Abstract
Although pancreatic neuroendocrine tumors (NETs) in von Hippel-Lindau (VHL) disease have been reported, their pathological features have not been characterized. In addition, it is unknown whether alterations of the VHL gene are responsible for pancreatic NET development. To evaluate NETs in VHL patients, we performed histopathological analysis of 30 pancreatic tumors in 14 patients. In addition, DNA from NETs and normal pancreatic tissue from 6 patients with documented germ-line VHL gene mutations was studied for allelic deletions of the second copy of the VHL gene by fluorescence in situ hybridization and polymerase chain reaction-based single-strand conformational polymorphism analysis. Morphologically, the tumors were characterized by solid, trabecular, and/or glandular architecture and prominent stromal collagen bands. Sixty percent of the tumors revealed at least focally clear-cell cytology. All tumors were positive for panendocrine immunohistochemistry markers (chromogranin A and/or synaptophysin); 35% of NETs demonstrated focal positivity for pancreatic polypeptide, somatostatin, insulin, and/or glucagon; and no immunostaining for pancreatic and gastrointestinal hormones was observed in 65% of tumors. Dense core neurosecretory granules were evident by electron microscopic examination, and the clear cells additionally revealed abundant intracytoplasmic lipid. All NETs that were subjected to genetic analysis showed allelic loss of the second copy of the VHL gene. We conclude that multiple, nonfunctional pancreatic NETs occur in VHL patients. Stromal collagen bands and clear-cell morphology are important histological features of VHL-associated NETs. The presence of allelic deletions of the VHL gene in pancreatic NETs provides direct molecular evidence for a role of the gene in their tumorigenesis and establishes NET as an independent tumor type of VHL disease.
Von Hippel-Lindau (VHL) disease is an autosomal dominant disorder in that affected individuals are predisposed to develop a variety of neoplasms in multiple target organs. The neoplasms are frequently multiple in a given organ and include hemangioblastomas of the central nervous system, retinal angiomas, renal cell carcinomas, pheochromocytomas, and cysts of the kidneys and epididymis. 1 The most common manifestations of pancreatic VHL disease are benign cysts and microcystic (serous) adenomas that occur in 35 to 75% of patients. 2,3 Pancreatic neuroendocrine tumors (NETs) have been reported to occur with an incidence of 17% in VHL patients in the radiological literature. 4 Until the present, gross features, histomorphology, and immunohistochemical and ultrastructural characteristics, as well as genetic alterations of VHL-associated NETs, have not been analyzed in a series of cases.
The gene for VHL disease has been localized to chromosome 3p25.5 and was identified in 1993. 5 The two-hit theory of Knudson 6 predicts that in a familial cancer syndrome such as VHL disease, the genotype of each neoplasm should consist of an allele with an inherited germ-line mutation and loss of the second wild-type allele through allelic deletion. In fact, genetic studies of different VHL disease-associated tumors, including hemangioblastomas, 7 renal cell carcinomas, 8-10 pheochromocytomas, 11 pancreatic microcystic adenomas, 12 and endolymphatic sac tumors, 13 demonstrated loss of heterozygosity (LOH) at chromosome 3p at the VHL gene region. VHL gene deletion, however, has never been studied in VHL-associated pancreatic NETs, and it is therefore unknown whether these tumors show genetic evidence of VHL disease-related pathogenesis.
In this study, we performed macroscopic, histopathological, and immunohistochemical evaluation of 30 pancreatic NETs in 14 VHL patients. Furthermore, to investigate VHL gene alterations in VHL NETs, DNA from tumor and normal pancreatic tissue in 6 patients was studied for allelic deletions of the VHL gene by fluorescence in situ hybridization (FISH) and/or polymerase chain reaction (PCR)-single-strand conformational polymorphism analysis (SSCP).
Materials and Methods
Patients
Fourteen VHL patients (eight females and six males; mean age, 35 years; range, 18 to 48 years) with solid pancreatic lesions were selected from the group of familial VHL patients followed at the National Cancer Institute, National Institutes of Health 14 (Table 1) ▶ . Each patient had a documented germ-line mutation in the VHL gene.
Table 1.
VHL-Associated Pancreatic NETs: Patient and Tumor Characteristics
| Patient | Age (years) Sex | Procedure | No. of tumors (tumor No.) | Size range (cm) | Location in pancreas | Other pancreatic lesions | Other VHL tumors |
|---|---|---|---|---|---|---|---|
| 1 | 38F | Partial pancr | 1 (1) | 3 | Body | None | ph, ra |
| 2 | 48M | Distal pancr; autopsy | 5 (2–6) | 0.5–2 | Head, body, tail | Cysts | h, ra, ph, rcc, rc |
| 3* | 34F | Expl. lap with biopsy | 2 (7–8) | 1.2–8 | Head-body | Cysts | h, ra, ph, rcc |
| 4 | 22M | Partial pancr | 2 (9–10) | 1–2 | Body, tail | Cysts | h, ra, ph, rcc |
| 5 | 43F | Total pancr | 3 (11–13) | 2–3 | Head, body, tail | Cysts | h, ra, ph, rc |
| 6 | 23F | Enucleation | 3 (14–16) | 1.5–4 | Head, body, tail | NA | rc |
| 7 | 29M | Enucleation | 1 (17) | 2 | Tail | Cysts | ph, h, ra, rcc, rc |
| 8 | 38F | Enucleation | 1 (18) | 3 | Head | NA | rcc |
| 9 | 39F | Enucleation | 1 (19) | 1.5 | Head | NA | ph |
| 10 | 42M | Distal pancr | 1 (20) | 3 | Tail | MCA | h |
| 11 | 42F | Total pancr | 1 (21) | 0.5 | Body-tail | MCA, Carcinoma | h |
| 12 | 34M | Pancreaticoduod | 2 (22–23) | 1–2 | Head, head | MCA | ph, h, ra, rc |
| 13 | 18M | Distal pancr | 3 (24–26) | 0.4–1.5 | Tail | None | None |
| 14 | 35F | Pancreaticoduod and enucleation | 4 (27–30) | 0.5–1.5 | Head, body, tail | None | ra |
Abbreviations: F, female; M, male; pancr, pancreatectomy; expl. lap, exploratory laparotomy; pancreaticoduod, pancreaticoduodenectomy (Whipple’s procedure); NA, adjacent pancreas is not available for evaluation; MCA, microcystic adenoma; ph, pheochromocytoma; ra, retinal hemangioma; h, hemangioblastoma; rcc, renal cell carcinoma; rc, renal cysts.
*Patient with liver metastasis (tumor 8).
Thirteen patients were caucasian, and one was from India. Two patients presented with abdominal pain, nausea, or diarrhea. Twelve patients were asymptomatic at presentation, and their tumors were discovered incidentally during screening by computed tomography or magnetic resonance imaging. All tumors were clinically nonfunctional. Radiologically, at least one solid tumor in each patient was enhanced on contrast computed tomography or magnetic resonance imaging scans; 3,14 additional smaller tumors were seen on intraoperative ultrasound or on serial sectioning of pancreatectomy specimens. Other nonpancreatic VHL-associated tumors included pheochromocytoma, retinal hemangioma, central nervous system hemangioblastoma, renal cell carcinoma, and renal cysts (Table 1) ▶ . Four patients were treated by enucleation, five patients were treated by distal/partial pancreatectomy, two patients underwent total pancreatectomy, two patients were treated by pancreaticoduodenectomy (Whipple’s procedure), and one patient had an exploratory laporotomy and biopsy of pancreatic and liver lesions. From one patient (patient 2), additional pancreatic NETs were obtained at autopsy (Table 1) ▶ .
Morphological Examination of Tumors
Thirty formalin-fixed, paraffin-embedded pancreatic tumors were obtained from the files of the Laboratory of Pathology, National Cancer Institute, National Institutes of Health. All tumors were grossly examined and measured. Tumors were evaluated on hematoxylin and eosin (H&E) stain, periodic acid-Schiff (PAS), and PAS-diastase (PAS-D) stains. Immunohistochemistry stains for cytokeratin AE1/AE3 (1:300/1:100) and chromogranin A (1:1600) (both from Boehringer Mannheim, Indianapolis, IN); synaptophysin (1:1000) (Zymed, San Diego, CA); neuron-specific enolase (NSE) (1:200), glucagon (1:900), pancreatic polypeptide (1:8000), somatostatin (1:3000), gastrin (1:2000) (DAKO, Carpinteria, CA) S100 (1:8000), and insulin (1:50) (BioGenex, San Ramon, CA) were performed in all tumors in which sufficient tissue was available (Table 2) ▶ . Tumor cells were scored as positive by immunohistochemistry stain when they showed moderate-to-marked intensity of staining in the appropriate distribution for the marker, with adequate tissue controls. Electron microscopy was performed in five cases (patients 1, 3, 4, 7, and 8). For electron microscopic examination, 2.5% glutaraldehyde-fixed, osmium-postfixed tumor tissue was embedded in Maraglas 655 (Ladd Research Industries, Burlington, VT). Thin sections were stained with uranyl acetate and lead citrate and were reviewed in a Philips CM10 transmission electron microscope.
Table 2.
VHL-Associated Pancreatic NETs: Histopathology and Immunohistochemistry Results
| Patient | Tumor No. | Architecture | Cytoplasm | Collagen/Atypia (yes or no) | Chr | Syn | S100 | NSE | Ker | Gluc | PPP | Som | Ins | Gastr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | solid/trabec | amph | Yes/Yes | + | + | + | + | + | − | foc+ | + | − | − |
| 2 | 2 | trabec | eos/clear | Yes/No | ||||||||||
| 3 | solid | eos/clear | No/Yes | |||||||||||
| 4 | solid | eos | No/Yes | |||||||||||
| 5 | solid | eos | Yes/Yes | |||||||||||
| 6 | solid | eos | Yes/No | + | + | + | + | + | − | + | − | − | − | |
| 3 | 7 | solid | clear | NA | + | + | + | + | + | − | − | − | − | − |
| 8† | solid | clear | NA | + | + | + | − | − | − | − | − | |||
| 4 | 9 | trabec | eos | Yes/Yes | − | + | + | + | + | − | − | − | − | − |
| 10 | solid | clear | Yes/No | |||||||||||
| 5 | 11 | trabec | eos* | No/Yes | + | + | + | + | + | − | foc+ | − | − | − |
| 12 | trabec/gland | amph/clear | Yes/Yes | |||||||||||
| 13 | trabec/gland | amph/clear | Yes/Yes | |||||||||||
| 6 | 14 | solid | clear | Yes/Yes | foc+ | − | foc+ | foc+ | − | |||||
| 15 | trabec | eos/clear | Yes/Yes | − | − | − | − | − | ||||||
| 16 | trabec | eos/clear | Yes/Yes | − | − | − | − | − | ||||||
| 7 | 17 | solid/trabec | clear/eos | Yes/Yes (calcification) | + | + | + | + | + | − | − | − | − | − |
| 8 | 18 | solid/trabec | clear | Yes/Yes | − | + | + | + | + | − | − | − | − | − |
| 9 | 19 | trabec | eos* | No/Yes | − | + | + | + | + | − | foc+ | foc+ | − | − |
| 10 | 20 | solid | amph/clear | Yes/No | foc+ | + | + | + | + | − | − | − | − | − |
| 11 | 21 | trabec | eos* | Yes/No | + | − | − | − | − | − | ||||
| 12 | 22 | trabec | eos | No/No | foc+ | + | − | − | − | − | − | |||
| 23 | solid | amph/clear | Yes/Yes | foc+ | + | − | − | − | − | − | ||||
| 13 | 24 | gland | amph/clear | Yes/Yes | + | + | foc+ | + | + | − | − | − | foc+ | − |
| 25 | solid | amph | Yes/No | − | + | + | + | + | − | + | − | − | − | |
| 26 | solid | amph | No/No | − | − | − | − | − | ||||||
| 14 | 27 | solid | amph | Yes/No | + | + | + | + | − | − | − | − | − | |
| 28 | solid | clear | Yes/No | + | + | foc+ | + | − | − | − | − | − | ||
| 29 | solid | clear | Yes/No | + | + | + | − | − | − | − | − | |||
| 30 | trabec | amph/clear | Yes/Yes | + | + | + | foc+ | − | + | foc+ | − | − |
NA, not applicable or not available; trabec, trabecular; gland, glandular; amph, amphiphilic cytoplasm; eos, eosinophilic cytoplasm; collagen, stromal collagen bands; Chr, chromogranin A; Syn, synaptophysin; Ker, cytokeratin AE1/AE3; Gluc, glucagon; PPP, pancreatic polypeptide; Som, somatostatin; Ins, insulin; Gastr, gastrin; foc, focal; +, positive immunohistochemistry stain; −, negative immunohistochemistry stain; blank, not done.
*Tumors composed of small cells.
†Liver metastasis.
Genetic Analysis of Tumors by PCR-SSCP and FISH
Six NETs from VHL patients 1, 4, and 11–14 and a pancreatic adenocarcinoma from patient 11 were analyzed for VHL gene deletion by PCR-SSCP analysis using a single nucleotide polymorphic marker (104/105) at the VHL gene locus. 10,15 Three patients (patients 1, 4, and 11) were informative for the marker; ie, normal DNA showed two different alleles (heterozygosity). Briefly, tumor and normal pancreatic cells were procured by a modified tissue microdissection procedure using 5-μm histological sections from frozen or formalin-fixed, paraffin-embedded tissue. 16 Cells were procured with a 30-gauge needle and immediately resuspended in 10-μL solution containing 10 mmol/L Tris-HCl, pH 8.0, 1 mmol/L ethylenediamine tetraacetic acid, pH 8.0, 1% Tween 20, 0.1 mg/ml proteinase K, and incubated overnight at 37°C. The mixture was boiled for 10 minutes to inactivate the proteinase K, and 10% of this solution was used for PCR analysis. The PCR amplification reaction was carried out for 30 cycles at 95°C for 30 seconds, 70°C for 30 seconds, using primers to the single-nucleotide polymorphism upstream of the coding region of the VHL gene: upstream, 5′-AGT GGA AAT ACA GTA ACG AGT TGG CCT-3′; downstream, 5′-GTC CCA GTT CTC CGC CCT CCG GGG CAT-3′. 15 Labeled amplified DNA was mixed with an equal volume of formamide loading dye (95% formamide, 20 mmol/L ethylenediamine tetraacetic acid, 0.05% bromphenol blue, and 0.05% xylene cyanol) and analyzed on SSCP gel. 10,15 The samples were denatured for 5 minutes at 95°C and loaded onto a gel consisting of 6% acrylamide (49:1 acrylamide:bis), 5% glycerol, and 0.6× Tris-borate ethylenediamine tetraacetic acid. Samples were electrophoresed at 8 W at room temperature overnight. Gels were transferred to 3-mm Whatman paper and dried, and autoradiography was performed with Kodak X-OMAT film (Eastman Kodak, Rochester, NY). The complete or near complete (90% decreased intensity) absence of one allele on acrylamide gel was interpreted as LOH. Each result was reproduced two to three times.
Four tumors from VHL patients 1, 2, 7, and 10 were analyzed for VHL gene deletion by FISH. Briefly, a touch preparation was performed from fresh or frozen tumors. FISH was performed using a P1 plasmid clone containing the entire VHL gene as a probe. 17 DNA was labeled with digoxigenin-11-dUTP by nick translation (Boehringer Mannheim). Biotin-labeled α-satellite centromeric probe specific for chromosome 3 (Oncor, Gaithersburg, MD) was used as a control. Slides were denatured in 70% formamide/2× standard saline citrate at 72°C for 2 minutes and dehydrated in ethanol series of 70, 80, 90, and 100%. The probes were denatured at 70°C for 10 minutes and then incubated at 37°C for 30 minutes for preannealing. DNA (250 μg) was applied to the slide and allowed to hybridize overnight in a humidified chamber at 37°C. Detection was performed using anti-digoxigenin rhodamine and avidin-fluorescein isothiocyanate. Hybridization signals were scored using a Zeiss Axiophot fluorescence microscope, and three-color images were captured on a Photometrics cooled-charge-coupled device camera (Photometrics, Tucson, AZ) using IP Lab image software (Signal Analytics Corporation, Vienna, VA). At least 100 interphases with strong hybridization signals were scored for each tumor. The presence of more than 20% cells with only one VHL signal was interpreted as an allelic deletion (loss of one copy of the VHL gene). The tumor cells retained both centromeric probes. Normal frozen pancreatic tissue control showed both centromeric probes and less than 2% of cells with one VHL signal.
Results
Tumor Gross Characteristics
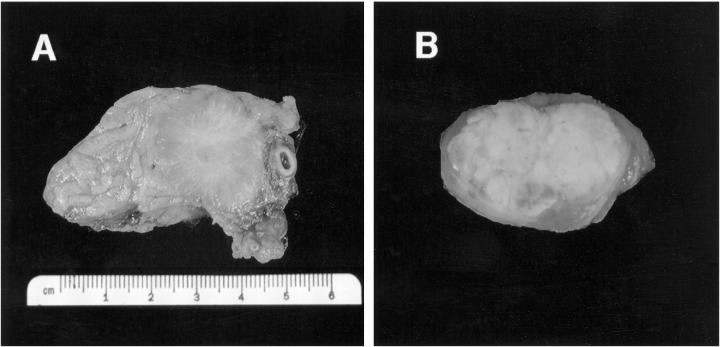
Thirty tumors were identified in 14 patients. Seven patients had multiple pancreatic tumors, and seven patients had a single tumor. The tumors were located in the head, body, and/or tail of the pancreas (Table 1) ▶ . All tumors were well circumscribed and varied in size from 0.4 to 8 cm (median, 2 cm). The color was tan, red/brown, gray, or yellow (Figure 1) ▶ . Invasion of adjacent organs was not observed.
Figure 1.
Gross photographs of two VHL-associated pancreatic NETs. A: A 3-cm solid, well-circumscribed, tan-gray tumor (tumor 1) with prominent collagen stroma removed from patient 1 by partial pancreatectomy. B: A 1-cm NET (tumor 29) removed from patient 14 by enucleation. The tumor displays a prominent yellow color secondary to abundant lipid content.
Tumor Microscopic Analysis
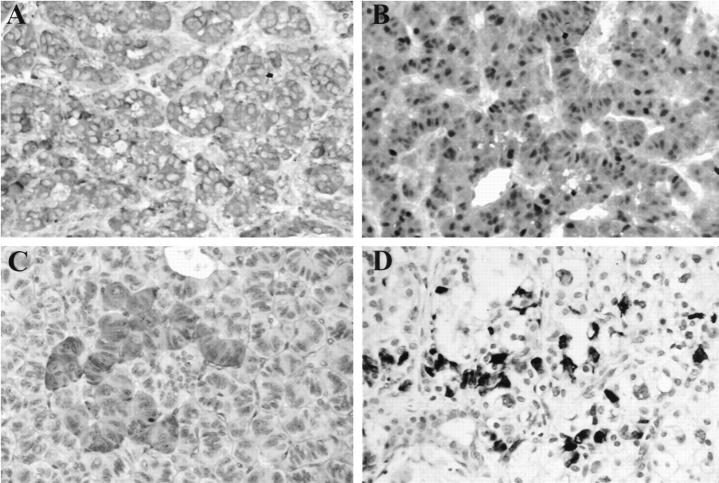
Histologically, 28 tumors were confined to the pancreas. One tumor in patient 1 showed a direct extension to a peripancreatic lymph node. Metastases to peripancreatic lymph nodes were not observed. Patient 3 had a biopsy-documented liver metastasis (tumor 8). Scattered islets, nerves, and exocrine ductules were seen within the tumor in 50% of the cases. The tumors showed solid, trabecular, and/or glandular architecture (Table 2 ▶ ; Figure 2, A and B ▶ ). Twenty-two tumors showed prominent stromal collagen bands (Figure 2C) ▶ , and one tumor demonstrated calcification (Table 2) ▶ . Congo red stain for amyloid was negative in the stroma. Angioinvasion was not present. The cytoplasm was eosinophilic in 8 tumors (Figure 2A) ▶ , amphiphilic in 4 tumors, clear in 7 tumors (Figure 2, B and C) ▶ , eosinophilic and clear in 5 tumors, and amphiphilic and clear in 6 tumors. Thus, 60% (18 of 30) of tumors demonstrated cells with clear cytoplasm (Figure 2, B and C) ▶ . PAS and PAS-D stains demonstrated glycogen in a minority of cases. The nuclear features characteristic for NETs were seen in all of the cases. Seventeen of 28 (61%) tumors showed prominent focal nuclear atypia (Figure 2C) ▶ . Mitoses were infrequent; the mitotic rate did not exceed 2 mitoses per 10 high-power fields. Evaluation of the pancreas for other lesions was possible in 11 cases. Additional lesions observed included benign serous cysts (5 cases), microcystic adenomas (3 cases), and a pancreatic adenocarcinoma (1 case) (Table 1) ▶ . Nesidioblastosis and hyperplasia of the islets of Langerhans were not observed in adjacent pancreas.
Figure 2.
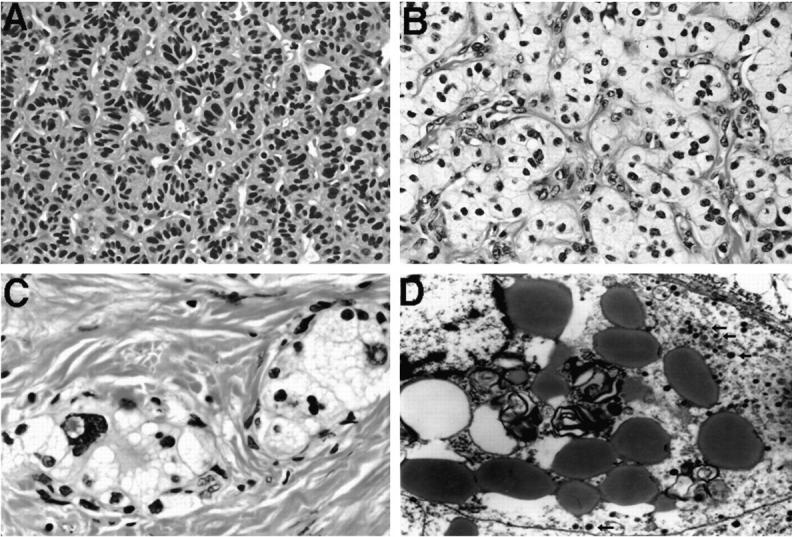
Histology and electron microscopy of representative VHL-associated pancreatic NETs. A: Tumor 19 (patient 9) shows trabecular architecture and small NET cells with eosinophilic cytoplasm (H&E, ×400). B: Tumor 18 (patient 8) shows solid architecture, small vessels, and cells with prominent clear cytoplasm (H&E, ×400). C: Tumor 14 (patient 6) demonstrates nests of tumor cells with clear cytoplasm and focal nuclear atypia surrounded by stromal collagen bands (H&E, ×630). D: Electron micrograph of a “clear” cell from tumor 18 (patient 8); prominent lipid globules and myelin figures and small dense core granules (arrows). Magnification, ×8900.
Immunohistochemistry
All 18 tumors that were evaluated by synaptophysin stain showed moderate-to-marked cytoplasmic positivity (Table 2 ▶ ; Figure 3A ▶ ). Chromogranin A stain was positive in 14 of 18 tumors evaluated. S100 (Figure 3B) ▶ and NSE stains were positive in all tumors evaluated (15 of 15 and 11 of 11 tumors, respectively). Cytokeratin AE1/AE3 demonstrated positivity in 16 of 16 tumors. Fifteen of 23 tumors evaluated with hormonal markers (glucagon, pancreatic polypeptide, somatostatin, insulin, and gastrin) showed negative results. Four tumors were positive with multiple hormonal markers. Six tumors were positive for pancreatic polypeptide, 4 for somatostatin, 2 for insulin, and 1 for glucagon. The majority of these stains showed only focal positivity in tumor cells (Figure 3, C and D) ▶ . All 23 tumors were negative for gastrin. Prominent small vessels in NETs were demonstrated by a vascular marker, CD34 (QBEND 10, Immunotech Inc., Westrock, MA).
Figure 3.
Immunohistochemistry results in representative VHL NETs. A: Positive synaptophysin stain in tumor 19 (patient 9) (×400). B: Positive S100 stain in tumor 19 (patient 9) (×400). C: Focally positive pancreatic polypeptide stain in tumor 11 (patient 5) (×400). D: Focally positive insulin stain in tumor 14 (patient 6) (×400).
Electron Microscopy
Electron microscopy was performed in six tumors from five patients. Electron-dense neurosecretory granules (Figure 2D) ▶ were demonstrated in all tumors. Neoplastic clear cells showed abundant intracytoplasmic lipid droplets. In addition, irregular, concentric, electron-dense phospholipid or lipoprotein membranes consistent with “myelin figures” were observed in clear cells (Figure 2D) ▶ . Both primary pancreatic and metastatic tumor in the liver in patient 3 showed tumor cells that were surrounded by basal laminae and contained large amounts of lipid and numerous neuroendocrine granules. Cytoplasmic glycogen was detected in one tumor.
VHL Gene Allelic Deletion Results
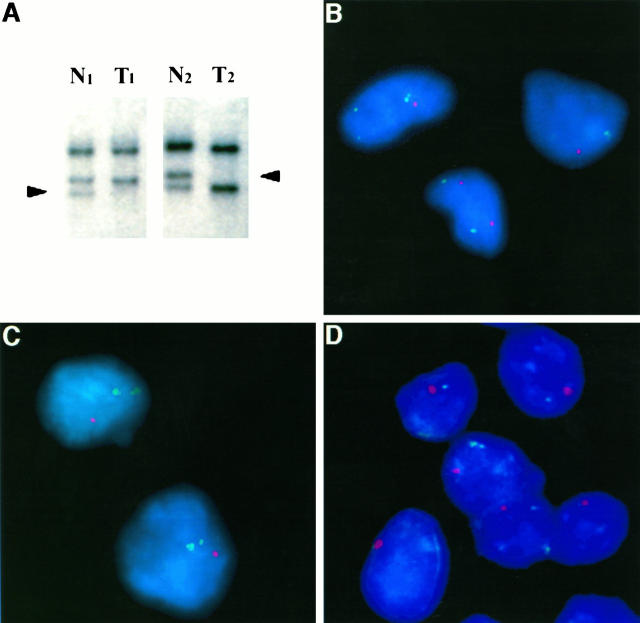
In each of the three informative patients (patients 1, 4, and 11) studied by PCR-SSCP analysis, LOH of one VHL gene allele was found in DNA from pancreatic NET and not in the DNA extracted from adjacent normal pancreatic parenchyma. Figure 4A ▶ illustrates representative results of SSCP analysis of DNA amplified across a single nucleotide polymorphic site at the VHL gene from two NETs in patients 1 and 4. For each patient, both alleles are present in lanes N1 and N2 (Figure 4) ▶ , respectively, containing DNA procured from the adjacent normal pancreas. In contrast, loss of the lower allele is detected in lane T1 in patient 1, and loss of the upper allele is detected in lane T2 in patient 4, containing DNA from pure populations of microdissected NET cells (Figure 4) ▶ . In patient 11, both pancreatic adenocarcinoma and NET were analyzed. LOH at the VHL gene was not detected in pancreatic adenocarcinoma in contrast to an NET in the same patient and to all NETs in the study. In four VHL patients (patients 1, 2, 7, and 10), allelic deletion of one copy of the VHL gene was detected by FISH in interphase touch preparations of pancreatic NETs. Figure 4 ▶ , B–D, illustrates representative results of FISH analysis in tumors from patients 1, 7 and 10.
Figure 4.
A: Detection of VHL gene (3p25.5) LOH by PCR-SSCP analysis in tumor 1 (T1; patient 1) and tumor 9 (T2; patient 4) using a single nucleotide polymorphic marker (104/105) upstream of the coding region of the VHL gene. Arrowheads: Loss of the lower allele is detected in lane T1 in patient 1, and loss of the upper allele is detected in lane T2 in patient 4, containing DNA from pure populations of microdissected NET cells as compared with matched normal DNA (lanes N1 and N2, respectively) procured from the adjacent pancreas. B, C, and D: VHL gene deletion detected by FISH in interphase touch preparations of three VHL NETs. Green signal: α-Satellite centromeric marker specific for chromosome 3; red signal: chromosome 3p25 P1 probe containing the VHL gene. Both centromeric probes are retained in tumor cells and are seen in normal somatic cells (green signals appear as dots or as a “blush” when out of focus). B: Tumor 1 (patient 1) showing allelic deletion of the VHL gene in two tumor cells one red signal as compared with a normal somatic cell with two red signals (bottom). C: Allelic deletion of the VHL gene in NET cells (tumor 17, patient 7; one red signal. D: Allelic deletion of the VHL gene in NET cells (tumor 20, patient 10; one red signal).
Discussion
Reports on NETs of the pancreas arising in VHL patients have been limited to case reports 18-22 and radiological series. 2-4,23 Although a relationship between pancreatic NETs and VHL disease has been suggested, neither pathological analysis of a series of tumors nor examination of genetic alterations in VHL-associated NETs have been reported.
Twelve percent of all VHL patients (n = 256) evaluated at the National Cancer Institute had solid pancreatic lesions by imaging studies. 14 VHL patients with pancreatic NETs in the current study were significantly younger (mean age, 35 years) than sporadic patients with NETs (mean age, 58 years). 24 All tumors were hormonally nonfunctional. The pancreatic NETs in VHL patients were often multiple, both microscopic and macroscopic in size, and were located throughout the pancreas (Table 1) ▶ .
Although usually well circumscribed and confined to the pancreas, VHL-associated NETs can metastasize, as evidenced by one case in this study. Recently, four other VHL patients referred to the National Cancer Institute had documented liver metastases from a pancreatic NET. These four cases are reported elsewhere, 14 but they were not included in the present study, because the primary pancreatic tumor was diagnosed by radiology only and was not available for evaluation. The median primary tumor diameter in patients with metastases was 5 cm, compared with a median primary tumor diameter of 2 cm for patients without evidence of metastatic disease. Therefore, the size of the primary VHL NET appears to be related to the risk of metastatic disease. 14 Similarly, three of the six NETs in VHL patients reported by the Mayo Clinic were malignant. 4
Histologically, all VHL-associated pancreatic lesions demonstrated an architecture characteristic for NETs. Neuroendocrine differentiation was confirmed by synaptophysin, chromogranin A, NSE, and S100 staining, and/or small, dense core granules shown by electron microscopy. Negative immunostaining for pancreatic and gastrointestinal hormones was observed in 15 of 23 of tumors (65%), and 8 of 23 (35%) NETs demonstrated focal positivity for pancreatic polypeptide, somatostatin, insulin, and/or glucagon. Small vessels, stromal collagen bands, and focal nuclear atypia were prominent in the majority of our VHL cases. The distinguishing feature of VHL NETs was clear-cell morphology that was present in 60% of the tumors, regardless of size. The cytoplasmic clearing was attributed to prominent lipid globules and myelin figures demonstrated by electron microscopy. A minority of VHL-associated NETs showed cytoplasmic glycogen on PAS and PAS-D stains or on electron microscopy.
NETs in VHL patients may be a diagnostic challenge because other VHL-associated tumors, such as renal cell carcinoma, hemangioblastoma, pancreatic microcystic (serous) adenoma, and epididymal cystadenoma, which contain numerous small vessels and clear cells, are histologically similar. The most common lesions to be considered in the differential diagnosis of VHL NETs are microcystic adenoma of the pancreas and metastatic renal cell carcinoma. Microcystic adenomas and NETs may occur in the same pancreas, and solid areas of microcystic adenoma can be hard to distinguish from clear-cell NETs in VHL patients. 14,25 However, pancreatic microcystic adenomas are usually glycogen rich and lack dense core granules. 26 Clear-cell renal cell carcinomas tend to form sheets of clear cells and microcysts separated by thin fibrovascular septae rather than the broad collagen bands common in NETs; renal cell carcinomas also have cytoplasmic glycogen or lipid and are negative for neuroendocrine immunohistochemistry markers. Two additional, uncommon pancreatic tumors may also be considered. The first, clear-cell “sugar” tumor of pancreas, similar to “sugar” tumor of lung, demonstrates abundant cytoplasmic glycogen and is HMB-45-positive and cytokeratin, NSE, and chromogranin A-negative. 27 The second, clear-cell carcinoma of the pancreas, is exceedingly rare and demonstrates significant pleomorphism and invasiveness. 26
The presence of pancreatic NETs, as well as pheochromocytomas in patients with VHL, suggests that VHL disease may represent a continuum of multiple endocrine neoplasia. 4,18,20 Multiple pancreatic NETs commonly occur in multiple endocrine neoplasia type 1 (MEN1). 24,28,29 However, along with similarities, several distinguishing features may be seen in pancreatic pathology of patients with VHL disease and MEN1. First, NETs are found in 82 to 100% of MEN1 patients, 24 as compared with an incidence of 12 to 17% in VHL disease. 4,14 Second, hormonally functional NETs are rare in VHL disease 4,21 but are relatively common in MEN1; 28 all tumors were nonfunctional in this study. Finally, pancreatic resection specimens in MEN1 patients demonstrate multiple microadenomas and nesidioblastosis in 30% of MEN1 cases. 29 VHL patients in this study had a mean of two pancreatic NETs. Although scattered islets and ductules were frequent within VHL-associated NETs, nesidioblastosis and/or NETs less than 0.4 cm in size were not observed in adjacent pancreas.
The two-hit tumor suppressor gene theory of Knudson 6 predicts that in a familial cancer syndrome such as VHL disease, the genotype of each neoplasm should consist of one allele with an inherited germ-line mutation and loss of the second wild-type allele, which occurs through chromosomal deletion. Therefore, LOH or mutation at the VHL gene should be detectable in the lesion if the lesion represents a VHL-associated neoplasm. LOH at chromosome 3p has been detected in VHL-associated renal cell carcinomas, 8-10 hemangioblastomas, 7 pheochromocytomas, 11 endolymphatic sac tumors, 13 and pancreatic microcystic adenomas. 12 In this study, allelic deletion of the second copy of the VHL gene was detected in NETs from all six VHL patients with known germ-line mutations. Furthermore, in one case (patient 11), VHL gene heterozygosity was retained in a pancreatic adenocarcinoma that was associated with a NET with a VHL gene deletion. The presence of allelic deletions of the VHL gene in pancreatic NETs provides direct molecular evidence for a role of the VHL gene in their tumorigenesis and establishes NET as an independent tumor type of VHL disease. In contrast, retention of heterozygosity in adenocarcinoma, coupled with the absence of other VHL patients with pancreatic carcinoma in our series, suggests that VHL gene alterations are unlikely to be associated with the development of pancreatic adenocarcinoma in VHL patients.
Acknowledgments
We thank Drs. H. Richard Alexander, David L. Bartlett, and Hernan A. Vargas (Surgery Branch, National Cancer Institute), who performed surgery on the subjects studied. We also thank Dr. Maria Tsokos (Laboratory of Pathology, National Cancer Institute) for assistance with electron microscopy and Cynthia A. Harris and Sarah A. Delay (Laboratory of Pathology, National Cancer Institute) for assistance with immunohistochemistry staining.
Footnotes
Address reprint requests to Zhengping Zhuang, M.D., Ph.D., Laboratory of Pathology, National Cancer Institute, NIH, Building 10, Room 2A33, 10 Center Drive, Bethesda, MD 20892. E-mail: zpzhuang@helix.nih.gov.
References
- 1.Lamiell JM, Salazar FG, Hsia YE: Von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore) 1989, 68:1-29 [DOI] [PubMed] [Google Scholar]
- 2.Neumann HPH, Dinkel E, Brambs H, Wimmer B, Friedburg H, Volk B, Sigmund G, Riegler P, Haag K, Schollmeyer P, Wiestler OD: Pancreatic lesions in the von Hippel-Lindau syndrome. Gastroenterology 1991, 101:465-471 [DOI] [PubMed] [Google Scholar]
- 3.Hough MT, Stephens DH, Johnson CD, Binkovitz LA: Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. Am J Roentgenol 1994, 162:1091-1094 [DOI] [PubMed] [Google Scholar]
- 4.Binkovitz LA, Johnson CD, Stephens DH: Islet cell tumors in von Hippel-Lindau disease: increased prevalence and relationship to the multiple endocrine neoplasias. Am J Roentgenol 1990, 155:501-505 [DOI] [PubMed] [Google Scholar]
- 5.Latif F, Tory K, Gnarra J, Yao M, Duh F-M, Orcutt M, Stackhouse T, Kuzmin I, Modi W, Geil L, Schmidt L, Zhou F, Li H, Wei M, Chen F, Glenn G, Choyke P, Walther M, Weng Y, Duan D, Dean M, glavac D, Richards F, Crossey P, Ferguson-Smith M, Paslier D, Chumakov I, Cohen D, Chinault A, Maher E, Linehan W, Zbar B, Lerman M: Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993, 260:1317-1320 [DOI] [PubMed] [Google Scholar]
- 6.Knudson AG: Hereditary cancer, oncogenes, and antioncogenes. Cancer Res 1985, 45:1437-1443 [PubMed] [Google Scholar]
- 7.Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, Zhuang Z: VHL gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol 1997, 28:540-543 [DOI] [PubMed] [Google Scholar]
- 8.Zbar B, Brauch H, Talmadge C, Linehan W: Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. Nature 1987, 327:721-724 [DOI] [PubMed] [Google Scholar]
- 9.Anglard P, Tory K, Brauch H, Weiss G, Merino M, Lerman M, Zbar B, Linehan W: Molecular analysis of genetic changes in the origin and development of renal carcinoma. Cancer Res 1991, 51:1071-1077 [PubMed] [Google Scholar]
- 10.Lubensky IA, Gnarra J, Bertheau P, Walther M, Linehan WM, Zhuang Z: Allelic deletions of the VHL gene detected in multiple microscopic clear cell renal lesions in von Hippel-Lindau disease patients. Am J Pathol 1996, 149:2089-2094 [PMC free article] [PubMed] [Google Scholar]
- 11.Zeiger M, Zbar B, Keiser H, Linehan WM, Gnarra JR: Loss of heterozygosity on the short arm of chromosome 3 in sporadic, von Hippel-Lindau disease-associated, and familial pheochromocytoma. Genes Chromosomes Cancer 1995, 13:151-156 [DOI] [PubMed] [Google Scholar]
- 12.Vortmeyer AO, Lubensky IA, Fogt F, Linehan WM, Khettry U, Zhuang Z: Allelic deletion and mutation of the VHL tumor suppressor gene in pancreatic microcystic adenomas. Am J Pathol 1997, 151:951-956 [PMC free article] [PubMed] [Google Scholar]
- 13.Vortmeyer AO, Choo D, Pack SD, Oldfield E, Zhuang Z: von Hippel-Lindau disease gene alterations associated with endolymphatic sac tumor (letter). J Natl Cancer Inst 1997, 89:970-972 [DOI] [PubMed] [Google Scholar]
- 14.Libutti SK, Choyke PL, Bartlett DL, Vargas HA, Walther M, Lubensky I, Glenn G, Steinberg S, Linehan WM, Alexander HR: Pancreatic neuroendocrine tumors associated with von Hippel-Lindau: diagnostic and management recommendations. Surgery (in press) [DOI] [PubMed]
- 15.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei H, Li H, Latif F, Liu S, Chen F, Duh F-M, Lubensky I, Duan D, Florence C, Pozzatti R, Walther M, Bander N, Grossman H, Brauch H, Pomer S, Brooks J, Isaacs W, Lerman M, Zbar B, Linehan W: Mutations of the VHL suppressor gene in renal carcinoma. Nat Genet 1994, 7:85-90 [DOI] [PubMed] [Google Scholar]
- 16.Zhuang Z, Bertheau P, Emmert-Buck M, Liotta L, Gnarra J, Linehan W, Lubensky I: A microdissection technique for archival DNA analysis of specific cell populations in lesions <1 mm in size. Am J Pathol 1995, 146:620-625 [PMC free article] [PubMed] [Google Scholar]
- 17.Pack S, Vortmeyer AO, Pak E, Liotta LA, Zhuang Z: Detection of gene deletion in single metastatic tumour cells in lymphnode tissue by fluorescent in situ hybridization. Lancet 1997, 350:264-265 [DOI] [PubMed] [Google Scholar]
- 18.Probst A, Lotz M, Heitz P: Von Hippel-Lindau’s disease, syringomyelia and multiple endocrine tumors: a complex neuroendocrinopathy. Virchows Arch A Pathol Anat Histol 1978, 378:265-272 [DOI] [PubMed] [Google Scholar]
- 19.Hull MT, Warfel KA, Muller J, Higgins JT: Familial islet cell tumors in von Hippel-Lindau’s disease. Cancer 1979, 44:1523-1526 [DOI] [PubMed] [Google Scholar]
- 20.Mulshine JL, Tubbs R, Sheeler LR, Gilford RW: Case report: clinical significance of the association of the von Hippel-Lindau disease with pheochromocytoma and pancreatic apudoma. Am J Med Sci 1984, 288:212-216 [DOI] [PubMed] [Google Scholar]
- 21.Cornish D, Pont A, Minor D, Coombs JL, Bennington J: Metastatic islet cell tumor in von Hippel-Lindau disease. Am J Med. 1984, 77:147-150 [DOI] [PubMed] [Google Scholar]
- 22.Mount SL, Weaver DL, Taatjes DJ, McKinnon WC, Hebert JC: von Hippel-Lindau disease presenting as pancreatic neuroendocrine tumour. Virchows Arch 1995, 426:523-528 [DOI] [PubMed] [Google Scholar]
- 23.Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B: von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology 1995, 194:629-642 [DOI] [PubMed] [Google Scholar]
- 24.Solcia E, Capella C, Klöppel G: Tumors of the endocrine pancreas. Tumors of the Pancreas. Rosai J eds. Atlas of Tumor Pathology. 1997, :pp 145-209 Armed Forces Institute of Pathology, Washington DC, [Google Scholar]
- 25.Perez-Ordonez B, Naseem A, Lieberman PH, Klimstra DS: Solid serous adenoma of the pancreas: the solid variant of serous cystadenoma? Am J Surg Pathol 1996, 20:1401-1405 [DOI] [PubMed] [Google Scholar]
- 26.Solcia E, Capella C, Klöppel G: Tumors of the exocrine pancreas. Tumors of the Pancreas. Rosai J eds. Atlas of Tumor Pathology. 1997, :pp 31-144 Armed Forces Institute of Pathology, Washington DC, [Google Scholar]
- 27.Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F: Clear cell “sugar” tumor of the pancreas: a novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol 1996, 20:722-730 [DOI] [PubMed] [Google Scholar]
- 28.Padberg B, Schröder S, Capella C, Frilling A, Klöppel G, Heitz PH: Multiple endocrine neoplasia type 1 (MEN1) revisited. Virchows Arch 1995, 426:541-548 [DOI] [PubMed] [Google Scholar]
- 29.Le Bodic MF, Heymann MF, Lecomte M, Berger N, Berger F, Louvel A, De Micco C, Patey M, De Mascarel A, Burtin F, Saint-Andre JP: Immunohistochemical study of 100 pancreatic tumors in 28 patients with multiple endocrine neoplasia, type I. Am J Surg Pathol 1996, 20:1378-1384 [DOI] [PubMed] [Google Scholar]