Abstract
The growth of solid tumors is dependent on angiogenesis, the formation of new blood vessels. Vascular endothelial growth factor (VEGF) is a secreted endothelial-cell-specific mitogen. We have recently characterized two novel endothelial growth factors with structural homology to VEGF and named them VEGF-B and VEGF-C. To further define the roles of VEGF-B and VEGF-C, we have studied their expression in a variety of human tumors, both malignant and benign. VEGF-B mRNA was detected in most of the tumor samples studied, and the mRNA and the protein product were localized to tumor cells. Endothelial cells of tumor vessels were also immunoreactive for VEGF-B, probably representing the binding sites of the VEGF-B polypeptide secreted by adjacent tumor cells. VEGF-C mRNA was detected in approximately one-half of the cancers analyzed. Via in situ hybridization, VEGF-C mRNA was also localized to tumor cells. All lymphomas studied contained low levels of VEGF-C mRNA, possibly reflecting the cell-specific pattern of expression of the VEGF-C gene in the corresponding normal cells. The expression of VEGF-C is associated with the development of lymphatic vessels, and VEGF-C could be an important factor regulating the mutual paracrine relationships between tumor cells and lymphatic endothelial cells. Furthermore, VEGF-C and VEGF-B can, similarly to VEGF, be involved in tumor angiogenesis.
Angiogenesis, the formation of new capillaries, is an important component of many biological processes, both physiological and pathological. In healthy adults, extensive angiogenesis occurs only during the female reproductive cycle. Angiogenesis also takes place in conditions such as wound healing, rheumatoid arthritis, and diabetic retinopathy and during tumor growth and metastasis formation (reviewed by Folkman1).
VEGF, also called vascular permeability factor, is a secreted, dimeric protein (Mr 46,000) that is active as an endothelial-cell-specific mitogen and as a vascular permeability factor (reviewed by Dvorak et al and Ferrara2,3). Five types of mRNA encoding VEGF species that differ in their molecular mass and in their biological properties are transcribed from a single gene as a result of alternative splicing (reviewed by Neufeld et al4). The shorter forms are efficiently secreted from cells, whereas the longest form remains mostly cell associated. VEGF has been shown to be hypoxia-inducible in vitro in a variety of cultured cells 5-7 and in vivo in a range of tissues. 8,9 Expression of VEGF has been shown to increase tumor growth and angiogenesis in vivo in a nude mouse model. 10-12 Also, anti-VEGF antibodies have the ability to inhibit the growth of several tumor cell lines in nude mice. 13-15
We have recently characterized two novel endothelial growth factors with homology to VEGF and named them VEGF-B and VEGF-C. 16,17 Depending on alternative splicing, VEGF-B is made as a 23-kd or a 34-kd polypeptide. The 23-kd polypeptide is unglycosylated, forms cell-surface-associated, disulfide-linked homodimers, and can heterodimerize with VEGF when the two are produced in the same cells. The 34-kd VEGF-B polypeptide is O-glycosylated and soluble, and forms similar dimers. VEGF-B is a mitogen for human endothelial cells. In human tissues, the most abundant expression of the 1.4-kb VEGF-B transcript was detected in the heart, skeletal muscle, pancreas, and prostate, and VEGF-B and VEGF were found to be co-expressed in many tissues. VEGF-C consists of proteolytically processed polypeptides of 29 and 31 kd. VEGF-C also forms dimers. 18 In Northern blotting and hybridization analysis, expression of VEGF-C mRNA was detected in human heart, placenta, muscle, and ovary and in the small intestine. VEGF-C stimulates endothelial cell migration in collagen gels, suggesting a role as a novel regulator of endothelia. Like VEGF, VEGF-C binds to VEGFR-2 (KDR), also indicating a function in endothelia. In addition, VEGF-C is a ligand for the VEGFR-3 (Flt-4), a receptor tyrosine kinase, which is mainly expressed in the endothelium of lymphatic vessels. 19 Recent experiments with transgenic mice show that the expression of VEGF-C is associated with the development of lymphatic vessels in normal tissues. 20
To define further the roles of VEGF-B and VEGF-C, we have now studied their expression in a variety of human tumors, both malignant and benign.
Materials and Methods
Tissue Specimens
Tumor tissue samples were obtained from patients at the Helsinki University Central Hospital. The samples were frozen in liquid nitrogen immediately after surgical excision and stored at −70°C. All specimens were inspected by a pathologist to confirm the diagnosis.
mRNA Isolation and Northern Analysis
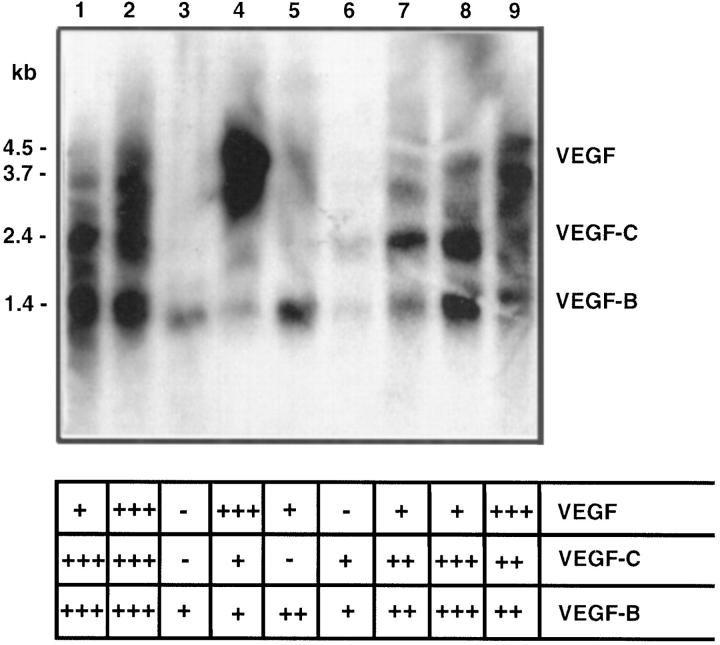
Poly(A)+ RNAs were isolated from 36 human tumors using affinity chromatography on oligo-dT cellulose. 21 Before the incubation with oligo-dT cellulose (Pharmacia, Uppsala, Sweden), the tumor tissue samples were homogenized with a Polytron PT3000 homogenizer (Kinematica, Littau, Switzerland) in 2% sodium dodecyl sulfate (SDS), 200 μg/ml proteinase K (Sigma Chemical Co., St. Louis, MO), 10 mmol/L Tris/HCL, 100 mmol/L NaCl, and 2 mmol/L EDTA at pH 7.4 and incubated for 24 hours at 56°C. The VEGF, VEGF-B, VEGF-C, and β-actin (Clontech, Palo Alto, CA) cDNAs were labeled using the random priming method and hybridized to Northern blots containing 8 μg of poly(A)+ RNA per lane. Poly(A)+ RNA extracted from human prostatic carcinoma cell line PC-3 was used as a positive control. The hybridization was carried out in 50% formamide, 5X Denhardt’s solution, 5X sodium chloride-sodium phosphate-EDTA buffer, 0.1% SDS, and 0.1 mg/ml sonicated salmon sperm DNA at 42°C for 24 hours. The filters were washed and exposed to Kodak XAR-5 film. The mRNA expression levels were elevated semiquantitatively based on visual evaluation. The criteria used are indicated in Figure 1 ▶ .
Figure 1.
Northern blot analysis of the expression of VEFG-B (1.4 kb), VEGF-C (2.4 kb), and VEGF (4.5 and 3.7 kb) mRNAs in human cancers. Hybridization was carried out with a pool of radiolabeled inserts for the three cDNAs, and the migration of the gene-specific mRNA bands are indicated. The criteria used for the semiquantitative evaluation of the mRNA expression levels are also shown. Lane 1, positive control PC-3 mRNA; lane 2, primary cutaneous melanoma; lane 3, melanoma metastasis; lane 4, ductal breast carcinoma; lane 5, ductal breast carcinoma; lane 6, squamous cell head and neck carcinoma, primary; lane 7, squamous cell head and neck carcinoma, metastasis; lane 8, sarcoma; lane 9, sarcoma. Note the differential regulation of the mRNAs, for example, the lack of VEGF mRNA in lanes 3 and 6.
In Situ Hybridization
Adjacent sections of a primary head and neck squamous cell carcinoma expressing both VEGF-B and VEGF-C mRNAs in Northern analyses were subjected to in situ hybridization analyses. In situ hybridization of the tissue sections was performed as described previously. 22 The human VEGF-C antisense RNA probe was generated from linearized pCRII plasmid (Invitrogen, San Diego, CA) containing an EcoRI fragment corresponding to nucleotides 287 to 696 of human VEGF-C cDNA. Radiolabeled RNA was synthesized using T7 polymerase and [35S]UTP (Amersham, Little Chalfont, UK). Human VEGF-B antisense and sense RNA probes were synthesized in a similar manner from linearized pCRII plasmid containing a fragment corresponding to nucleotides 1 to 382 of human VEGF-B cDNA. Radiolabeled antisense RNA was synthesized using the SP6 polymerase and sense strand using the T7 polymerase. The high-stringency wash was for 90 minutes at 65°C in a solution containing 50 mmol/L dithiothreitol and 2X SSC. The slides were exposed for 28 days, developed, and stained with hematoxylin. Ten grains per tumor cell was required as a minimal criterion for a positive reaction.
Immunohistochemistry
A glutathione-S-transferase (GST) fusion protein containing exon 6B-encoded sequences of mouse VEGF-B186 was purified and used to immunize rabbits. The antisera obtained after the fourth booster injection were affinity purified on a GST-VEGF-B186 column. In addition, a polyclonal antiserum raised against a branched 23-mer oligopeptide comprising the amino-terminal region of mouse VEGF-B was used. Five-micron frozen sections from six tumor samples expressing the highest amounts of VEGF-B mRNA in Northern analyses were subjected to the immunohistochemical analyses. The sections were rehydrated and incubated for 30 minutes in 0.8% hydrogen peroxidase in methanol at 4°C and for 30 minutes in 5% normal horse serum at room temperature. The sections were then incubated for 24 hours at 4°C with the affinity-purified Ig at a concentration of 35 μg/ml. A subsequent incubation for 30 minutes in biotinylated anti-rabbit/mouse serum was followed by a 30-minute incubation using reagents of the StrepABComplex/HRP Duet, mouse/rabbit kit (Dako, Glostrup, Denmark). Peroxidase activity was developed with 3-amino-9-ethyl carbazole (Sigma) for 10 minutes. Finally, the sections were stained with hematoxylin for 5 minutes. Negative controls were done by staining with the preimmune serum or with anti-VEGF-B antibodies incubated for 5 hours in eightfold molar exess of the recombinant GST-VEGF-B polypeptide and by omitting the primary antibody or by using irrelevant primary antibodies. After the staining procedures, all samples were examined by a pathologist.
Results
Expression of VEGF-B mRNA in Tumor Tissue
Expression of the 1.4-kb mRNA for VEGF-B was observed in 31 of 35 of the tumors analyzed (Figure 1 ▶ ; Table 1 ▶ ). Notably, VEGF-B was expressed in all of the benign and malignant tumors studied, with the exception of three of eight breast carcinomas and one primary adenocarcinoma, for which no VEGF-B mRNA could be shown in Northern hybridization analysis. The highest levels of VEGF-B mRNA were detected in primary and metastatic malignant melanomas. In a squamous cell head and neck carcinoma sample, VEGF-B mRNA was detected via in situ hybridization in carcinoma cells (Figure 2) ▶ . The sense probe did not give any specific signal.
Table 1.
Comparison of VEGF-B, VEGF-C, VEGF, and β-Actin mRNA Expression in Human Tumors
| Tumor | mRNA | |||
|---|---|---|---|---|
| VEGF-B | VEGF-C | VEGF | β-Actin | |
| Adenocarcinoma | − | − | − | ++ |
| Primary Metastasis | + | + | + | ++ |
| Breast carcinoma | ||||
| Ductal Grade 1 | − | − | − | ++ |
| Grade 2 | ++ | ++ | − | ++ |
| Grade 3 | + | + | +++> | +++ |
| Grade 3 | + | − | − | + |
| Grade 3 | − | − | − | ++ |
| Grade 3 | − | − | − | ++ |
| Grade 3 | ++ | − | + | ++ |
| Grade 3 | ++ | ++ | ++ | +++ |
| Breast carcinoma, lobular | ++ | + | NT | NT |
| Breast carcinoma, intraductal (large) | ++ | − | ++ | NT |
| Squamous cell carcinoma | ||||
| Primary | ++ | + | + | ++ |
| Primary | + | ++ | ++ | ++ |
| Primary | + | + | − | +++ |
| Primary | + | +++ | NT | + |
| Metastasis | + | − | − | ++ |
| Metastasis | ++ | ++ | + | +++ |
| Metastasis | + | − | NT | ++ |
| Lymphoma | ||||
| Non-Hodgkin | +++ | + | − | ++ |
| Non-Hodgkin | ++ | + | + | ++ |
| Non-Hodgkin | ++ | + | NT | + |
| Melanoma | ||||
| Primary | +++ | +++ | +++ | +++ |
| Metastasis | + | − | − | ++ |
| Metastasis | +++ | ++ | ++ | ++ |
| Metastasis | +++ | − | NT | ++ |
| Metastasis | ++ | − | + | + |
| Sarcoma | ||||
| Fibrosarcoma | +++ | +++ | + | ++ |
| Schwannoma | +++ | − | − | ++ |
| MFH | ++ | ++ | +++ | ++ |
| NUD | + | + | − | +++ |
| Head and neck tumor | ||||
| Benign adenoma (from salivary glands) | + | − | + | ++ |
| Benign papilloma | + | − | + | ++ |
| Benign papilloma | + | − | + | ++ |
| Benign thymoma | ++ | + | ++ | ++ |
NT, not tested; NUD, not determined, MFH, malignant fibrous histiocytoma.
Figure 2.
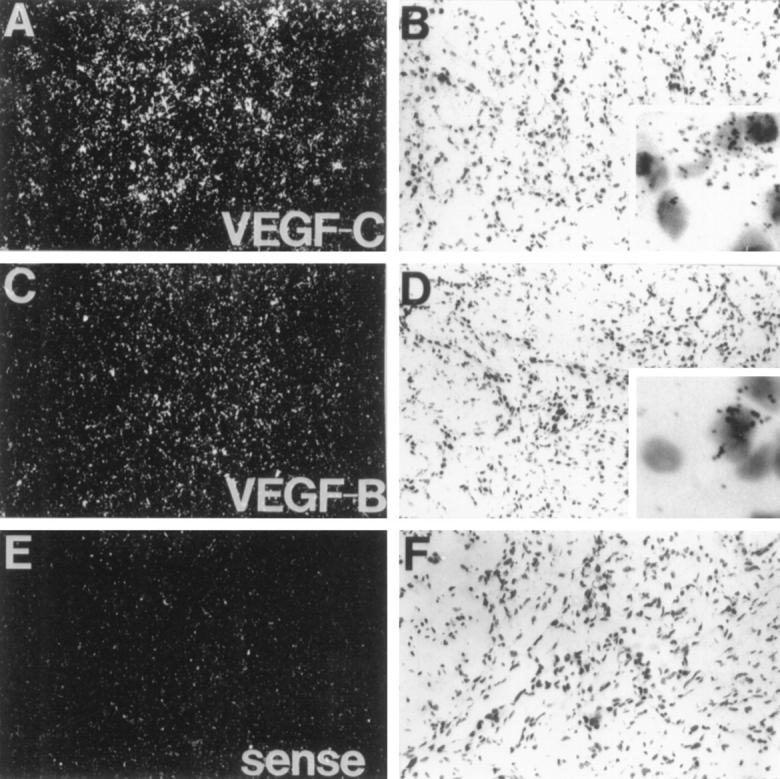
In situ hybridization analysis of VEGF-B and VEGF-C mRNAs in a head and neck carcinoma. Dark-field (A, C, and E) and light-field (B, D, and F) exposures are shown. Magnification, ×110. Insets: Higher magnification (×800). Note that the signals originate from the squamous cell carcinoma cells.
Immunohistochemical Detection of the VEGF-B Protein in Tumor Tissue
Strong immunoperoxidase staining for VEGF-B protein was observed in the cytoplasm of cancer cells in the six tumor samples studied expressing high levels of the VEGF-B mRNA in the Northern analysis (Figure 3) ▶ . Interestingly, the endothelial cells lining blood vessels in these samples were also positive for VEGF-B in immunostaining (arrows in Figure 3 ▶ ). The negative controls and staining with the preimmune serum did not give signal (Figure 3, C and D) ▶ .
Figure 3.
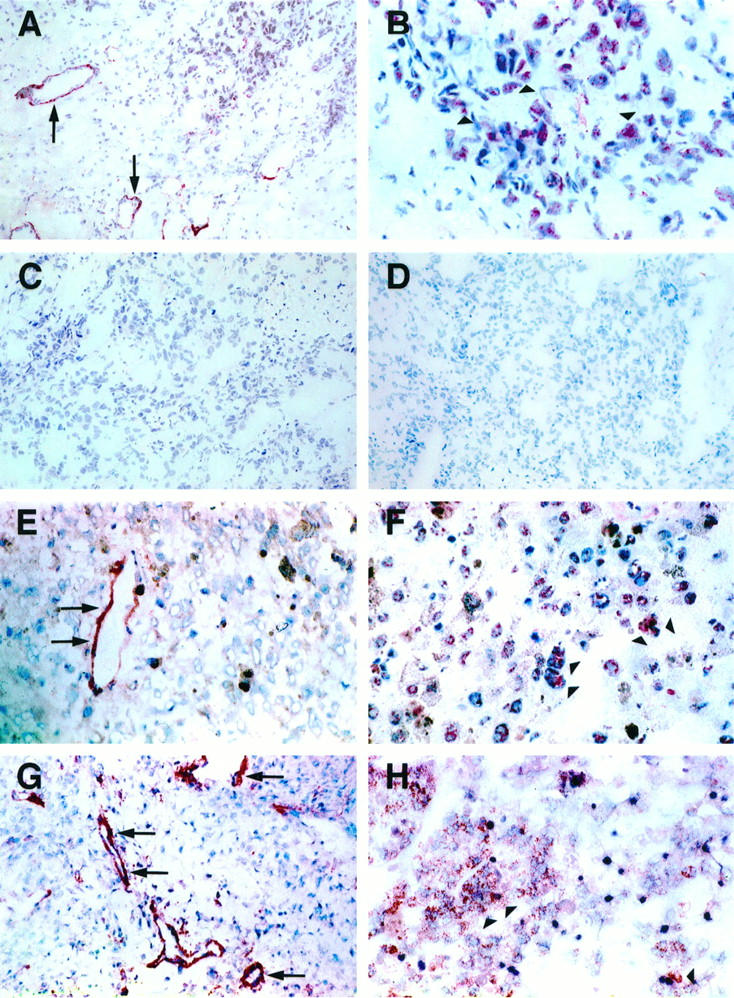
Immunohistochemical analysis of VEGF-B protein. Immunohistochemical staining of ductal breast carcinoma (A and B), malignant melanoma (E and F), and fibrosarcoma (G and H). VEGF-B protein is observed in the carcinoma cells (arrowheads) and in the endothelial cells of tumor microvessels (arrows). Negative controls include staining of the ductal breast carcinoma with preimmune serum (C) as well as with VEGF-B polypeptide-blocked antiserum (D). Magnification, ×110 (A, C, D, E, and G) and ×250 (B, F, and H).
Expression of VEGF-C mRNA in Tumor Tissue
Expression of VEGF-C mRNA was detected in 19 of the 35 tumor samples (Figure 1 ▶ and Table 1 ▶ ). All lymphomas examined contained the VEGF-C mRNA. Some of the squamous cell carcinomas, melanomas, sarcomas, and breast carcinomas examined also expressed VEGF-C mRNA. VEGF-C mRNA was also detected in one benign thymoma, whereas the other benign tumors were negative. In a squamous cell head and neck carcinoma sample, VEGF-C mRNA was detected via in situ hybridization in carcinoma cells (Figure 2) ▶ . The VEGF-C probe gave a more intense signal in comparison with VEGF-B via in situ hybridization of the same tumor sample. The sense probe did not give any specific signal.
Discussion
In this study, we show that VEGF-B is commonly present in both benign and malignant tumors. VEGF-B mRNA and its protein product were observed in the cytoplasm of the tumor cells. Moreover, endothelial cells of tumor vessels also stained for VEGF-B, probably as a result of VEGF-B diffusion from producer cells and concentration in endothelial cells containing VEGF-B receptors. Such findings have previously been published for VEGF immunoreactivity in a variety of tumor tissues. 23-26 The results suggest that VEGF-B could contribute to tumor angiogenesis, particularly as it is known to form heterodimers with VEGF, 16 which is also made by tumor cells.
The VEGF-B and VEGF-C mRNAs were distributed rather evenly in the tumor cells via in situ hybridization analysis. This contrasts with the published distribution patterns of the VEGF mRNA, with highly elevated amounts in hypoxic areas of the tumors, particularly surrounding areas of tumor necrosis (reviewed by Dvorak et al2). Although a major stimulus for VEGF expression is hypoxia, thus far there is no evidence that hypoxia would regulate either VEGF-B or VEGF-C mRNAs. In contrast, cytokines and growth factors induce VEGF-C mRNA expression in cultured cells, 27 and they could be significant factors contributing to the expression of VEGF-C in tumors.
Interestingly, all lymphomas studied in the present series contained low levels of VEGF-C mRNA. It may be that this reflects the cell-specific pattern of expression of the VEGF-C gene in the corresponding normal cells. The expression of a receptor for VEGF-C, VEGFR-3 (Flt-4), becomes restricted largely to lymphatic endothelium during development, and increased expression of VEGFR-3 has been observed in metastatic lymph nodes and in lymphangiomas. 19 Recent experiments with transgenic mice show that the expression of VEGF-C is associated with the development of lymphatic vessels in normal tissues. 20 VEGF-C could also be an important factor regulating the mutual paracrine relationships between tumor cells and endothelial cells in lymphatic vascular metastases and possibly also in primary tumors. An interesting question is whether lymphangiogenesis occurs in the tumors showing the highest levels of VEGF-C mRNA. Such tumors could also be predisposed to metastatic spread via the lymphatics.
The growth and dissemination of solid tumors is dependent on the formation of new blood vessels. During tumorigenesis, the quiescent vasculature can become activated to grow new capillaries in response to an appropriate stimulus (reviewed by Hanahan and Folkman 28 and Risau29). Several inducers of angiogenesis have been described, including basic fibroblast growth factor and its relative, acidic FGF, as well as VEGF. In this study, most of the cancers examined expressed mRNAs of several members of the VEGF growth factor gene family. However, some cancers did not express any of the known VEGFs. These include some ductal breast carcinomas and adenocarcinomas of the gastrointestinal tract. In these tumors, angiogenic stimuli may be provided by angiogenic growth factors other than VEGF, VEGF-B, or VEGF-C. It is tempting to speculate that there may exist other, still unknown VEGF-related growth factors that may be up-regulated in these cases.
Note added in proof
While this manuscript was being reviewed, a novel VEGF-related molecule was characterized and named VEGF-D (Yamada Y, et al. Genomics 1997, 42:483–488; Achen MG, et al. Proc Natl Acad Sci USA 1998, 95:548–553).
Acknowledgments
We thank Elina Roimaa, Kati Konola, and Tapio Tainola for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Petri Salven, Department of Oncology, Helsinki University Central Hospital, P.O. Box 180, 00029 HYKS, Finland. E-mail: petri.salven@helsinki.fi.
Supported by grants from the Academy of Finland, the Finnish Cancer Foundation, and Helsinki University Central Hospital Research Funds.
References
- 1.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995, 146:1029-1039 [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara N: Vascular endothelial growth factor. Eur J Cancer 1996, 32A:2413-2422 [DOI] [PubMed] [Google Scholar]
- 4.Neufeld G, Tessler S, Gitay-Goren H, Cohen T, Levi BZ: Vascular endothelial growth factor and its receptors. Prog Growth Factor Res 1994, 5:89-97 [DOI] [PubMed] [Google Scholar]
- 5.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843-845 [DOI] [PubMed] [Google Scholar]
- 6.Shweiki D, Neeman M, Itin A, Keshet E: Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA 1995, 92:768-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, Varticovski L, Isner JM: Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem 1995, 270:31189-31195 [DOI] [PubMed] [Google Scholar]
- 8.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E: Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc Res 1994, 28:1176-1179 [DOI] [PubMed] [Google Scholar]
- 9.Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E: Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest 1995, 72:638-645 [PubMed] [Google Scholar]
- 10.Ferrara N, Winer J, Burton T, Rowland A, Siegel M, Phillips HS, Terrell T, Keller GA, Levinson AD: Expression of vascular endothelial growth factor does not promote transformation but confers a growth advantage in vivo to Chinese hamster ovary cells. J Clin Invest 1993, 91:160-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang HT, Craft P, Scott PA, Ziche M, Weich HA, Harris AL, Bicknell R: Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst 1995, 87:213-219 [DOI] [PubMed] [Google Scholar]
- 12.Claffey KP, Brown LF, Aguila L, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF: Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res 1996, 56:172-181 [PubMed] [Google Scholar]
- 13.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N: Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362:841-844 [DOI] [PubMed] [Google Scholar]
- 14.Asano M, Yukita A, Matsumoto T, Kondo S, Suzuki H: Inhibition of tumor growth and metastasis by an immunoneutralizing monoclonal antibody to human vascular endothelial growth factor/vascular permeability factor121. Cancer Res 1995, 55:5296-5301 [PubMed] [Google Scholar]
- 15.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N: Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995, 95:1789-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson RF, Alitalo K, Eriksson U: Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA 1996, 93:2576-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 18.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K: Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 1997, 16:3898-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, ed 2. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 1989
- 22.Västrik I, Kaipainen A, Penttilä TL, Lymboussaki A, Alitalo R, Parvinen M, Alitalo K: Expression of the Mad gene during cell differentiation in vivo and its inhibition of cell growth in vitro. J Cell Biol 1995, 128:1197-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van de Water L, Senger DR: Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med 1991, 174:1275-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, Senger DR: Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol 1993, 143:1255-1262 [PMC free article] [PubMed] [Google Scholar]
- 25.Salven P, Heikkilä P, Joensuu H: Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer 1997, 76:930-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salven P, Heikkilä P, Anttonen A, Kajanti M, Joensuu H: Vascular endothelial growth factor in squamous cell head and neck carcinoma: expression and prognostic significance. Mod Pathol 1997, 10:1128-1133 [PubMed] [Google Scholar]
- 27.Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefström J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K: Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14:2475-2483 [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 29.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]