Abstract
Carbonic anhydrase isoenzyme IX, MN/CA IX, is a recently discovered member of the carbonic anhydrase (CA) gene family with a suggested function in acid-base balance, intercellular communication, and cell proliferation. Increased expression of MN/CA IX has been observed with certain epithelial tumors. We investigated the expression of MN/CA IX in 69 colorectal neoplasms, consisting of 1 juvenile polyp, 8 hyperplastic polyps, 39 adenomatous lesions, 21 carcinomas, and 7 metastases. Tissue sections were immunostained with a monoclonal antibody specific to MN/CA IX. The proliferative activity of the tumor cells was evaluated by Ki-67 antigen immunoreactivity. The hyperplastic polyps showed a weak or moderate reaction for MN/CA IX only in the cryptal epithelium, as did the normal intestinal mucosa. The adenomas showed immunoreactivity mainly in the superficial part of the mucosa, whereas the distribution in the carcinomas and metastases was more diffuse. Comparative immunostaining of serial sections for Ki-67, a well established marker of cell proliferation, confirmed that MN/CA IX is expressed in areas with high proliferative capacity. Our results show abnormal MN/CA IX expression in colorectal neoplasms, suggesting its involvement in their pathogenesis. The co-occurrence of MN/CA IX and Ki-67 in the same tumor cells indicates its potential for use as a marker of increased proliferation in the colorectal mucosa.
The carbonic anhydrases (CAs) catalyze the interconversion of carbon dioxide and bicarbonate. They participate in a variety of physiological processes that involve pH regulation, CO2 and HCO3− transport, and water and electrolyte balance. 1-3 There is now reliable evidence for seven distinct CA isoenzymes with catalytic activity. They are expressed in many organs, including those of the gastrointestinal tract, in which they function as important components of regulatory pathways, maintaining physiological equilibrium. 4-7
The novel membrane-associated CA isoenzyme CA IX was initially described as a tumor-associated membrane antigen (MN). Recently, it was reported to contain an extracellular domain with the essential structural features and activity of CAs. 8,9 It was originally detected in the human cervical carcinoma cell line HeLa and in a number of human carcinomas but not in the corresponding normal tissues. The expression of MN/CA IX in vitro is positively regulated by the cell density and correlates with the tumorigenic phenotype of hybrids between HeLa and normal human fibroblasts. 10 Expression of MN/CA IX in NIH3T3 cells promotes cell proliferation. 8 An immunohistochemical study of cervical carcinomas and a polymerase chain reaction-based survey of renal cell carcinomas identified a close association of MN/CA IX expression with these neoplasias and suggested its potential utility as a tumor biomarker. 11-13
MN/CA IX has been detected in normal gastric, intestinal, and biliary mucosa. 7 Immunohistochemical analysis of the normal large intestine revealed moderate staining in the proximal colon, with the reaction becoming weaker distally. The staining was confined to the basolateral surfaces of the cryptal epithelial cells, the area of greatest proliferative capacity. As MN/CA IX is much more abundant in the proliferating cryptal epithelium than in the upper part of the mucosa, it may play a role in control of the proliferation and differentiation of intestinal epithelial cells.
Cell proliferation increases abnormally in premalignant and malignant lesions of the colorectal epithelium, and therefore it is considered as an indicator of colorectal tumor progression. 14,15 A nuclear protein, Ki-67, has been reported as a reliable marker of cell proliferation in the gastrointestinal mucosa. 16,17
In the present study, we performed a comparative analysis of MN/CA IX and Ki-67 expression in colorectal tumors. The results allowed us to propose a potential value of MN/CA IX as a marker for cell proliferation in the colorectal mucosa. The increase and abnormal localization of MN/CA IX expression observed in some colorectal tumors suggests that it may be functionally involved in their pathogenesis.
Materials and Methods
Specimens
We studied 69 colonic lesions from 60 patients, including 8 hyperplastic polyps, 1 juvenile polyp, 39 adenomas, and 21 adenocarcinomas. Seven metastases of colorectal adenocarcinomas were also analyzed, including six mesenteric lymph node metastases and one liver metastasis. The adenomatous lesions included 18 tubular, 11 tubulovillous, and 4 villous tumors. The grade of dysplasia was low in 13 lesions, moderate in 19, and high in 8. Some tumors showed varying dysplasia or adenomatous and invasive histology in the same lesion. There were three patients with familial adenomatous polyposis disease from whom two samples were analyzed, one from the cecum and the other from the rectum.
The group of 21 malignant colorectal tumors consisted of 6 well differentiated, 9 moderately differentiated, and 6 poorly differentiated adenocarcinomas. There were 6 adenocarcinomas with a mucinous component. There were 2 carcinomas of stage A in Dukes’ classification, 10 at stage B, 7 at stage C, and 2 at stage D. The primary lesions had been isolated from the right colon (n = 13), transverse colon (n = 19), descending colon (n = 8), sigmoid colon (n = 13), and rectum (n = 16).
Preparation of the Samples
The tissue samples were obtained together with routine histopathological specimens taken during colonscopies and surgical operations for colonic tumors carried out at Oulu University Hospital. The study was approved by the Research Ethics Committee of Oulu University Hospital. The samples were fixed with 4% formaldehyde in 0.1 mol/L phosphate-buffered saline and embedded in parafin. Sections of 5 μm were mounted on gelatin-coated microscope slides. Routine histological examination was carried out after hematoxylin and eosin (H&E) staining of the sections.
Antibodies and the Immunostaining Method
The monoclonal antibody M75 recognizing the amino-terminal domain of MN/CA IX protein has been described previously by Pastoreková et al. 18 As the secretory isoenzyme CA VI is not expressed in the gut 5 but has the greatest homology to MN/CA IX, 9 a previously described polyclonal anti-CA VI serum 19,20 was used for negative control staining. Additional controls were obtained by omitting the first antibody from immunostaining. Sections were immunostained according to the biotin-streptavidin complex method as described previously. 7 The intensity of staining for MN/CA IX was scored by two of the investigators (J. Saarnio and T.J. Karttunen) on a scale of 0 to 2 as follows: 0, absent or weak focal reaction; 1, weak reaction; 2, moderate to strong reaction. The distribution of immunoreactivity between the superficial and deep parts of the mucosa was recorded separately.
To assess proliferative activity, the serial sections were stained with primary antibody to Ki-67 (clone MIB-1, PharMingen, San Diego, CA) and detected by a biotin-streptavidin-peroxidase system, and the sections were counterstained with Harris’ hematoxylin. The number of MIB-1-positive cells was counted (by J. Saarnio and T.J. Karttunen) in both the superficial and the deep half of the mucosa. All positively stained nuclei, regardless of intensity, were regarded as positive. An approximation to the Ki-67 score (labeling index) was obtained for each sample as the percentage of tumor cells counted that had positively stained nuclei.
Sixteen samples containing areas of normal mucosa were used to calculate the control labeling index for Ki-67. The mean value of the score for the proliferative zone of the normal mucosa was 37% with a 95% confidence interval from 28% to 46%. To allow comparison with plasma-membrane-associated MN/CA IX, the immunoreactivities of the nuclear Ki-67 were adjusted to the same scale of 0 to 2, on which 0 represents less than 28% (weak staining), 1 represents 28% to 46% (moderate staining), and 2 represents more than 46% (strong staining).
Results
An outline of the MN/CA IX and Ki-67 immunoreactivities estimated in the colorectal lesions is presented in Tables 1 and 2 ▶ ▶ . The intensity of MN/CA IX immunostaining was compared with Ki-67 in the superficial and deep parts of the lesions. There was a significant correlation between MN/CA IX and Ki-67 immunoreactivity for superficial (C = 0.30, P < 0.01) and for cryptal (C = 0.31, P < 0.01) staining (Spearman).
Table 1.
Summary of the Immunohistochemical Staining for MN/CA IX in the Normal Colorectal Mucosa, Colorectal Tumors, and Metastases of Colorectal Carcinomas
| n | Mean MN/CA IX SF (median, range) | Mean MN/CA IX DE (median, range) | Mean MN/CA IX SF/DE | |
|---|---|---|---|---|
| Normal epithelium | 16 | 0 (0, 0–0) | 0.3 (0, 0–1) | 0 |
| Hyperplastic polyps | 8 | 0.1 (0, 0–1) | 1.1 (1, 0–2) | 0.1 |
| Adenomatous lesions | 39 | 1.1 (1, 0–2) | 0.5 (0, 0–2) | 2.3 |
| SD | 13 | 0.9 (1, 0–2) | 0.2 (0, 0–2) | 4.5 |
| MD | 19 | 1.3 (1, 0–2) | 0.6 (10, 0–2) | 2.2 |
| GD | 8 | 1.0 (1, 0–2) | 0.7 (1, 0–2) | 1.7 |
| Malignant lesions | 21 | 1.2 (2, 0–2) | 1.3 (2, 0–2) | 1.0 |
| Grade I | 6 | 1.7 (2, 1–2) | 1.5 (2, 1–2) | 1.0 |
| Grade II | 9 | 1.1 (2, 0–2) | 1.2 (2, 0–2) | 0.9 |
| Grade III | 6 | 0.8 (1, 0–2) | 0.8 (1, 0–2) | 1.0 |
| Metastases | 7 | 0.6 (0, 0–2) | 0.6 (0, 0–2) | 1.0 |
SF, superficial epithelial staining; DE, deep epithelial staining; SD, slight dysplasia; MD, moderate dysplasia; GD, grave dysplasia.
Table 2.
Summary of the Immunohistochemical staining for Ki-67 in the Normal Colorectal Mucosa, Colorectal Tumors, and Metastases of Colorectal Carcinomas
| n | Mean Ki-67 SF (median, range) | Mean Ki-67 DE (median, range) | Mean Ki-67 SF/DE | |
|---|---|---|---|---|
| Normal epithelium | 16 | 0 (0, 0–0) | 0.9 (1, 0–2) | 0 |
| Hyperplastic polyps | 8 | 0 (0, 0–0) | 2.0 (2, 2–2) | 0 |
| Adenomatous lesions | 39 | 1.7 (2, 1–2) | 0.5 (0, 0–2) | 3.4 |
| SD | 13 | 1.7 (2, 1–2) | 0.2 (0, 0–1) | 8.5 |
| MD | 19 | 1.6 (2, 1–2) | 0.5 (0, 0–2) | 3.2 |
| GD | 8 | 1.9 (2, 1–2) | 1.1 (1,5, 0–2) | 1.7 |
| Malignant lesions | 21 | 1.8 (2, 1–2) | 1.5 (2, 0–2) | 1.2 |
| Grade I | 6 | 2.0 (2, 2–2) | 1.7 (2, 1–2) | 1.2 |
| Grade II | 9 | 1.9 (1.5, 1–2) | 1.7 (1.5, 1–2) | 1.1 |
| Grade III | 6 | 1.4 (1, 0–2) | 1.2 (1, 0–2) | 1.2 |
| Metastases | 7 | 0.6 (1, 0–2) | 0.6 (1, 0–2) | 1.0 |
SF, superficial epithelial staining; DE, deep epithelial staining; SD, slight dysplasia; MD, moderate dysplasia; GD, grave dysplasia.
Normal Mucosa
Sixteen samples contained normal mucosa in which MN/CA IX staining was generally quite weak or absent. The staining was localized to the basolateral plasma membrane of the cryptal epithelial cells, and it was also these cells that showed high Ki-67 immunoreactivity. Occasional epithelial cells in the superficial mucosa expressed Ki-67 (mean, 3%), whereas no reaction was detected for MN/CA IX.
Nonneoplastic Polyps of the Large Intestine
The expression of MN/CA IX was studied in one juvenile polyp and eight hyperplastic polyps. The immunostaining of juvenile polyp for MN/CA IX was negative. Most hyperplastic polyps showed a weak or moderate reaction only in the deep parts of the cryptal epithelium (Figure 1) ▶ , and two of them did not show any immunoreactivity at all. There were no differences in staining intensity between the proximal and distal polyps. The cryptal cells also showed an intense immunoreaction for Ki-67.
Figure 1.
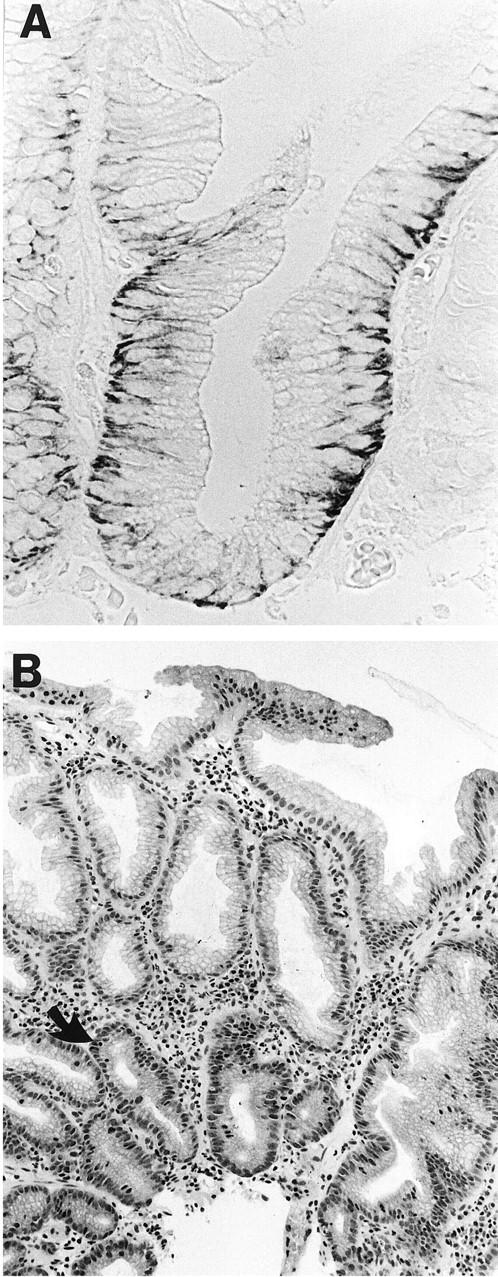
Immunohistochemical staining of a hyperplastic polyp for MN/CA IX (A) and Ki-67 (B). A positive reaction for MN/CA IX is seen in the basolateral surfaces of the cryptal epithelial cells. The nuclear staining for Ki-67 is located mainly in the cryptal epithelium (arrow). Original magnification ×330, (A) and ×100 (B).
Adenomatous Polyps
The 39 adenomatous lesions were obtained from 30 patients, including 3 with familial adenomatous polyposis disease. No staining for MN/CA IX was found in eight lesions (20%), which were located mainly in the distal part of the colon and rectum. Nineteen lesions (49%) showed weak staining and twelve (31%) a moderate to strong reaction, located mainly in the basolateral plasma membrane of the superficial epithelium (Figure 2) ▶ . The MN/CA-IX-positive lesions were evenly distributed along with the cranial-caudal axis of the large intestine. No differences in the immunoreactivity were observed between the adenomas obtained from patients with familial adenomatous polyposis and sporadic adenomas.
Figure 2.
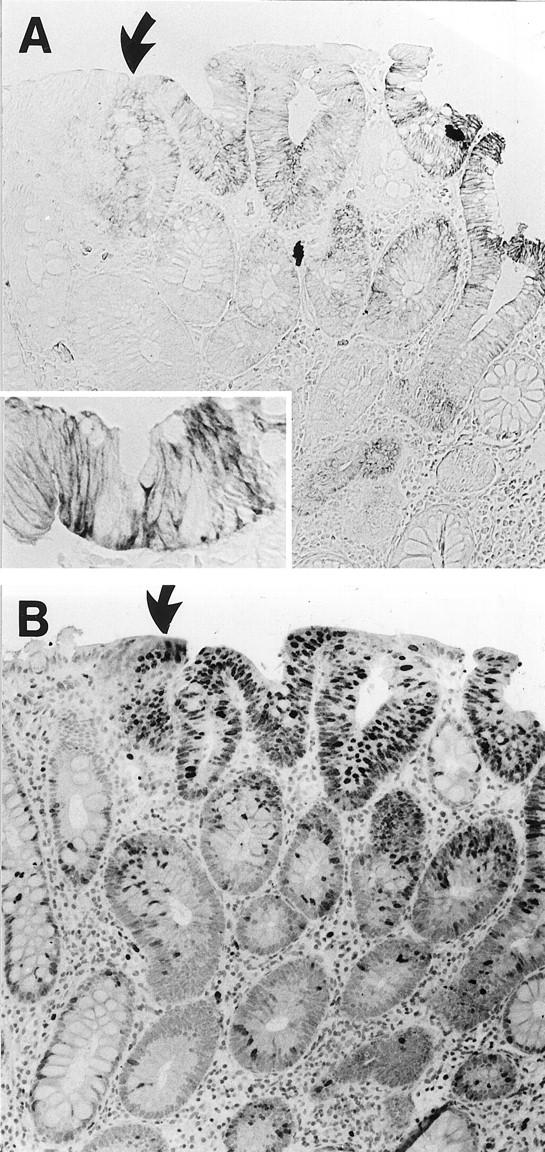
Immunohistochemical demonstration of MN/CA IX (A) and Ki-67 (B) in colonic adenoma (low dysplasia). Immunoreactions to both MN/CA IX and Ki-67 are mainly localized in the superficial epithelial cells. The (arrow) marks the border of the normal and adenomatous mucosa. Original magnification, ×100 and ×330 (inset).
There were 13, 19, and 8 lesions showing low, moderate, or severe dysplasia, respectively. In the group with low dysplasia, eight lesions stained for MN/CA IX in the superficial part of the mucosa but only two in the cryptal area as well. Furthermore, 17 adenomas with moderate dysplasia were stained superficially, whereas the cryptal epithelium was also positive in 9 lesions. Six of the eight adenomas with severe dysplasia gave a positive signal. The staining for MN/CA IX was more diffuse in these more dysplastic adenomas, all of which expressed it in the superficial epithelium and five in the cryptal area. Differences in the staining intensity, however, were not statistically significant (Mann-Whitney test).
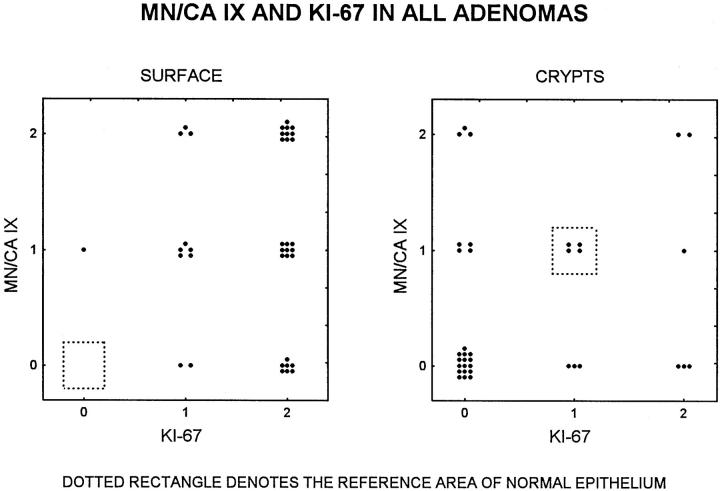
The correlations between Ki-67 and MN/CA IX expression in the superficial and deep parts of the lesions are summarized in Figure 5 ▶ . Both markers show an extension in staining from the crypts to the surface in adenomatous lesions relative to the normal epithelium.
Figure 5.
Correlation between MN/CA IX and Ki-67 expression in the superficial (left panel) and deep (right panel) parts of 37 adenomatous lesions. Dotted rectangles denote the reference area of the normal epithelium (see Materials and Methods).
Malignant Lesions
The material included 21 malignant colorectal tumours, 6 well differentiated adenocarcinomas, 9 moderately differentiated, and 6 poorly differentiated. The stage according to Dukes’ classification was A in 2 carcinomas, B in 10, C in 7, and D in 2.
No staining for MN/CA IX was observed in 5 carcinomas (24%), whereas 6 (29%) showed a weak positive reaction and 10 (47%) a moderate to strong reaction. The immunostaining was localized to the plasma membrane of the tumor cells. Interestingly, the most intense signals were seen in five of the six adenocarcinomas with a mucinous component (Figure 3) ▶ . Staining intensity did not correlate with the location of the carcinoma, but it showed a trend to correlate with the histological grade, being higher in the well differentiated tumors. The mean staining intensities were 1.7, 1.2, and 0.8 in grades 1, 2, and 3, respectively (grade 1/grade 3, P = 0.05, Mann-Whitney). Similarly, the mean staining intensity was higher (1.5) in less advanced tumors (Dukes A plus B, n = 12) than in more advanced ones (1.0; Dukes C plus D, n = 9; P < 0.047, Mann-Whitney test). The MN/CA IX staining was generally diffuse, being present in both the superficial and deep parts of the mucosa (Figure 4) ▶ . A diffuse staining pattern was also characteristic of Ki-67. Interestingly, desmoplastic connective tissue in the malignant lesions occasionally showed prominent immunostaining for MN/CA IX (not shown).
Figure 3.
Immunohistochemical staining shows that most cells in a mucinous adenocarcinoma are intensively positive for MN/CA IX (A) and Ki-67 (B). Original magnification ×400.
Figure 4.
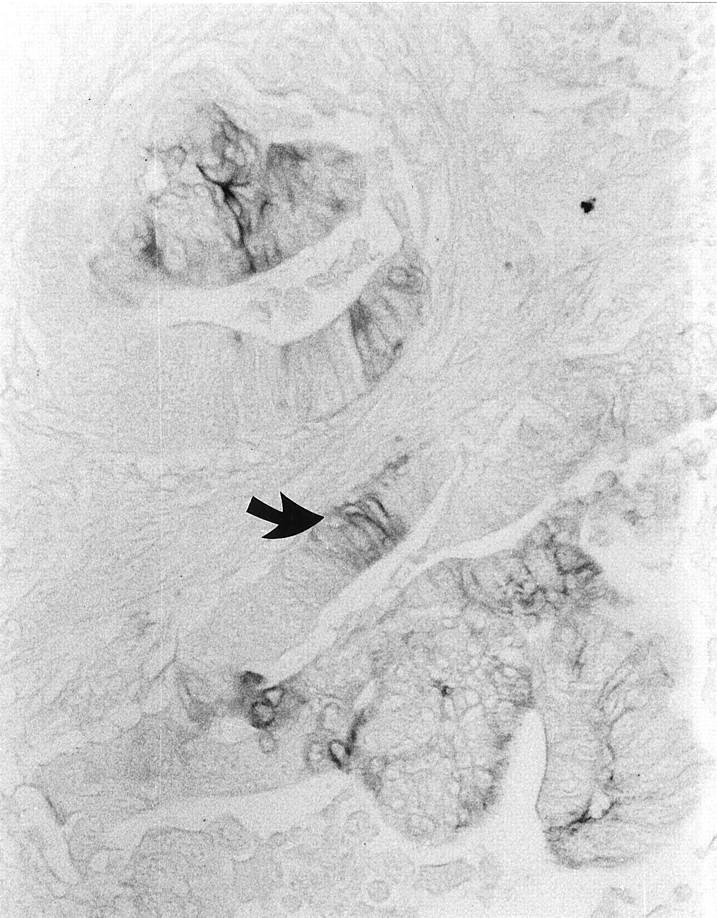
Immunohistochemical demonstration of MN/CA IX in an adenocarcinoma with moderate differentiation. Note a focal expression of MN/CA IX protein (arrow). Original magnification, ×200.
The data on simultaneous expression of Ki-67 and MN/CA IX in superficial and deep parts of the malignant lesions are summarized in Figure 6 ▶ . Both the superficial and deep parts of the mucosa seem to stain for both antigens more intensely in malignant tumors than in the normal mucosa, with only a few exceptions.
Figure 6.
Correlation of MN/CA IX and Ki-67 expression in the superficial (left panel) and deep (right panel) parts of 19 adenocarcinomas. Dotted rectangles denote the reference area of the normal epithelium (see Materials and Methods).
We studied six mesenteric lymph node metastases of colorectal carcinoma and one liver metastasis. Three of six lymph node metastases were positive in the same manner as their primary tumors. The liver metastasis and its primary carcinoma also showed an intense immunoreaction for MN/CA IX.
Discussion
An adenoma-carcinoma morphological model has been proposed that involves a sequence of histological changes from a normal colonic epithelium through benign adenomas that become increasingly dysplastic and finally develop into cancer. 21 Colorectal cancers typically develop over decades and appear to require several genetic events for the completion of the malignant phenotype. 22,23 One important consequence of these genetic aberrations is that cell proliferation is abnormally increased in premalignant and malignant lesions of the colorectal epithelium. 14
MN/CA IX is a transmembrane protein that is widely expressed in the gastrointestinal tract. 7 When its full-length cDNA was cloned, this was found to contain a central part with sequence homology to the CAs, on which basis it was thought to participate in the regulation of carbon dioxide homeostasis like the other CAs. 8,9 However, this protein has some unique features that suggest a role in intercellular communication or cell proliferation: it shows cell-density-dependent expression in HeLa cells, 10 it promotes cell proliferation when transfected into NIH3T3 cells, 8 and it is localized to the basolateral membranes of polarized epithelial cells. 7 Moreover, expression of MN/CA IX is closely associated with certain human carcinomas. 11-13 The purpose of our investigation was to study MN/CA IX expression in colorectal tumors and metastases and to correlate it with the biological behavior of the tumors.
Several important aspects of MN/CA IX expression emerged from this investigation: 1) hyperplastic polyps showed immunoreaction in the cryptal area, as reported earlier in normal mucosa, 7 2) MN/CA IX was more widespread and abundant in the surface epithelium of adenomas, 3) the staining pattern was more diffuse in carcinomas than in benign lesions, 4 of all the carcinoma samples, the most prominent reactions were observed in the tumors with a mucinous component, and 5) the desmoplastic connective tissue of some malignant lesions revealed an intense immunoreaction. Furthermore, the results confirmed that MN/CA IX is expressed on the basolateral membranes of enterocytes in hyperplastic polyps and adenomas, whereas it was the polarized expression of MN/CA IX on cell surfaces that was affected in carcinomas.
To demonstrate more precisely the association of MN/CA IX expression with proliferation, we used immunohistochemistry for Ki-67, which is one of the most reliable markers of cell proliferation. 17 Ki-67 and MN/CA IX were co-expressed in the same area in these lesions, suggesting that MN/CA IX expression is indeed related to cell proliferation.
In summary, the present results show that the majority of colorectal tumors display abnormal expression of MN/CA IX, suggesting its involvement in their pathogenesis. Furthermore, the co-occurrence of MN/CA IX and Ki-67 at the site of rapid cell proliferation indicates that MN/CA IX could be used as a biomarker of increased cell proliferation in the colorectal mucosa. High expression of MN/CA IX in premalignant lesions such as adenomas suggest that it might be useful in early diagnosis of colorectal tumors.
Footnotes
Address reprint requests to Dr. Juha Saarnio, Department of Surgery, University of Oulu, Kajaanintie 52 A, SF-90220 Oulu, Finland. E-mail: juha.saarnio@oulu.fi.
Supported by grants from the Sigrid Juselius Foundation to S. Parkkila, from the Maud Kuistila Foundation to A.-K. Parkkila, and from Chiron Diagnostics Corp., Alameda, CA, and the Slovak Scientific Grant Agency to S. Pastorekovà and J. Pastorek.
References
- 1.Maren TH: Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev 1967, 47:595-781 [DOI] [PubMed] [Google Scholar]
- 2.Sly WS, Hu PY: Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995, 64:375-401 [DOI] [PubMed] [Google Scholar]
- 3.Hewett-Emmett D, Tashian RE: Functional diversity, conservation and convergence in the evolution of the alpha-, beta- and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol 1996, 5:50-77 [DOI] [PubMed] [Google Scholar]
- 4.Lönnerholm G, Selking Ö, Wistrand PJ: Amount and distribution of carbonic anhydrases CA I and CA II in the gastrointestinal tract. Gastroenterology 1985, 88:1151–1161 [DOI] [PubMed]
- 5.Parkkila S, Parkkila A-K, Juvonen T, Rajaniemi H: Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut 1994, 35:646-650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkkila S, Parkkila A-K: Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol 1996, 31:305–317 [DOI] [PubMed]
- 7.Pastorekovà S, Parkkila S, Parkkila A-K, Opavský R, Zelnìk V, Saarnio J, Pastorek J: Immunohistochemical demonstration of MN/CA IX, carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1996, 112:398-408 [DOI] [PubMed] [Google Scholar]
- 8.Pastorek J, Pastorekovà S, Callebaut I, Mornon JP, Zelnìk V, Opavský R, Zatòvilovà M, Liao S, Portetelle D, Stanbridge EJ, Zàvada J, Burny A, Kettman R: Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 1994, 9:2877-2888 [PubMed] [Google Scholar]
- 9.Opavský R, Pastorekovà S, Zelník V, Gibadulinovà A, Stanbridge EJ, Zàvada J, Kettman R, Pastorek J: MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics 1996, 33:480-487 [DOI] [PubMed] [Google Scholar]
- 10.Zàvada J, Zàvadovà Z, Pastorekovà S, Chiampor F, Pastorek J, Zelník V: Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer 1993, 54:268-274 [DOI] [PubMed] [Google Scholar]
- 11.Liao SY, Brewer C, Zàvada J, Pastorek J, Pastorekovà S, Manetta A, Bermann ML, DiSaia PJ, Stanbridge EJ: Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinomas. Am J Pathol 1994, 145:598-609 [PMC free article] [PubMed] [Google Scholar]
- 12.Liao SY, Stanbridge EJ: Expression of the MN antigen in cervical Papanicolau smears is a diagnostic biomarker of cervical dysplasia. Cancer Epidemiol Biomarkers Prev 1996, 5:549-557 [PubMed] [Google Scholar]
- 13.McKeman JM, Buttyan R, Bander NH, Stifelman MD, Katz AE, Chen MW, Olsson CA, Sawczuk IS: Expression of the tumor-associated gene MN: a potential biomarker for human renal cell carcinoma. Cancer Res 1997, 57:2362-2365 [PubMed] [Google Scholar]
- 14.Risio M: Cell proliferation in colorectal tumor progression: an immunohistochemical approach to intermediate biomarkers. J Cell Biochem 1992, 16G:79-87 [DOI] [PubMed] [Google Scholar]
- 15.Moss SF, Liu TC, Petrotos A, Hsu TM, Gold LI, Holt PR: Inward growth of colonic adenomatous polyps. Gastroenterology 1996, 111:1425-1432 [DOI] [PubMed] [Google Scholar]
- 16.Lee CS: Differences in cell proliferation and prognostic significance of proliferating cell nuclear antigen and Ki-67 antigen immunoreactivity in in situ and invasive carcinomas of the extrahepatic biliary tract. Cancer 1996; 78:1881–1887 [DOI] [PubMed]
- 17.Holt PR, Moss SF, Kapetanakis AM, Petros A, Wang S: Is Ki-67 a better proliferative marker in the colon than proliferating cell nuclear antigen? Cancer Epidemiol Biomarkers Prev 1997, 6:131–135. [PubMed]
- 18.Pastoreková S, Závadová Z, Kostál M, Babusiková O, Závada J: A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992, 187:620-626 [DOI] [PubMed] [Google Scholar]
- 19.Parkkila S, Kaunisto K, Rajaniemi H: Location of the carbonic anhydrase isoenzymes VI and II in human salivary glands by immunohistochemistry. Botrè F Gros G Storey BT eds. Carbonic Anhydrase: From Biochemistry and Genetics to Physiology and Clinical Medicine. 1991, :pp 254-257 Verlagsgesellschaft, Weinheim, Germany, Weinheim [Google Scholar]
- 20.Parkkila A-K, Parkkila S, Juvonen T, Rajaniemi H: Carbonic anhydrase isoenzymes II and I are present in the zona glomerulosa cells of the human adrenal gland. Histochemistry 1993, 99:37-41 [DOI] [PubMed] [Google Scholar]
- 21.Faeron ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 [DOI] [PubMed] [Google Scholar]
- 22.Lengauer C, Kinzler KW, Vogelstein B: Genetic instability in colorectal cancers. Nature 1997, 386:623-627 [DOI] [PubMed] [Google Scholar]
- 23.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]