fraction. Nuclei were lysed in 20 mmol/L Hepes (pH 7.9), 1.5 mmol/L MgCl2, 420 mmol/L NaCl, and 0.2 mmol/L ethylenediaminetetraacetate. After centrifugation at 13,000 × g for 5 minutes, the supernatant was diluted in an equal volume of 20 mmol/L Hepes (pH 7.9), 100 mmol/L KCl, 0.2 mmol/L ethylenediaminetetraacetate, and 20% glycerol and used as nuclear extract. Protein concentration of nuclear and cystolic extracts was determined using a protein assay (Pierce, Rockford, IL). Processed samples were applied to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, and protein was transferred to nitrocellulose membranes (Millipore, Bedford, MA) using semidry blotting for 1 hour, as described previously. 10
Membranes were blocked overnight in Tris-buffered saline-Tween with 5% dry milk and incubated with goat anti-human PPARγ monoclonal antibodies (N-20, Santa Cruz Biotechnology, San Diego, CA) for 1 hour. After washing, membranes were stained with horseradish-conjugated rabbit anti-goat monoclonal antibodies. Antigen detection was performed with a chemiluminescent detection system (NEN, Boston, MA). Similar methods were used to perform Western blots on MMP-9 in monocyte-derived macrophage supernatants using a specific rabbit anti-human MMP-9 antibody (Oncogene Science, Cambridge, MA).
Substrate Gel Zymography
Gelatinolytic activity of MMP-9 from conditioned medium (10 μl/500 μl total supernatant loaded) of monocytes or monocyte-derived macrophages was analyzed by zymography on gelatin-containing polyacrylamide. 10 Equal amounts were loaded in each lane. After washing in 2.5% Triton X-100, gels were incubated overnight at 37°C in 50 mmol/L Tris-HCl (pH 7.4) containing CaCl2 and 0.05% Brij 35. Gels were stained in 0.1% Colloidal Brilliant Blue (Sigma), 10% acetic acid, and 40% methanol for 2 hours and destained in 10% acetic acid and 40% methanol. Proteins having gelatinolytic activity were visualized as clear zones in an otherwise blue gel. Photographs of the gels were scanned by an imaging densitometer and quantified using the NIH Image 1.6 software program. To ensure that differences in protein amounts did not account for the differences seen, the zymographic data were normalized to the total amount of protein applied to each lane. Of note, the total amount of protein did not vary significantly from sample to sample.
Results
Macrophages in Human Atheroma Contain PPARγ
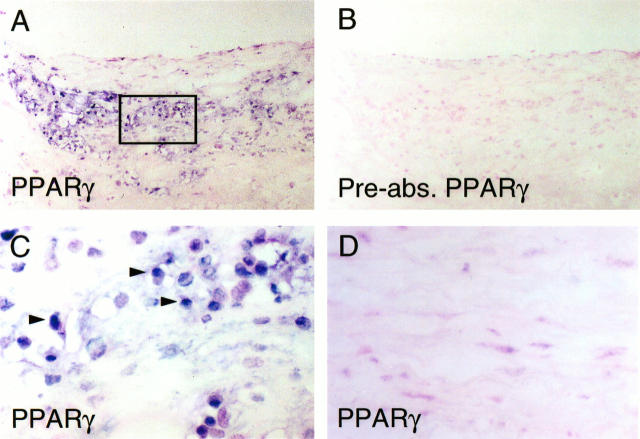
Analysis of human carotid atheroma (n = 12) demonstrated immunoreactive PPARγ co-localizing with macrophages (Figure 1, A and C) ▶ , so identified by morphology and by staining of parallel sections with the macrophage-specific antibody anti-CD68 (data not shown). PPARγ staining was mainly localized in the macrophage-rich shoulder region of the plaque (Figure 1A) ▶ , with 35 ± 5% of this area positive for PPARγ and 54 ± 6% positive for macrophages, as determined by color image analysis. Quantification of staining in nonshoulder regions revealed a 2 ± 1% area positive for PPARγ and 8 ± 1% for macrophages. Preabsorption of anti-PPARγ antibodies with the peptide antigen abrogated staining in adjacent sections (Figure 1B) ▶ , indicating the specificity of the immunostaining. Higher-power views of PPARγ-stained plaque (indicated by the rectangle in Figure 1A ▶ ) showed staining predominantly in nuclei of macrophages (Figure 1C) ▶ . Only occasional endothelial cells or smooth muscle cells in lesions showed immunoreactive PPARγ (data not shown). Study of nonatheromatous arterial specimens (n = 6) showed scant PPARγ in nuclei of vascular smooth muscle cells (Figure 1D) ▶ .
Figure 1.
Expression of PPARγ in human atherosclerotic lesions. A: Low-power view of frozen section of human carotid lesions shows immunoreactive PPARγ next to the lipid core in the shoulder region with abundant macrophages present. Similar results were seen in other atherosclerotic specimens (n = 12). B: No immunoreactive PPARγ is detectable in parallel sections stained with PPARγ antibodies preabsorbed with the immunizing peptide, indicating that staining for PPARγ in (A) and (C) is specific. Similar results were seen with immunoglobulin G controls (not shown). C: High-power view of the area indicated by the rectangle in (A) shows PPARγ staining restricted to macrophage nuclei (see quantification in Results). D: In nonatherosclerotic arteries (n = 6), little PPARγ could be detected, with scant staining in occasional vascular smooth muscle cells.
Cells of the Monocyte/Macrophage Lineage Express PPARγ mRNA and Protein
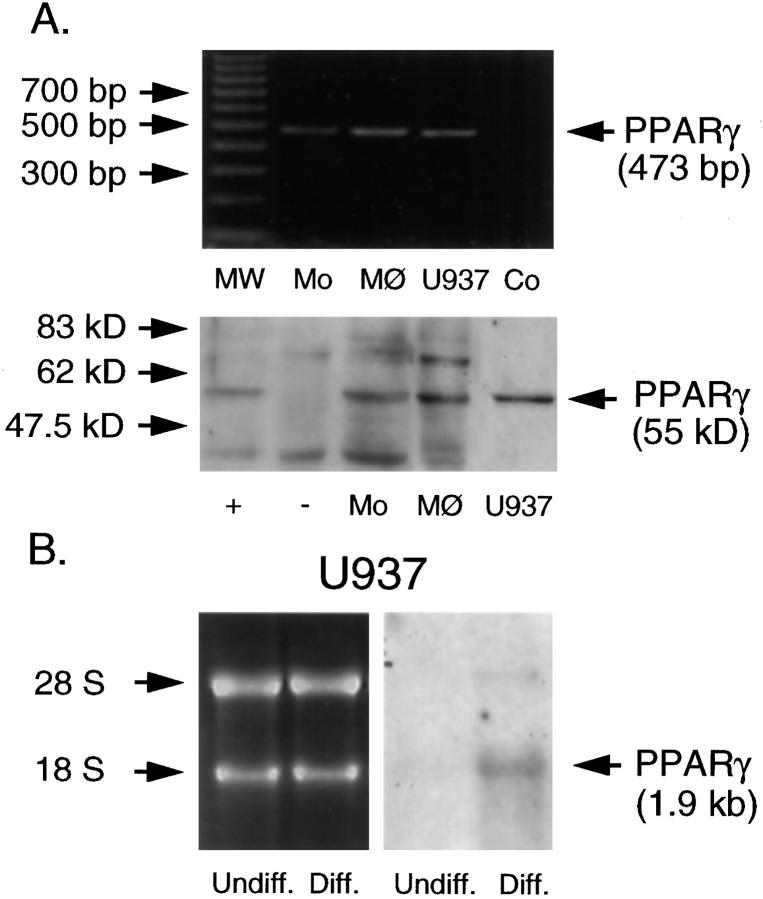
Freshly prepared monocytes, monocyte-derived macrophages, and PMA-treated U937 cells all contained PPARγ mRNA as detected by a 473-bp reverse transcription-polymerase chain reaction product (Figure 2A ▶ , upper panel). For the detection of PPARγ protein, we prepared separate nuclear and cytosolic fractions of the above-mentioned cells, as well as untransfected and PPARγ-transfected fibroblasts, and performed Western blot analysis. Nuclear extracts of monocytes, monocyte-derived macrophages, and differentiated U937 cells (Figure 2A ▶ , lower panel), but not cytosolic fractions (data not shown), contained PPARγ protein. The identity of this band as PPARγ was supported by its lack of cytosolic expression, its expected apparent molecular weight (55 kd), and co-migration with a signal in fibroblasts transfected with a PPARγ expression construct. Untransfected fibroblasts demonstrate no cross-reacting band of the appropriate size (Figure 2A ▶ , lower panel).
Figure 2.
A: PPARγ mRNA and protein are expressed in cells of the monocyte/macrophage lineage. Upper panel: reverse transcription-polymerase chain reaction of PPARγ mRNA in freshly prepared monocytes (Mo), monocyte-derived macrophages (MØ), and PMA-differentiated U937 cells (U937) reveals a cDNA fragment of the expected size. Also shown are a 100-bp DNA ladder (MW) and negative control without cDNA (Co). Lower panel: Western blot analysis of PPARγ protein expression in nuclear extracts of freshly prepared monocytes (Mo), monocyte-derived macrophages (MØ), and U937 cells (U937) reveals a band of the appropriate size. The identity of this band is confirmed by co-migration with a band seen in PPARγ-transfected human skin fibroblasts (+) but not in similar but untransfected fibroblasts (−). All results shown were reproduced in three independent experiments. B (right): PPARγ mRNA expression in undifferentiated (Undiff.) and PMA-differentiated U937 cells (Diff.), as shown by Northern blot analysis. Differentiated U937 cells show increased PPARγ mRNA expression compared with undifferentiated cells. B (left): Ethidium bromide staining demonstrates equal loading of intact RNA. Results shown were reproduced in three independent experiments.
Increased PPARγ Expression during PMA-Induced Differentiation of U937 Cells
To investigate further the regulation of PPARγ during differentiation of cells of the monocytic lineage, U937 cells were stimulated with PMA (10 μg/L) for 12 hours, and Northern blotting was performed. This treatment increased PPARγ mRNA expression (Figure 2B) ▶ . Western blot analysis showed a parallel rise in protein levels in nuclear fractions of PMA-treated U937 cells (data not shown). Neither undifferentiated nor differentiated U937 cells demonstrate PPARγ in the cytosol (data not shown).
PPARγ Activators Troglitazone and PGJ2 Decrease Both Protein Levels and Gelatinolytic Activity of MMP-9 Secreted from Monocyte-Derived Macrophages
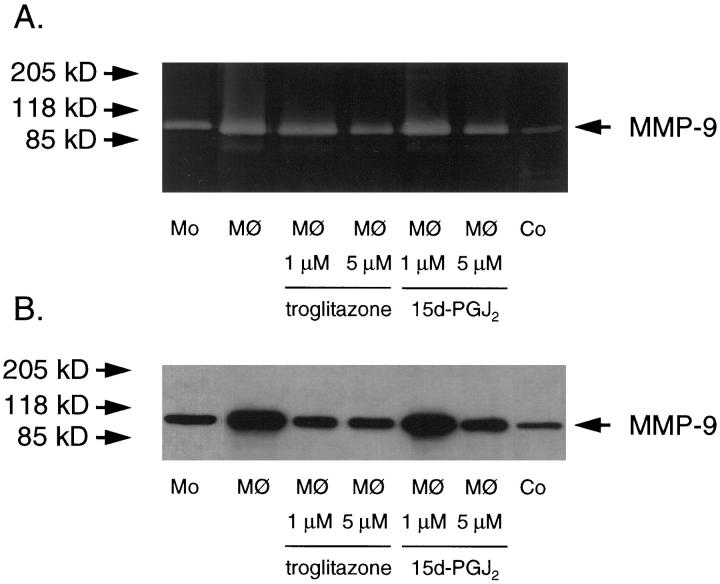
To elucidate the functional relevance of PPARγ activation, we investigated the effect of the selective PPARγ activators troglitazone and 15 d-PGJ2 on MMP-9 activity in monocyte-derived macrophages. Monocytes were cultured initially in human serum and then transferred to serum-free medium in the absence or presence of troglitazone or 15 d-PGJ2. Secreted MMP-9 gelatinolytic activity was then measured using substrate zymography. As previously reported, culture of monocytes for 6 days with human serum substantially increases gelatinolytic activity in the supernatant compared with freshly prepared monocytes (Figure 3A) ▶ . Concurrent treatment of monocytes with troglitazone or 15 d-PGJ2 in serum-free medium during this transition toward monocyte-derived macrophages inhibited the increase in gelatinolytic activity in a concentration-dependent manner (Figure 3A) ▶ . Quantification of gelatinolytic areas by densitometry revealed a reduction of MMP-9 activity after treatment with troglitazone (1 μM and 5 μM) to 73.5 ± 1.9% and 53.3 ± 12.2% (P < 0.01), respectively, and with 15 d-PGJ2 (1 μM and 5 μM) to 79.0 ± 14.6% and 45.5 ± 8.9% (P < 0.01), respectively, in both cases relative to control cells (n = 3). Similar results were seen in U937 cells (data not shown). Treatment with either troglitazone or 15 d-PGJ2 after 6 days of culture in human serum had no effect (data not shown), suggesting that PPARγ activation was required during differentiation for inhibition of MMP-9 levels/activity to occur. The identity of the observed gelatinolytic area was confirmed by the mobility of the band and co-migration with the known 92-kd gelatinolytic activity in PMA-treated human fibroblast supernatants (Figure 3A) ▶ . Treatment of monocyte-derived macrophages with either PPAR activator, troglitazone or 15 d-PGJ2, reduces supernatant MMP-9 protein levels from monocyte-derived macrophages (Figure 3B) ▶ . Co-migration with the known MMP-9 band of supernatants from PMA-stimulated vascular smooth muscle cells confirms identity of the detected band (Figure 3B) ▶ .
Figure 3.
A: PPARγ activators decrease MMP-9 gelatinolytic activity in supernatants from monocyte-derived macrophages (MØ) in a concentration-dependent fashion, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis zymography. Monocytes were cultured in 5% human serum in the presence or absence of troglitazone or 15 d-PGJ2 as indicated. Supernatants of PMA-treated fibroblasts served as a control (Co). Gelatinolytic activity of freshly prepared monocytes is also shown (Mo). Equal amounts of lysates were loaded. Results shown were reproduced in three independent experiments. B: PPARγ activators decrease MMP-9 protein levels in supernatants from monocyte-derived macrophages (MØ). Vascular smooth muscle cells treated with PMA at 50 μg/L served as a control (Co). Supernatants from freshly prepared monocyte supernatant (Mo) are also shown. Similar results were reproduced in three independent experiments.
Discussion
This study demonstrates PPARγ expression in macrophages in human atherosclerotic lesions. Furthermore, we find differentiation-dependent regulation of PPARγ expression in cells of the monocyte/macrophage lineage in vitro. Treatment of differentiated monocyte-derived macrophages in vitro with two different PPARγ activators, 15 d-PGJ2 or troglitazone, decreased both MMP-9 protein levels and MMP-9 gelatinolytic activity in a concentration-dependent manner.
Coronary atherosclerosis is typically a diffuse process. 26 Nevertheless, despite systemic risk factors, such as low-density lipoprotein levels, and the presence of macrophages and inflammatory cytokines in most plaques, some arterial plaques rupture, whereas others do not. The factors accounting for this variability are unclear. Certainly, monocytes/macrophages are intimately involved in plaque rupture. 2,27 As monocytes differentiate in the subintima into macrophages and foam cells, the atherogenic microenvironment influences transcriptional regulation of genes, the products of which will determine the natural history of the lesion. 1 MMPs furnish one example of proteins, the induction and expression of which by monocytes/macrophages likely contributes to subsequent plaque rupture. 2 Several lines of evidence indicate that lesional macrophages synthesize MMPs de novo.28,29. These proteins are enzymatically active, as shown by zymographic analysis of human arterial specimens. 30 Secretion of these MMPs, with MMP-9 prominent among them, favors destabilization of the plaque’s fibrous cap. 1
Our findings suggest that PPARγ, as a nuclear transcription factor present in lesional macrophages, may inhibit MMP expression and activity. Furthermore, PPARγ may control expression of other target genes within the arterial wall, thus modulating a cascade of responses in monocytes/macrophages after activation by its endogenous or synthetic ligand(s).
15 d-PGJ2, a naturally occurring ligand for PPARγ, likely interacts with monocytes/macrophages. J2 prostanoids, and the immediate upstream precursors of 15 d-PGJ2, are found in vivo. 31 Prostaglandins themselves are synthesized from fatty acids, with arachadonic acid as the primary source. 15 d-PGD2, the major prostaglandin in most tissues, is converted to 15 d-PGJ2. 32 This process requires 15 d-PGD2 synthetase, an enzyme produced primarily by macrophages and other antigen-presenting cells. 33 Thus, 15 d-PGJ2, acting through PPARγ, may be an important regulator of macrophage function. Troglitazone, a synthetic PPARγ activator, is currently in clinical use as an antidiabetic agent. 34,36 The implications of its effects on inhibiting macrophage MMP-9 matrix degradation merit further consideration in a clinical context.
A very recent report showed that the PPARγ activator 15 d-PGJ2 inhibits expression of an MMP-9 promoter luciferase construct when transfected into U937 cells. 25 Our findings support a critical role for PPARγ in atherosclerosis, demonstrating its presence in monocytes/macrophages of human atheroma and increased expression during differentiation in vitro. Furthermore, the present study of MMP-9 gelatinolytic activity illustrates directly the functional relevance of PPARγ in these cells. These results establish a rationale for further study of PPARγ in monocyte/macrophage biology, particularly in the context of atherosclerosis.
Acknowledgments
We thank Eugenia Schvartz for her expert technical assistance and our colleagues in the Vascular Medicine and Atherosclerosis Unit for their insightful technical and intellectual advice.
Footnotes
Address reprint requests to Jorge Plutzky, Vascular Medicine and Atherosclerosis Unit, Cardiovascular Division, Brigham and Women’s Hospital, 221 Longwood Avenue, Boston, MA 02115. E-mail: jplutzky@bics.bwh.harvard.edu.
Supported by Deutsche Forschungsgemeinschaft Grant MA 2047/1-1 (to NM) and National Institutes of Health Grants HL 03107 and P50HL56985 (to JP), and P50HL56985 and R37HL34636 (to PL).
References
- 1.Libby P, Geng Y, Aikawa M, Schoenbeck U, Mach F, Clinton S, Sukhova G, Lee R: Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996, 7:330-335 [DOI] [PubMed] [Google Scholar]
- 2.Dollery C, McEwan J, Henney A: Matrix metalloproteinases and cardiovascular disease. Circ Res 1995, 77:863-868 [DOI] [PubMed] [Google Scholar]
- 3.Davies M, Richardson P, Woolf N, Katz D, Mann J: Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cells content. Br Heart J 1993, 69:377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lendon CL, Davies MJ, Born GV, Richardson PD: Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 1991, 87:87-90 [DOI] [PubMed] [Google Scholar]
- 5.Nikkari S, O’Brien K, Ferguson M, Hatsukami T, Welgus H, Clowes A: Interstitial collagenase (MMP-1) expression in human carotid atherosclerotics. Circulation 1995, 92:1393-1398 [DOI] [PubMed] [Google Scholar]
- 6.Galis Z, Sukhova G, Lark M, Libby P: Increased expression of matrix metalloproteinases and matrix degradation activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994, 94:2493-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetzl E, Banda M, Leppert D: Matrix metalloproteinases in immunity. J Immunol 1996, 156:1-4 [PubMed] [Google Scholar]
- 8.Welgus H, Campbell E, Cury J, Eisen A, Senior RM, Goldberg G: Neutral metalloproteinases produced by human mononuclear phagocytes: enzyme profile, regulation, and expression during cellular development. J Clin Invest 1990, 86:1496-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saren P, Welgus H, Kovanen P: TNF-α and IL-1β selectively induce expression of 92-kd gelatinase by human macrophages. J Immunol 1996, 157:4159-4165 [PubMed] [Google Scholar]
- 10.Schoenbeck U, Mach F, Sukhova G, Murphy C, Bonnefoy J-Y, Fabunmi R, Libby P: Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes. Circ Res 1997, 81:448-454 [DOI] [PubMed] [Google Scholar]
- 11.Schroen DJ, Brinckerhoff C: Nuclear hormone receptors inhibit matrix metalloproteinases (MMP) gene expression through diverse mechanisms. Gene Exp 1996, 6:197-207 [PMC free article] [PubMed] [Google Scholar]
- 12.Schoonjans K, Martin G, Staels B, Auwerx J: Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr Opin Lipidol 1997, 8:159-166 [DOI] [PubMed] [Google Scholar]
- 13.Vidal-Puig AJ, Considine RV, Jimenez-Linan MA, Pories WJ, Caro JF, Flier JS: Peroxisome proliferator-activated receptor gene expression in human tissues: effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 1997, 99:2416-2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tontonoz P, Hu E, Spiegelman BM: Stimulation of adipogenesis in fibroblasts by PPAR γ2, a lipid-activated transcription factor. Cell 1994, 79:1147-1156 [DOI] [PubMed] [Google Scholar]
- 15.Brun R, Tontonoz P, Forman B, Ellis RJ, Evan R, Spiegelman BM: Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev 1996, 10:974-984 [DOI] [PubMed] [Google Scholar]
- 16.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R: The nuclear receptor superfamily: the second decade. Cell 1995, 83:835-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans R: M. 15-Deoxy-delta 12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR γ. Cell 1995, 83:803-812 [DOI] [PubMed] [Google Scholar]
- 18.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris D, Lehmann JM: A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995, 83:813-819 [DOI] [PubMed] [Google Scholar]
- 19.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA: Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem 1995, 270:23975-23983 [DOI] [PubMed] [Google Scholar]
- 20.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA: An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ. J Biol Chem 1995, 270:12953-12956 [DOI] [PubMed] [Google Scholar]
- 21.Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM: The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 1996, 39:665-668 [DOI] [PubMed] [Google Scholar]
- 22.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM: Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci USA 1997, 94:4318-4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene M, Blumberg B, McBride O, Kronquist K, Kwan K, Hsieh L, Greene G, Nimer S: Isolation of the human peroxisome proliferator activated receptor γ cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expression 1995, 4:281-299 [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Ting A, Seed B: PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391:82-86 [DOI] [PubMed] [Google Scholar]
- 25.Ricote M, Li A, Wilson T, Kelly C, Glass C: The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 1998, 391:79-82 [DOI] [PubMed] [Google Scholar]
- 26.Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801-809 [DOI] [PubMed] [Google Scholar]
- 27.Raines E, Ross R: Is overamplification of the normal macrophage defensive role critical to lesion development? Ann NY Acad Sci 1997, 811:76-85 [DOI] [PubMed] [Google Scholar]
- 28.Galis Z, Sukhova G, Kranzhoefer R, Clark S, Libby P: Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci USA 1995, 92:402-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henney A, Wakeley P, Davies M, Foster K, Hembry R, Murphy G, Humphries S: Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 1991, 88:8154-8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galis Z, Sukhova G, Libby P: Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J 1995, 9:974-980 [DOI] [PubMed] [Google Scholar]
- 31.Fukushima M: Biological activities and mechanisms of action of PGJ2 and related compounds: an update. Prostaglandins Leukotrienes Essent Fatty Acids 1992, 47:1-12 [DOI] [PubMed] [Google Scholar]
- 32.Giles H, Leff P: The biology and pharmacology of PGD2. Prostaglandins 1988, 35:277-300 [DOI] [PubMed] [Google Scholar]
- 33.Urade Y, Ujihara M, Horigushi Y, Ikai K, Hayaishi O: The major source of endogenous prostaglandin D2 is likely antigen-presenting cells. J Immunol 1989, 143:2982-2989 [PubMed] [Google Scholar]
- 34.Nolen J, Ludvik B, Beerdsen P: Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med 1994, 331:1188-1193 [DOI] [PubMed] [Google Scholar]
- 35.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI: Efficacy and metabolic effects of merformin and troglitazone in type II diabetes mellitus. N Engl J Med 1998, 338:867-872 [DOI] [PubMed] [Google Scholar]
- 36.Schwartz S, Raskin P, Fonseca V, Graveline JF: Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. N Engl J Med 1998, 338:861-866 [DOI] [PubMed] [Google Scholar]