Abstract
In the present study we analyzed, by immunohistochemistry, a panel of human melanomas for protein expression of the cyclin-dependent kinase (cdk) inhibitor p27Kip1 and evaluated whether deregulated expression correlates with clinical outcome for this type of cancer. We found that p27Kip1 was strongly expressed by normal melanocytes and benign nevi, whereas in malignant melanoma, a heterogeneous expression pattern was observed. In the case of nodular melanomas, the level of p27Kip1 was found to correlate significantly with the thickness of the tumor, with less protein expressed in thicker lesions. We also found that patients having tumors with fewer than 5% p27Kip1-staining cells had a significantly higher risk of early relapse of their disease compared with those expressing moderate or high levels. In contrast, the level of p27Kip1 did not correlate with tumor thickness or disease-free survival in patients with superficial spreading melanomas, suggesting that p27Kip1 may play different roles in these two major pathological subgroups of malignant melanoma. Furthermore, p27Kip1 did not appear to have an influence on overall survival for either subgroup. When we examined the combined effect of p21WAF1/CIP1 (another cdk inhibitor) and p27Kip1 on clinical outcome, we found that analysis of these two cdk inhibitors together may have greater prognostic potential than either alone. In conclusion, our results suggest that virtually complete loss of p27Kip1 protein expression has potential importance as a prognostic indicator of early relapse in patients with nodular melanoma. The results, furthermore, underscore the value of analyzing multiple cell cycle regulatory proteins to obtain the most reliable indication of prognosis.
Normal cell cycle progression is governed by a family of cyclin-dependent kinases (cdks), the activity of which is regulated by association with positive effectors, the cyclins, by phosphorylation and dephosphorylation of the cdk subunit, and by association with small inhibitor molecules, of which p15CDKN2b and p16CDKN2a of the INK4 family and p21WAF1/CIP1 and p27Kip1 of the KIP family are the best characterized. 1-3
p21WAF1/CIP1 was the first mammalian cdk inhibitor to be identified and was found to be induced by wild-type p53 in response to DNA damage, thereby suggesting a role in the coordination of p53-mediated growth arrest and DNA repair. 4,5 However, p21WAF1/CIP1 has also been demonstrated to be involved in cellular senescence, terminal differentiation, and apoptosis through p53-independent mechanisms. 6-9 Notably, the gene encoding p21WAF1/CIP1 has been recently cloned and identified as a melanoma differentiating antigen (mda6), the expression of which is up-regulated in more differentiated melanoma cell lines and in melanocytes grown in vitro. 10 In contrast, decreased p21WAF1/CIP1 mRNA and protein levels have been detected in cell lines derived from advanced melanomas and in tumor specimens obtained from melanoma metastases, as compared with the levels in early-stage malignant lesions, suggesting that loss of p21WAF1/CIP1 may contribute to the progression of this type of cancer. 10,11
p27Kip1 was originally identified as a cdk inhibitor, the activity of which is up-regulated in vitro by transforming growth factor (TGF)-β, by contact inhibition, or by serum depletion. 12-15 Furthermore, p27Kip1 levels are increased during differentiation of cultured cells. 16,17 p27Kip1 has been demonstrated to play an important role in regulating progression through G1 and entrance into the S phase of the cell cycle by binding to and preventing premature activation of cdk4/cyclin D and cdk2/cyclin E complexes. 14,18 In addition, constitutive overexpression of p27Kip1 causes cell cycle arrest in the G1 phase. Recent studies have demonstrated that transgenic p27Kip1 knockout mice develop multi-organ hyperplasia and are larger than their normal littermates. 19-21 This finding supports an important role for p27Kip1 in the negative regulation of normal cellular proliferation.
In contrast to the INK4 inhibitors, members of the KIP family, p21WAF1/CIP1 and p27Kip1, are rarely mutated in human cancer. 22,23 The level of p27Kip1 has been shown to be regulated primarily at the post-transcriptional level through the ubiquitin-proteasome-mediated pathway. 24 In colorectal carcinomas, decreased levels of p27Kip1 are associated with increased ubiquitin-mediated degradation of p27Kip1 and with a less favorable prognosis. 25 Furthermore, low levels of p27Kip1 have also been associated with decreased survival of patients with breast, 26-28 gastric, 29 and non-small-cell lung cancer. 30 These findings led us to study whether altered expression of p27Kip1 could also be detected in tumor samples obtained from patients with malignant melanoma and, if so, whether such alterations in p27Kip1 levels may have value as a prognostic marker. Recently, our panel of primary and metastatic melanomas has been used to analyze protein expression of p21WAF1/CIP1. In the aforementioned study, we observed reduced p21WAF1/CIP1 protein expression in advanced melanomas; however, no correlation between p21WAF1/CIP1 levels and clinical outcome was detected. In another recent study, by Porter et al, 27 p27Kip1 and cyclin E proteins were both analyzed in a panel of breast carcinomas. The finding that low p27Kip1 and high cyclin E levels appeared to have a powerful synergistic effect as prognostic indicators spurred us to address whether analysis of p21WAF1/CIP1 and p27Kip1 levels together may be of greater prognostic value for melanoma than either alone.
Materials and Methods
Specimens
Formalin-fixed, paraffin-embedded tissue sections were obtained from 113 primary malignant melanomas, 45 melanoma metastases of which 36 had distant location, and 4 benign nevi. From 32 patients, both primary and metastatic material were collected. Of the primary tumors, 79 were classified as superficial and 34 as nodular. Clinical follow-up was available for 109 patients, and for 104 of these the combined value of p21WAF1/CIP1 and p27Kip1 could be examined.
Immunohistochemical Analysis
Sections of formalin-fixed, paraffin-embedded tissue were immunostained using the biotin-streptavidin-peroxidase method (Supersensitive Immunodetection System, LP000-UL, Biogenex, San Ramon, CA) and the Optimax Plus Automated cell staining System (Biogenex). Deparaffinized sections were microwaved in 10 mmol/L citrate buffer (pH 6.0) for 20 minutes (four times for 5 minutes each) to unmask epitopes and treated with 1% hydrogen peroxidase (H2O2) for 10 minutes to block endogenous peroxidase. The sections were subsequently incubated with monoclonal p27Kip1 antibody (K25020, Transduction Laboratories, Lexington, KY) diluted 1:200 (1.25 μg of IgG1/ml) for 30 minutes at room temperature and incubated thereafter with biotin-labeled secondary antibody (1:40) and streptavidin-peroxidase (1:40) for 20 minutes each. Tissue was stained for 5 minutes with 0.05% 3,3′-diaminobenzidine tetrahydrochloride freshly prepared in 0.05 mol/L Tris/HCl buffer, pH 7.6, containing 0.024% H2O2 and then counterstained with hematoxylin, dehydrated, and mounted in Diatex. All the dilutions of antibody, biotin-labeled secondary antibody, and streptavidin-peroxidase were made with phosphate-buffered saline, pH 7.4, containing 5% bovine serum albumin. All series included normal skin as positive controls. Negative controls included substitution of the monoclonal antibody with mouse myeloma protein of the same subclass and concentration as the monoclonal antibody. All controls gave satisfactory results. Four semiquantitative classes were used to describe the number of positive stained cells: −, none; +, <5%; ++, 5 to 50%; +++, >50%. The p27Kip1 staining was evaluated by one and, in cases that were not obvious, by two observers. In the latter cases a good concordance was always achieved. Based on previous studies, p27Kip1 protein expression was classified as high when more than 50% of the tumor cells stained positive 25,26 or, alternatively, when more than 5% were p27Kip1 immunoreactive.
Statistical Analysis
The relationship between the expression of p27Kip1 and the median tumor thickness was evaluated nonparametrically using the Mann-Whitney two-sample test. Kaplan-Meier survival analysis was used to evaluate the relapse-free and overall survival data. A value of P < 0.05 was considered significant.
Results
p27Kip1 Levels in Primary and Metastatic Melanoma Lesions
Formalin-fixed, paraffin-embedded tissue sections from 113 primary and 45 metastatic malignant melanomas were immunohistochemically analyzed for p27Kip1 protein expression. Nuclear p27Kip1 staining was detected in 87% (98 of 113) of the primary malignant melanomas (Figure 1A) ▶ and in 67% (30 of 45) of the metastases (Figure 1B ▶ ; Table 1 ▶ ). Furthermore, a higher fraction of primary tumors as compared with metastatic lesions showed p27Kip1 immunoreactivity in more than 5% of the cells (54% versus 42%).
Figure 1.
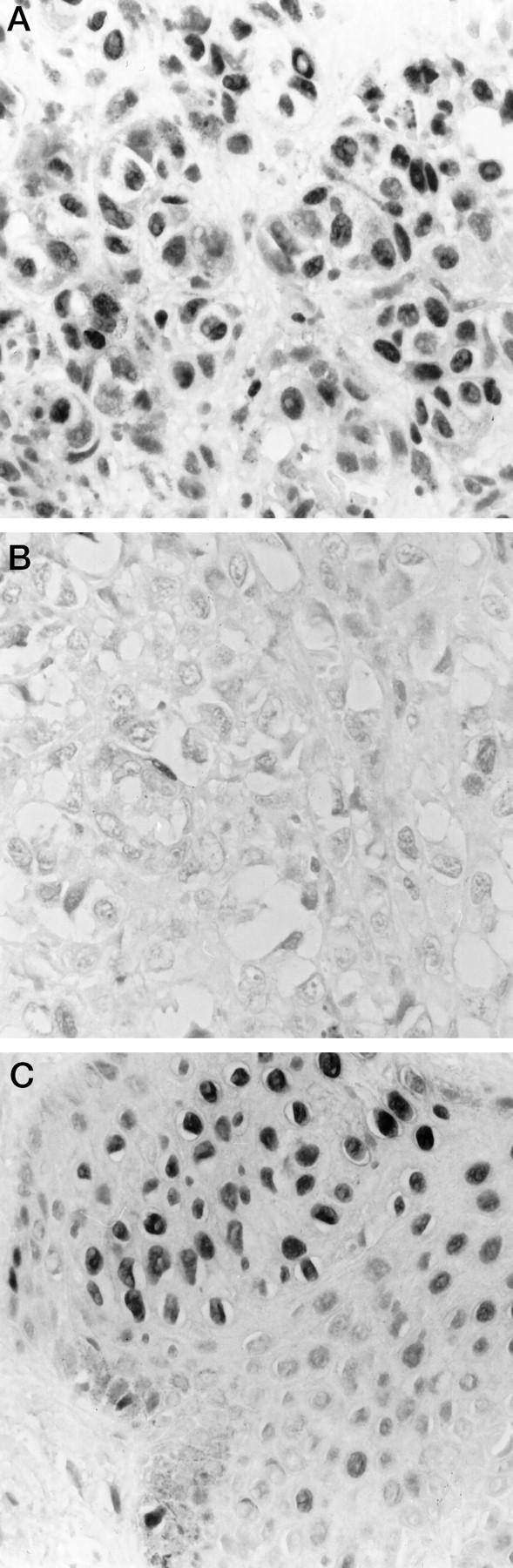
Immunohistochemical analysis showing the level of p27Kip1. A: Primary melanoma. B: Metastasis from the same patient as shown in A. C: Benign nevi, including normal skin.
Table 1.
Number (Percentage) of Melanocytic Tumors Expressing p27Kip1
| Tumor type | Number examined | p27Kip1 protein level* | |||
|---|---|---|---|---|---|
| − | + | ++ | +++ | ||
| Primary melanomas | 113 | 15 (13%) | 38 (34%) | 23 (20%) | 37 (33%) |
| Superficial | 79 | 10 (13%) | 31 (39%) | 14 (18%) | 24 (30%) |
| Nodular | 34 | 5 (15%) | 7 (21%) | 9 (26%) | 13 (38%) |
| Metastatic melanoma | 45 | 15 (33%) | 11 (25%) | 13 (29%) | 6 (13%) |
| Benign nevi | 4 | – | – | – | 4 (100%) |
*Measured as described in Materials and Methods.
In 29 cases, both primary and metastatic tumor samples from the same patient could be examined. A decrease in the number of p27Kip1-positive cells was observed in 14 of 29 (45%) of the metastatic tumors when compared with the corresponding primary lesion (Figure 1, A and B) ▶ . In another eight patients, with the same proportion of cells stained in the primary and metastatic tumor, a lower intensity of p27Kip1 staining was found in four of the latter. Combining these results, altogether 18 of 29 (62%) of the metastases expressed lower levels of p27Kip1 than the corresponding primary tumor (Table 2) ▶ . In normal skin, distinct protein staining was present in all nucleus-containing layers of the squamous epidermis and underlying adnexal structures. A high p27Kip1 level was also observed in the four benign nevi examined (Figure 1C) ▶ .
Table 2.
Relationship between the Level of p27Kip1 Protein in Primary and Metastatic Melanoma from the Same Patient
| p27Kip1 protein level* | ||||
|---|---|---|---|---|
| Metastasis | Primary tumor† | |||
| − | + | ++ | +++ | |
| − | 2‡ | 5 | 2 | 3 |
| + | 2 | 2 | 0 | 1 |
| ++ | 1 | 2 | 3 | 3 |
| +++ | 1 | 0 | 1 | 1 |
*Measured as described in Materials and Methods.
†In 29 cases, primary and metastatic tumor from the same patient could be analyzed.
‡In eight cases with the same proportion of cells stained in the primary and metastatic tumor, a lower staining intensity was found in four of the metastases.
Expression of p27Kip1 Protein in Relation to Clinical Parameters of Malignancy
We found that there was no significant difference in the distribution of low and high p27Kip1 staining between nodular and superficial melanomas using either a 50% or a 5% cutoff to describe high and low p27Kip1 levels (Table 1 ▶ and results not shown). Interestingly, however, within the nodular subtype, there was a significant variation in p27Kip1 staining as a function of the vertical growth of the tumors, with lower levels in thicker lesions (P = 0.05, 5% cutoff; Table 3 ▶ ). Using a 50% cutoff to discriminate between high and low p27Kip1 levels did not change the results (P = 0.05, data not shown).
Table 3.
Relationship between p27Kip1 Levels and Depth of Tumor Growth of Primary Melanomas
| Tumor type | Immunohisto- chemistry* | Number of patients | Depth of growth† (mm) |
|---|---|---|---|
| Superficial | High | 24 | 1.15 P = 0.56 |
| Low | 55 | 1.15 | |
| Nodular | High | 13 | 2.30 P = 0.05 |
| Low | 21 | 5.20 |
*Scored as described in Materials and Methods: <50% cells stained, low expression; >50% cells stained, high expression. Changing the cutoff to high when more than 50% of the cells were p27 positive did not alter the outcome. A total of 113 tumors were analyzed.
†Measured as the median thickness in each group.
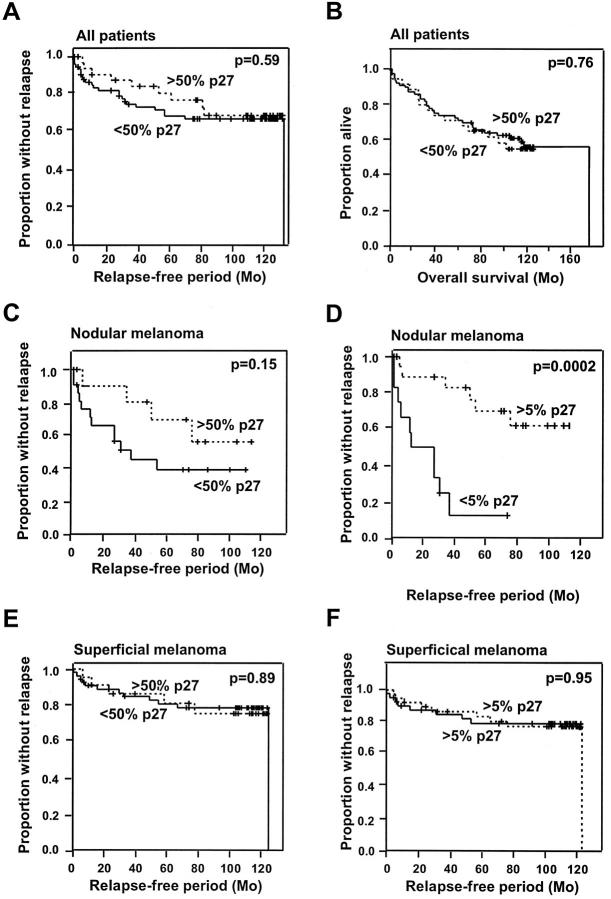
Clinical follow-up data were available for 109 patients (superficial, n = 76; nodular, n = 33), of which 35 developed distant metastases. For the total group of patients, there was no correlation between the level of p27Kip1 using a 50% cutoff and relapse-free or overall survival (P = 0.59 and P = 0.76, respectively; Figure 2, A and B ▶ ). Changing the cutoff (low p27Kip1 when <5% cells stained positive) did not alter the outcome of the evaluation (data not shown). When the superficial and the nodular spreading melanomas were examined separately, there was a trend toward increased disease-free survival in patients whose nodular tumor expressed higher levels of p27Kip1 than those having less than 50% of the cells stained positive for the inhibitor (P = 0.15; Figure 2C ▶ ). Interestingly, when the cutoff between high and low p27Kip1 protein expression was changed (high when >5% of the cells were p27Kip1 positive), the disease-free survival was clearly reduced for those patients expressing p27Kip1 in less than 5% of the tumor cells (P = 0.0002; Figure 2D ▶ ). Notably, the level of p27Kip1 in the primary tumor did not influence overall survival for patients with nodular melanoma (P = 0.68 with 50% cutoff versus P = 0.41 with 5% cutoff; data not shown). p27Kip1 was not found to have any impact on either disease-free or overall survival for patients with the superficial spreading subtype (Figure 2E ▶ and data not shown), and changing the cutoff did not influence the outcome of the evaluation (Figure 2F ▶ and data not shown), possibly due to the long duration of follow-up required for the demonstration of survival benefit for this subtype of melanoma. Altogether, these results suggest that p27Kip1 may play different roles in the malignant progression of the two major subgroups of melanoma, and furthermore, nodular melanomas, which express virtually no p27Kip1 in the tumor cell nuclei, have the highest risk of early recurrence.
Figure 2.
Kaplan-Meier curves demonstrating the relationship between the expression of p27Kip1 and relapse-free (A) and overall (B) survival for the entire panel of melanoma patients (n = 109) and relapse-free survival divided by the following subtypes: nodular (C and D; n = 33) and superficial (E and F; n = 76). p27Kip1 was considered as high when more than 50% of the tumor cells stained positive (A, B, C, and E) or high when more than 5% showed positive immunoreactivity (D and F).
Comparison between p27Kip1 and p21WAF1/CIP1 Protein Expression
A total of 104 primary tumors and 31 metastases could be examined for expression of both p21WAF1/CIP1 and p27Kip1 proteins. Only one primary tumor showed positive immunoreactivity for both proteins in more than 50% of the cells. In contrast, 36 tumors (31%) showed both p27Kip1 and p21WAF1/CIP1 staining in fewer than 5% of the cells. High (>50% cells stained) staining of both proteins was never seen in metastatic samples, and indeed, 13 (42%) showed almost complete loss (<5% cells stained) of both p27Kip1 and p21WAF1/CIP1 staining.
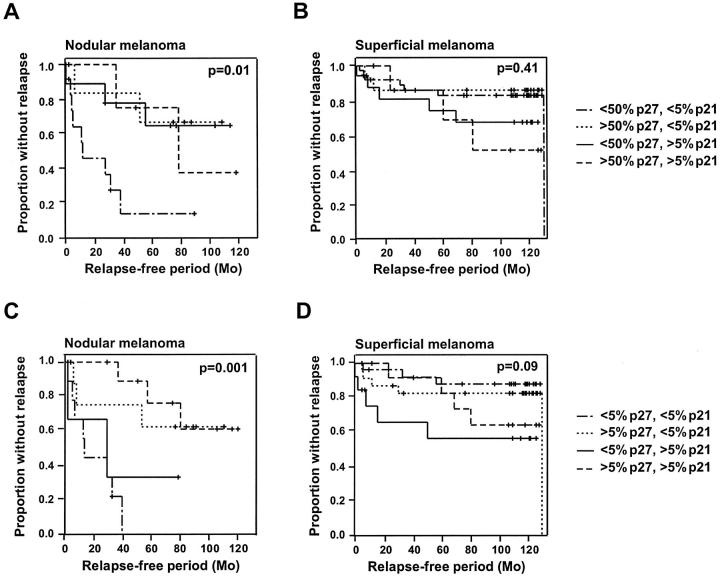
When assessing the effect of reduced levels of both p21WAF1/CIP1 and p27Kip1 proteins on clinical outcome for the whole group of patients, we observed that moderate to high levels of both proteins in the primary tumors did not correlate with increased disease-free and overall survival (data not shown). When pathological subgroups of melanoma were analyzed separately, however, we found that, in nodular melanoma, low levels of p21WAF1/CIP1 staining in cases that also showed reduced p27Kip1 (<50% cells stained positive) was a stronger predictor of relapse of disease (P = 0.01) than low p27Kip1 alone (P = 0.15; Figures 3A and 2C ▶ ▶ ). However, with a 5% cutoff between high and low p27Kip1 expression, less than 5% p27Kip1-positive cells was still the strongest indicator of early relapse (P = 0.0002), and p21WAF1/CIP1 did not add any additional power to the evaluation (P = 0.001; Figures 2D and 3C ▶ ▶ ).
Figure 3.
Kaplan-Meier curves demonstrating the association between relapse-free survival and expression of p21WAF1/CIP1 and p27Kip1 in nodular (A and C) and superficial (B and D) melanoma. In A and B, p27Kip1 was considered as high when more than 50% of the tumor cells stained positive, and in B and D, when more than 5% expressed the protein. In all cases, p21WAF1/CIP1 was considered as high when more than 5% of the cells showed positive p27Kip1 immunoreactivity. The different combinations of high and low p21WAF1/CIP1 and p27Kip1 are indicated.
Recently, in primary superficial melanomas, we observed a significant correlation between p21WAF1/CIP1 protein expression and tumor thickness, with thin lesions showing the lowest protein levels (P = 0.005). 11 Neither p21WAF1/CIP1 nor p27Kip1 levels examined alone were found to have any impact on the clinical outcome for this group of patients. However, when p27Kip1 and p21WAF1/CIP1 were assayed together, we found that low levels of p27Kip1 (<5% cells stained) together with high levels of p21WAF1/CIP1 (>5% cells stained) was a stronger predictor of early recurrence than p27Kip1 alone (P = 0.09 versus P = 0.95; Figures 3D and 2F ▶ ▶ ).
In conclusion, we have shown that almost complete loss of p27Kip1 protein has prognostic value for relapse of disease for patients with nodular melanoma, but not for those with superficial malignant melanoma. p27Kip1 was not predictive of overall survival in either group. Furthermore, a combined analysis of p21WAF1/CIP1 and p27Kip1 proteins may have a greater prognostic potential for relapse-free survival in both of these pathological subgroups.
Discussion
In the present study we examined the protein level of p27Kip1 in a panel of primary and metastatic human melanoma specimens and evaluated whether deregulated expression of this cdk inhibitor was correlated with clinical outcome. Our panel of melanoma specimens has been previously analyzed for the protein expression of p21WAF1/CIP1, another member of the KIP family of cdk inhibitors. 11 We therefore made a comparison between the protein levels of p21WAF1/CIP1 and p27Kip1 and evaluated their combined prognostic value for this group of patients.
In agreement with previous reports for normal tissue of epithelial origin, 26,30,31 we found that p27Kip1 was highly expressed in normal skin, including keratinocytes, melanocytes, and benign nevi, supporting the notion that one important function of p27Kip1 may be to regulate quiescence in normal cells. 32-34 This is in contrast to p21WAF1/CIP1, the expression of which is not detectable in melanocytes and benign nevi in vivo 11,35 and which is also very low in G0-arrested melanoma cell lines in vitro. 32 Thus, these two cdk inhibitors may play different roles in proliferation of cells of the melanocytic linage.
In agreement with what has been observed for other types of tumors, 26,27,30,31 p27Kip1 was found to be heterogeneously expressed in malignant melanomas, with a trend toward less p27Kip1 immunoreactivity in metastatic lesions as compared with the levels seen in the corresponding primary tumors. However, when the panel of malignant melanomas was divided into the two most common pathological subtypes, the superficial and the nodular, important differences were observed. In nodular melanomas, thicker tumors had significantly less p27Kip1 than thinner, and potentially less aggressive, lesions. Furthermore, we found that patients whose tumors showed virtually no p27Kip1 immunoreactivity had a significantly shorter relapse-free period than those whose tumors expressed higher p27Kip1 levels. This finding is in accordance with recent reports indicating that p27Kip1 is a prognostic indicator of disease-free survival for patients with breast, colon, gastric, and non-small-cell lung cancers. 25-30 Notably, Ki-67 immunostaining of a limited number of the melanomas revealed no clear correlation between the p27 levels and cell proliferation (data not shown), a finding that has been demonstrated also by others, 25,26,36,37 suggesting that p27 may have additional functions that are not strictly related to cell cycle progression.
The most reliable prognostic marker for patients with melanoma is considered to be the vertical thickness of the primary tumor. 38 Due to the low number of cases analyzed, we were, however, unable to determine whether the observed impact of p27Kip1 on disease-free survival for patients with nodular melanoma was independent of tumor thickness. Notably, p27Kip1 was not found to have any impact on overall survival for this group of patients. This could be due to the need for longer follow-up or to limitations in the power of the study. p27Kip1 has been suggested to have functions related to cell adhesion 39-41 and may also play a role in tumor invasion. 29 Based on these findings, reduced p27Kip1 levels may correlate with other events that favor metastasis formation by allowing cells to escape from the primary site.
In superficial melanomas, no association between p27Kip1 levels, tumor thickness, and relapse-free period could be detected. In contrast to nodular melanomas, the superficial spreading lesions are characterized by an initial radial growth phase that is generally considered to be significantly less aggressive than the vertical growth phase of superficial spreading melanoma. 38 It may be speculated, therefore, that one reason for the observed differences in growth pattern between these two pathological subtypes may be related to differences in their responsiveness to altered levels of p27Kip1.
We next evaluated the prognostic potential of combined analysis of p21WAF1/CIP1 and p27Kip1. In nodular melanomas, low p21WAF1/CIP1 levels in combination with less than 50% of the cells showing p27Kip1 immunoreactivity was a better indicator of disease failure (P = 0.01) than p27Kip1 alone (P = 0.15). In this regard, it is of note that Porter et al 27 found that a combined analysis of p27Kip1 and cyclin E had a greater prognostic value than either factor alone. However, the strongest predictor of disease-free survival for patients with nodular melanoma was the virtual complete loss of p27Kip1 protein (<5% tumor cells stained, P = 0.0002) as compared with those having higher levels of the cdk inhibitor. Altogether, our results suggest that p27Kip1 is a more important predictor of the aggressiveness of nodular melanomas than p21WAF1/CIP1.
We have shown previously that the protein level of p21WAF1/CIP1 increases with tumor thickness of superficial melanomas. 11 One reason for the observed differences in aggressiveness between superficial spreading and nodular melanomas could be that p21WAF1/CIP1 functions to delay cell cycle progression and thereby tumor growth in the former. However, when evaluating the combined effect of p21WAF1/CIP1 and p27Kip1 in superficial melanomas, we found that moderate to high levels of p21WAF1/CIP1 (>5% cells stained) in combination with a low level of p27Kip1 (<5% cells stained) was a stronger indicator of disease recurrence than either inhibitor alone. Thus, in accordance with reports suggesting that p21WAF1/CIP1 may act as an assembling factor, 42 it might be speculated that p21WAF1/CIP1 in early-stage superficial melanomas plays a role in activating cdk complexes, thereby facilitating cell cycle progression. Given the small sizes of the melanoma subsets studied, the prognostic potential of combined analysis of p21WAF1/CIP1 and p27Kip1 needs, however, to be verified in larger cohorts of both superficial or nodular melanoma subtypes.
In summary, our results suggest that virtually complete loss of p27Kip1 protein may have potential as an indicator of early relapse of disease in patients with nodular melanoma. Studies with larger sample sizes are needed to assess whether p27Kip1 has significant prognostic value independent of tumor thickness.
Acknowledgments
We gratefully thank Ellen Hellesylt and Mette Ingrud for technical assistance and Cassandra Cheng for secretarial help.
Footnotes
Address reprint requests to Dr. Ruth Holm, Department of Pathology, Institute for Cancer Research, The Norwegian Radium Hospital, Montebello, N0310 Oslo, Norway. E-mail: ruth.holm@labmed.uio.no.
Supported by stipends from The Norwegian Research Council (V. A. Flørenes) and Lillemor Grobstocks Legacy (V. A. Flørenes and G. M. Mælandsmo), by funding from the Norwegian Cancer Society (J. M. Nesland and R. Holm), and by grants from the National Institutes of Health (CA41223 to R. S. Kerbel) and the National Cancer Institute of Canada (to J. M. Slingerland). R. S. Kerbel is a Terry Fox Research Scientist of the National Cancer Institute of Canada. V. A. Flørenes was supported by a Postdoctoral Fellowship from the Medical Research Council of Canada.
References
- 1.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 2.Morgan DO: Principles of CDK regulation. Nature 1995, 374:131-134 [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, Roberts JM: Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 1995, 9:1149-1163 [DOI] [PubMed] [Google Scholar]
- 4.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 5.Di Leonardo A, Linke SP, Clarkin K, Wahl GM: DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 1994, 8:2540-2551 [DOI] [PubMed] [Google Scholar]
- 6.Zeng YX, El-Deiry WS: Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene 1996, 12:1557-1564 [PubMed] [Google Scholar]
- 7.Shao ZM, Dawson MI, Li XS, Rishi AK, Sheikh MS, Han QX, Ordonez JV, Shroot B, Fontana JA: p53 independent G0/G1 arrest and apoptosis induced by a novel retinoid in human breast cancer cells. Oncogene 1995, 11:493-504 [PubMed] [Google Scholar]
- 8.Jiang H, Lin J, Su ZZ, Collart FR, Huberman E, Fisher PB: Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21, WAF1/CIP1, expression in the absence of p53. Oncogene 1994, 9:3397-3406 [PubMed] [Google Scholar]
- 9.Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR: Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 1994, 211:90-98 [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Lin J, Su Z, Herlyn M, Kerbel RS, Weissman BE, Welch DR, Fisher PB: The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene 1995, 10:1855-1864 [PubMed] [Google Scholar]
- 11.Mælandsmo GM, Holm R, Fodstad Ø, Kerbel RS, Flørenes VA: Cyclin kinase inhibitor p21WAF1/CIP1 in malignant melanoma: reduced expression in metastatic lesions. Am J Pathol 1996, 149:1813-1822 [PMC free article] [PubMed] [Google Scholar]
- 12.Koff A, Masahiko O, Polyak K, Roberts JM, Massague J: Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-β. Science 1993, 260:536-539 [DOI] [PubMed] [Google Scholar]
- 13.Slingerland JM, Hengst L, Pan C-H, Alexander D, Stampfer MR, Reed SI: A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor β-arrested epithelial cells. Mol Cell Biol 1994, 14:3683-3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J: Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 15.Polyak K, Kato J-Y, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 16.Hengst L, Reed SI: Translational control of p27Kip1 accumulation during the cell cycle. Science 1996, 271:1861-1864 [DOI] [PubMed] [Google Scholar]
- 17.Wang QM, Jones JB, Studzinski GP: Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res 1996, 56:264-267 [PubMed] [Google Scholar]
- 18.Coats S, Flanagan WM, Nourse J, Roberts JM: Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272:877-880 [DOI] [PubMed] [Google Scholar]
- 19.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY: Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85:707-720 [DOI] [PubMed] [Google Scholar]
- 20.Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A: Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 1996, 85:721-732 [DOI] [PubMed] [Google Scholar]
- 21.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai L-H, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM: A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 1996, 85:733-744 [DOI] [PubMed] [Google Scholar]
- 22.Shiohara M, El-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R, Chen D-L, Vogelstein B, Koeffler HP: Absence of WAF1 mutations in a variety of human malignancies. Blood 1994, 84:3781-3784 [PubMed] [Google Scholar]
- 23.Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilczynski S, Lee YY, Bartram CR, Koeffler HP: Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 1995, 55:2266-2269 [PubMed] [Google Scholar]
- 24.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 25.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degration of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nature Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 26.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nature Med 1997, 3:227-230 [DOI] [PubMed] [Google Scholar]
- 27.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nature Med 1997, 3:222-225 [DOI] [PubMed] [Google Scholar]
- 28.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M: The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 1997, 57:1259-1263 [PubMed] [Google Scholar]
- 29.Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T: p27 expression and gastric carcinoma. Nature Med 1997, 3:593. [DOI] [PubMed] [Google Scholar]
- 30.Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 31.Lloyd RV, Jin L, Qian X, Kulig E: Aberrant p27kip1 expression in endocrine and other tumors. Am J Pathol 1997, 150:401-407 [PMC free article] [PubMed] [Google Scholar]
- 32.Flørenes VA, Bhattacharya N, Bani MR, Ben-David Y, Kerbel RS, Slingerland JM: TGF-β mediated G1 arrest in a human melanoma cell line lacking p15INK4B: evidence for cooperation between p21Cip1/WAF1 and p27Kip1. Oncogene 1996, 13:2447-2457 [PubMed] [Google Scholar]
- 33.Sandhu C, Garbe J, Bhattacharya N, Daksis J, Pan CH, Yaswen P, Koh J, Slingerland JM, Stampfer MR: Transforming growth factor beta stabilizes p15INK4B protein, increases p15INK4B-cdk4 complexes, and inhibits cyclin D1-cdk4 association in human mammary epithelial cells. Mol Cell Biol 1997, 17:2458-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon RYC, Toyoshima H, Hunter T: Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell 1995, 6:1197-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trotter MJ, Tang L, Tron VA: Overexpression of the cyclin-dependent kinase inhibitor p21(WAF1/CIP1) in human cutaneous malignant melanoma. J Cutaneous Pathol 1997, 24:265-271 [DOI] [PubMed] [Google Scholar]
- 36.Fredersdorf S, Burns J, Milne AM, Packham G, Fallis L, Gillett CE, Royds JA, Peston D, Hall PA, Hanby AM, Barnes DM, Shousha S, O’Hare MJ, Lu X: High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc Natl Acad Sci USA 1997, 94:6380-6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan R, Bradley G, Slingerland J: Reduced levels of the cell-cycle inhibitor p27Kip1 in epithelial dysplasia and carcinoma of the oral cavity. Am J Pathol 1998, 152:585-590 [PMC free article] [PubMed] [Google Scholar]
- 38.Koh HK: Cutaneous melanoma. N Engl J Med 1991, 325:171-182 [DOI] [PubMed] [Google Scholar]
- 39.St Croix B, Flørenes VA, Rak JW, Flanagan M, Bhattacharya N, Slingerland JM, Kerbel RS: Impact of the cyclin dependent kinase inhibitor p27Kip1 on adhesion-dependent resistance of tumor cells to anticancer agents. Nature Med 1996, 2:1204-1210 [DOI] [PubMed] [Google Scholar]
- 40.Fang F, Orend G, Watanabe N, Hunter T, Ruoslathi E: Dependence of cyclin E-Cdk2 kinase activity on cell anchorage. Science 1996, 271:499-502 [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Ohtsubo M, Mohmer RM, Roberts JM, Assoian RK: Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol 1996, 133:391-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E: New functional activities for the p21 family of CDK inhibitors. Genes Dev 1997, 11:847-862 [DOI] [PubMed] [Google Scholar]