Abstract
Although the functional role of TRPM-2/clusterin in the prostate remains controversial, it has been postulated that transcriptional activation of the gene is an important mechanism in castration-induced prostatic involution and perhaps is a means for prostatic cells to escape apoptotic induction. In the present study, we have measured expression levels of TRPM-2/clusterin and apoptotic activities in the prostates of castrated Noble (NBL) rats and those treated with testosterone (T) and estradiol-17β (E2) for 16 weeks. We have previously shown that the combined sex hormone treatment (T+E2) induces dysplasia, a purported preneoplastic lesion, exclusively in the dorsolateral prostates (DLPs) of all treated rats. In the present study, we demonstrate that, as expected, castration readily induced enhanced TRPM-2/clusterin expression, which was accompanied by increased apoptotic activity in the epithelia of DLP and ventral prostate (VP). The increase in TRPM-2/clusterin expression appeared earlier and was more dramatic in the VP than in the DLP. In sharp contrast, treatment of rats with T+E2 for 16 weeks induced augmentation of TRPM-2/clusterin expression selectively in the dysplastic lesions of the DLP but not in the lesion-free VP. The enhanced expression of TRPM-2/clusterin in the dysplastic epithelium was, however, not attended by an increase in apoptotic activity within the lesion. Thus, the observed up-regulation of TRPM-2/clusterin expression in the dysplastic foci of T+E2-treated rats occurred in animals whose androgen status remained normal and, despite the increased level of expression of this gene, apoptotic activity in these lesions was unchanged from basal values measured in the DLPs of untreated rats. These findings suggest that TRPM-2/clusterin expression in dysplastic lesions was no longer repressed by androgen nor was it associated with apoptosis. We propose that overexpression of the gene is likely a phenotype of neoplastic transformation. In addition, we speculate that TRPM-2/clusterin may serve as a survival factor, which could favor accumulation of transformed cells in dysplastic foci and thus promote the carcinogenic process.
Testosterone-repressed prostatic message-2 gene (TRPM-2) was first identified and cloned from the regressing rat prostate after castration. 1 The gene encodes a sulfated glycoprotein that is closely related to sulfated glycoprotein-2 (SGP-2) secreted by rat Sertoli cells, 2,3 clusterin in ram rete testicular fluid, 4 glycoprotein III in bovine adrenal chromaffin granules, 5 and apolipoprotein J, SP-40,40, and complement lysis inhibitor in human serum. 6,7 The TRPM-2/clusterin gene product and its related proteins share similar primary amino acid sequences but undergo significantly different post-translational modifications. 8,9 Apart from having a broad tissue distribution, these related proteins have been implicated in a wide variety of cellular processes and functions (for reviews see Refs. 8 and 10 ). Perhaps the most common postulated role for TRPM-2/clusterin is its involvement in the regulation of apoptosis. In this regard, expression of TRPM-2/clusterin is induced or greatly enhanced in a number of tissues undergoing apoptosis. 11-19
In the prostate, TRPM-2/clusterin was first considered to be an androgen-repressed gene with its protein product playing a role in regression of the gland after castration. 1,11-13 A number of past studies have demonstrated that a close association exists between castration-induced apoptosis and the induction of TRPM-2/clusterin expression in the epithelium of the rat ventral prostate (VP). 1,11-13 However, in several recent investigations, enhanced TRPM-2/clusterin expression was found to be dissociated from apoptosis and/or from androgen regulation. For example, prostatic involution, induced by treating rats with anti-androgens, 5α-reductase inhibitors, or luteinizing hormone releasing hormone (LHRH) agonists, was not attended by the induction of TRPM-2/clusterin expression in the rat VP. 14,20 Similarly, in several prostatic neoplasms and cancer cell lines 21-23 as well as in the VPs of aging rats, 24 TRPM-2/clusterin up-regulation occurred in the absence of increased apoptotic activity or diminished androgen stimulation. It has therefore been suggested that TRPM-2/clusterin expression, under these physiological or pathological conditions, reflects the escape of prostatic cells from androgen regulation or apoptotic control.
We have reported that treatment of Noble (NBL) rats with testosterone (T) and estradiol-17β (E2) for 16 weeks induces dysplasia, a purported preneoplastic lesion, exclusively in the dorsolateral prostates (DLPs) of all treated rats. 25,26 The sex-hormone-induced lesion is morphologically similar to a premalignant lesion, prostatic intraepithelial neoplasia (PIN), in the human gland. 27,28 Longer-term treatment with T+E2 has been reported to induce adenocarcinomas in the DLPs of a majority of treated animals. 29,30 In the present study, we now report a marked elevation of TRPM-2/clusterin expression, localized in dysplastic foci but not observed in adjacent normal epithelia of the DLP, nor was it found in the lesion-free VP. Moreover, high levels of TRPM-2/clusterin expression were not attended by increased apoptotic activity in these DLP lesions.
Materials and Methods
Animals and Steroid Treatment
Male NBL rats were purchased from Charles River Laboratories (Wilmington, MA) at 5 to 6 weeks old. Animals were housed at the departmental animal facility until they reached a size of 280 to 300 g or 11 to 12 weeks of age before they were used in these studies. All surgery was done under light isoflurane (Ohmeda Caribe, Guayama, PR) anesthesia. Animals were separated into four groups (n = 6 animals per group). The first group was surgically implanted with two 2-cm Silastic capsules (catalog number 602–205; 1.0-mm inner diameter × 2.2-mm outer diameter, Dow Corning Corp., Midland, MI) filled with T (Sigma Chemical Co., St. Louis, MO) and one 1-cm capsule filled with E2 (Sigma) as previously described. 26,27 We previously showed that this treatment maintained normal levels of circulating T but caused a moderate elevation in plasma E2. 25 These rats were killed by an overdose of isoflurane (Ohmeda Caribe) followed by decapitation 16 weeks after implantation. The second group consisted of untreated age-matched rats that served as controls. The last two groups were orchiectomized via the scrotal route. 25 One group of castrates was killed on day 3, and the others were killed on day 7 after castration. Three animals from each group were used for tissue total RNA preparation, and the remaining three were used for histology. The VPs and DLPs were excised from individual animals and used to generated total RNA and multiple serial histology sections. All animal treatment protocols were previously approved by Tufts Animal Care and Usage Committee and are in accordance with National Institutes of Health animal usage guidelines.
Buffers and Chemicals
All chemicals used were of reagent grade and were purchased from Sigma or from Fisher (Pittsburgh, PA) unless otherwise indicated. Restriction enzymes were from New England Biolabs (Beverly, MA). The 10X borate buffer (pH 8.0) contained 0.5 mol/L boric acid, 50 mmol/L sodium borate, 100 mmol/L sodium sulfate, 10 mmol/L EDTA. The 1 mol/L phosphate buffer (pH 7.0) was 1 mol/L dibasic sodium phosphate adjusted to pH 7.0 using phosphoric acid.
Northern Hybridization with a TRPM-2/Clusterin cDNA Probe
Total RNA was prepared by a modified single-step method 31 using RNAzol B (Tel-Test, Friendswood, TX). Ten micrograms of total RNA from each sample was size fractionated through a 0.8% agarose (electrophoresis grade, Gibco-BRL, Gaithersburg, MD)/1 mol/L formaldehyde gel in 1X borate buffer, transferred to a Nytran membrane (Schleicher & Schuell, Keene, NH) by a downward alkaline capillary transfer method, 32 and ultraviolet cross-linked onto the membrane using a UV Stratalinker 1800 (Stratagene, La Jolla, CA). A cDNA probe, pG21–04, containing a 300-bp insert corresponding to the 3′ end of rat TRPM-2/clusterin sequence, 1,12 was kindly provided by Dr. Martin Tenniswood at the University of Ottawa, Canada. The cDNA (20 to 40 ng) was routinely radiolabeled to a specific activity of approximately 1 × 10 9 cpm/μg of cDNA.
Northern blots were prehybridized for 2 to 3 hours in 0.5 mol/L phosphate buffer, 7% sodium dodecyl sulfate SDS, 1 mmol/L EDTA, 100 μg/ml low molecular weight salmon testis DNA (pH 7.0) at 60°C to 62°C. The blots were then hybridized with 10 6 cpm/ml 32P-labeled cDNA probes for approximately 16 hours at 62°C in a buffer similar to the prehybridization buffer with the exclusion of the low molecular weight salmon testis DNA. After hybridization, the blots were washed in 40 mmol/L phosphate buffer, 1% SDS, and 1 mmol/L EDTA, twice at room temperature for 15 minutes each and then once at the hybridization temperature for 15 minutes, and rinsed once in 40 mmol/L phosphate buffer without SDS. The washed blots were wrapped in plastic and exposed with Fuji x-ray film at −70°C with intensifying screens for autoradiography. To verify equal loading of RNA in each lane, blots were stripped in boiling diethylpyrocarbonate-treated H2O for 15 minutes and reused for hybridization with an end-labeled 30-mer oligonucleotide (5′-d(CGGCATGTATTAGC-TCTAGAATTACCACAG)-3′) 33 complementary to part of the 18 S rRNA at 45°C.
Quantitation of Hybridization Signals
Hybridization signals were quantitated by densitometric scanning and normalized with respect to the corresponding 18 S rRNA signal to correct for loading variations. Normalized signal intensities of samples obtained from the DLPs of untreated rats were designated as controls and arbitrarily assigned a value of 1. Signal intensities of samples obtained from the VPs of control and treated animals and DLPs of T+E2-treated rats were compared with a contiguous DLP control sample within the same Northern blot and expressed as folds of the control value (set as 1) to obtain relative mRNA levels. The prominent 4.5-kb TRPM-2/clusterin transcript signal was used for quantitation of this message.
Data points in Figures 1 and 2 ▶ (histograms) are group mean values derived from three separate experiments. In each experiment, total RNA was prepared from individual animals and used to obtain at least two Northern blots. After hybridization, signal intensities were quantitated for each blot, and values from the two blots were averaged to give the mean signal intensity for each sample.
Figure 1.
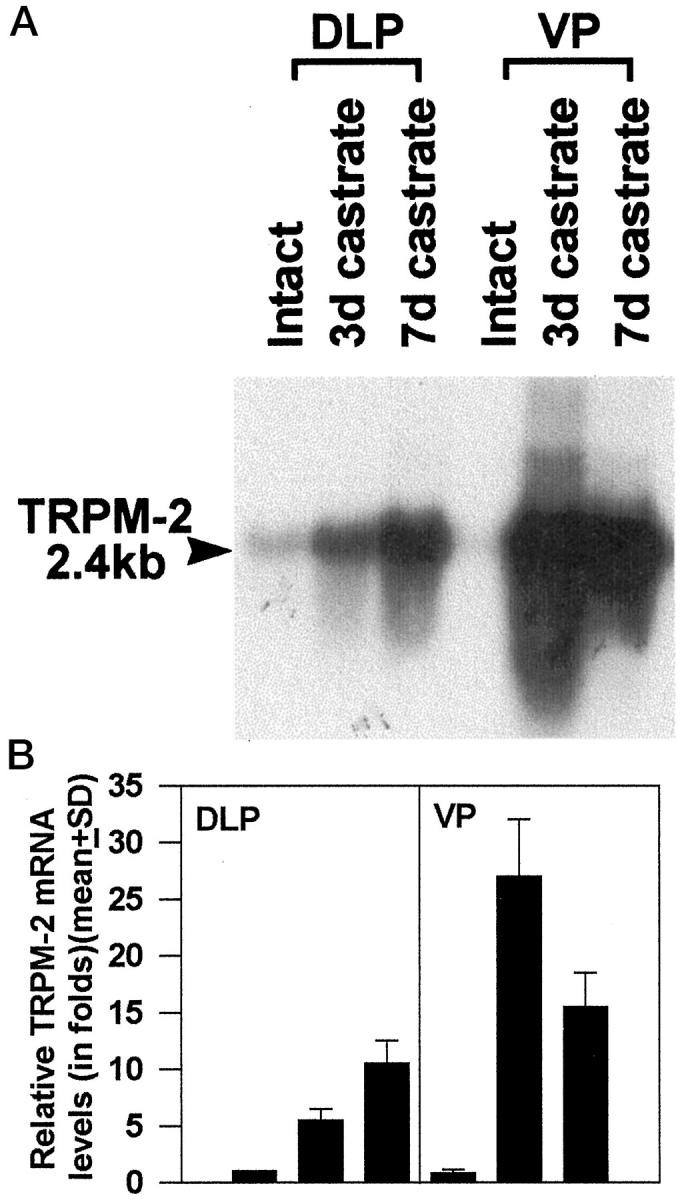
Effects of castration on TRPM-2/clusterin mRNA expression in the DLP and VP of NBL rats. Mature male rats were orchiectomized and sacrificed 3 and 7 days after the procedure. Total RNA was isolated from DLPs and VPs of individual animals (n = 3 animals per group). Aliquots (10 μg) of RNA were analyzed by Northern blot analyses using a 32P-labeled TRPM/clusterin probe (pG21–04). 1,12 Blots were washed and rehybridized with a 30-mer oligonucleotide complementary to part of the 18 S rRNA. After each hybridization, x-ray film was exposed by the washed blots at −70°C with intensifying screens for autoradiography. Hybridization signals of the TRPM-2/clusterin mRNA (size, 2.4 kb) and the 18 S rRNA were quantified by densitometric scanning. TRPM-2/clusterin hybridization signals were first normalized with respect to the corresponding 18 S rRNA signal to correct for loading variations. The normalized signal intensities of samples were compared with the contiguous DLP sample prepared from intact rats to arrive at a relative message level. The relative TRPM-2/clusterin mRNA levels in DLPs of intact rats were arbitrarily assigned a value of 1. A: A representative autoradiograph. B: Histograms are relative message levels expressed as group means ± SE derived from three separate experiments.
In Situ Hybridization
A modified procedure adapted from Höfler et al 34 and Komminoth et al 35 was used on 6- to 10-μm-thick frozen sections. To detect the TRPM-2/clusterin, a 35S-labeled riboprobe was prepared from linearized pG21–04 using a Riboprobe R system kit from Promega (Madison, WI) following the manufacturer’s protocol. The riboprobe was diluted in hybridization buffer to deliver 6 × 10 5 cpm/20 μl/slide. As negative controls, an unrelated riboprobe, derived from a 550-bp cDNA encoding the human preproparathyroid hormone (prepro-PTH) sequence, 36 was similarly applied to consecutive sections. Photomicrographs were taken on a Zeiss universal microscope. The dark-field images were produced with the aid of a dark-field fiberoptics illuminator (Microvideo Instrument, Avon, MA).
Immunohistochemistry
For immunohistological detection of the translation product of the TRPM-2/clusterin gene, a polyclonal antibody against rat sulfated glycoprotein-2 was used as the primary anitbody. The antibody was a generous gift from Dr. C. Y. Cheng of the Population Council, Center for Biomedical Research, New York, NY. It had been characterized 37 and previously used to localize TRPM-2/clusterin translational product in rat prostatic tissues. 38
Prostatic tissues were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin. Sections were cut at 6 μm and deparaffinized through a graded series of xylenes and ethanol and then rehydrated in water and phosphate-buffered saline (PBS). After deparaffinization, sections were treated for 30 minutes with normal rabbit serum. The blocking serum was drained off, and the sections were rinsed twice in PBS. The primary antibody, at a dilution of 1:1000, was applied to the sections. Incubation was carried out overnight at 4°C in humid chambers. After rinsing off the primary antibody with PBS, the sections were incubated with a biotinylated goat anti-rabbit antiserum (ABC Kit, Vector Laboratories, Burlingame, CA) at a 1:200 dilution for 30 minutes at room temperature. After a rinse in PBS, the sections were covered with peroxidate-conjugated streptavidin for 30 minutes at room temperature, washed with PBS, and incubated with diaminobenzidine/H2O2 substrate until the desired density of the reaction product was achieved (2 to 10 minutes). Sections were then lightly counterstained with 5% hematoxylin. Negative controls were processed in parallel with normal rabbit IgG substituted for the primary antibody. Estimates of the intensity of immunostaining was evaluated by F. Merk and K. Mallery in a double-blinded manner. Intensity was scored as 1+ to 4+ with the higher number indicating the strongest positivity.
In Situ Localization of Apoptotic Cells
Apoptotic cells were detected in either paraffin or frozen sections (5 to 6 μm thick) using the ApopTag in situ nuclear DNA fragmentation detection kit (Oncor, Gaithersburg, MD), following instructions described in the company’s literature. This assay uses terminal deoxynucleotide transferase to catalytically link digoxigenin-labeled nucleotides to 3′-OH ends of DNA, fragmented during apoptosis. Digoxigenin-labeled nuclei were recognized by immunoperoxidase staining. Four replicate sections from each prostatic specimen were stained with the ApopTag protocol. Using a 25× objective, 500 cells were counted for each specimen. The number of positively stained cells were then divided by 500 to estimate the percentage of apoptotic cells in each specimen. Apoptotic indices (AIs) were calculated as group means ± SD derived from values obtained from sections of individual animals of the group (data were derived from individual animals; n = 3 animals per group).
Results
Effects of Castration on Prostatic TRPM-2/Clusterin mRNA Levels
TRPM-2/clusterin message levels in untreated intact VP were approximately 0.8 ± 0.3-fold (80%) of that found in the DLP (Figure 1) ▶ . Three days after castration a 5.5 ± 1.0-fold increase in TRPM-2/clusterin expression was observed in the DLP whereas a 27 ± 5-fold elevation occurred in the VP. At 7 days after castration, the level of TRPM-2/clusterin mRNA in the DLP continued to rise to 10.5 ± 2.0-fold whereas values in the VP declined to approximately 15.5 ± 3.0-fold. Thus, castration induced a slower increase of TRPM-2/clusterin mRNA in the DLP than in the VP. The magnitude of increase in the DLP was also of a lower magnitude than was the level of expression in the VP. Values reported were group means ± SD with three animals in a group.
Effects of Long-Term T+E2 Treatment on Prostatic TRPM-2/Clusterin mRNA Levels
After 16 weeks of T+E2 treatment, a 21 ± 4-fold increase in TRPM-2/clusterin mRNA levels was observed in the DLPs harboring dysplasia whereas no increase in expression was found in the lesion-free VPs of these animals (Figure 2) ▶ . Studies with shorter-term treatments (1, 4, or 8 weeks) revealed no increase in TRPM-2/clusterin transcript expression in the DLP before 16 weeks (data not shown). Values reported were group means ± SD with three animals in a group.
Figure 2.
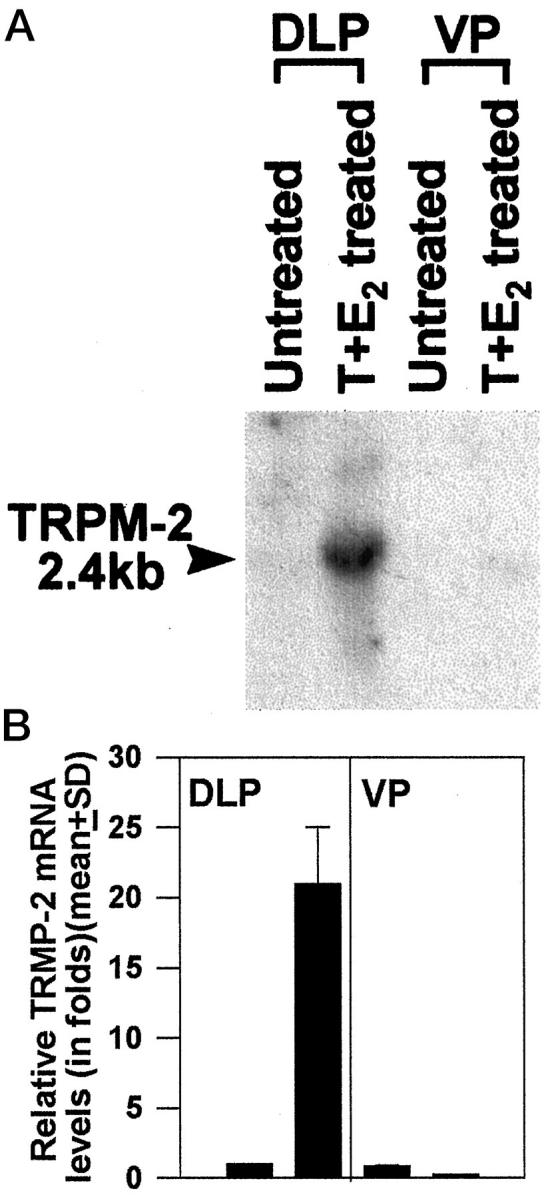
Effects of 16-week T+E2 treatment on prostatic TRPM-2/clusterin mRNA expression. Northern blot analysis was used to estimate relative message levels in the DLPs and VPs of untreated and T+E2-treated NBL rats. Experimental protocol and message quantitation methods were similar to those described for Figure 1 ▶ . A: A representative autoradiograph. B: Histograms are relative message levels expressed as group means ± SE derived from three separate experiments.
In Situ Detection of Prostatic TRPM-2/Clusterin Transcripts
In situ hybridization localized TRPM-2/clusterin transcripts primarily to the epithelia of both the VP and DLP. Hybridization signal intensities for TRPM-2/clusterin mRNA closely paralleled transcript level estimates obtained from Northern hybridization studies. Low signal intensities were observed in the glandular cells of DLPs (Figure 3A) ▶ and VPs (not illustrated) of untreated controls. Castration caused dramatic increases in TRPM-2/clusterin mRNA hybridization signal intensity in glandular cells of both the DLP (Figure 3B ▶ , from 7-day castrate) and the VP (not illustrated). After 16 weeks of T+E2 treatment, strong TRPM-2/clusterin transcript signals were detected in the dysplastic epithelia of DLPs (Figure 4, A and B) ▶ but not in the adjacent morphologically normal acini nor in the lesion-free VPs (not illustrated). The signal intensity in these dysplastic lesions often exceeded that visualized in the regressing epithelia of DLPs from orchiectomized rats (Figure 3B ▶ , 7-day castrate) and approximated that found in the VPs (not illustrated) of sexually ablated animals. Sections of VPs and DLPs were negative when the unrelated antisense prepro-PTH probe was used or when the sections were pretreated with RNAse A (not illustrated).
Figure 3.
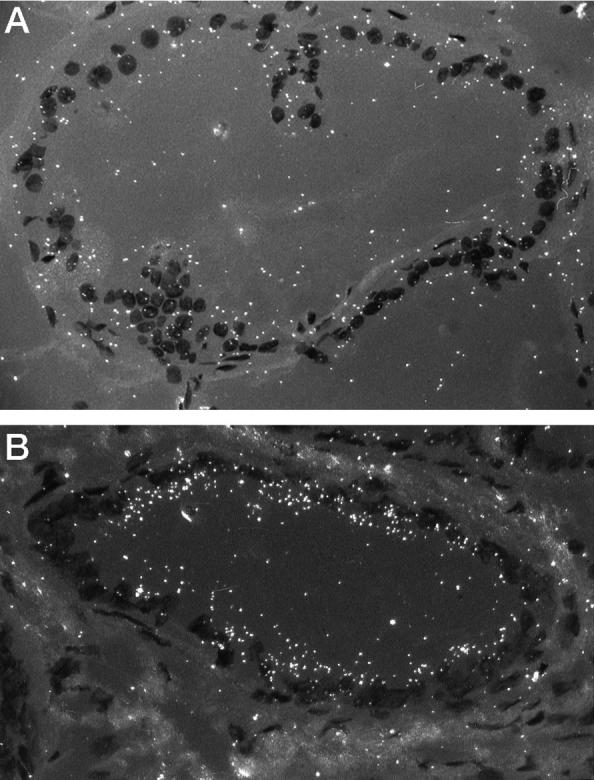
In situ hybridization of TRPM-2/clusterin mRNA in prostatic epithelia. Dark-field photomicrographs. A: Expression of TRPM-2/clusterin is weakly expressed in epithelium of this DLP duct from an intact untreated animal. Magnification, ×160. B: Labeling is strong over the epithelium of this DLP duct from a 7-day castrate. Magnification, ×160.
Figure 4.
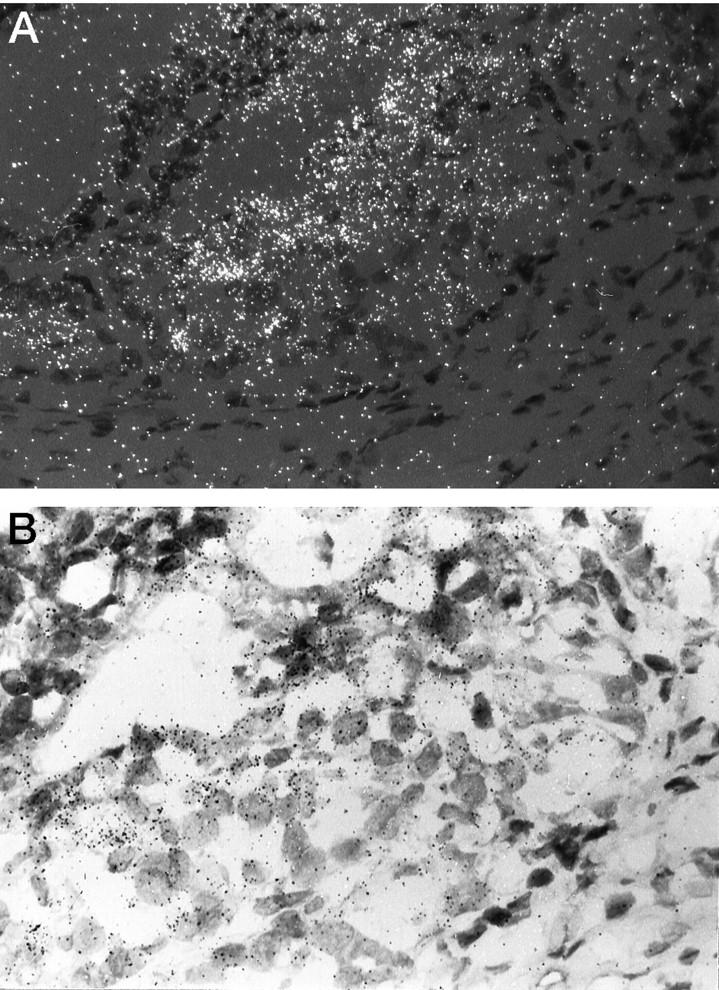
A: Dark-field micrograph showing marked expression of TRPM-2/clusterin mRNA in this DLP duct from a T+E2-treated rat. Signal intensity is especially strong in the area of the duct (right) with extensive dysplasia. Enhancement of expression is in general localized only to the dysplastic foci with neighboring normal acini expressing low signal intensity. Magnification, ×160. B: Bright-field micrograph of the same section illustrated in A. Magnification, ×240.
Immunohistochemical Detection of TRPM-2/Clusterin Translational Product in Rat Prostatic Lobes
Clusterin was localized almost exclusively in prostatic epithelia. Minimal immunostaining was detected in sections of VPs from intact, untreated rats where it was localized along the apical borders of secretory cells. Similarly, light immunostaining was present in the glandular cells of the DLP, but in these cells the clusterin was predominantly localized in apical vacuoles or was seen as fine cytoplasmic granules (1+ intensity; Figure 5A ▶ ). Clusterin immunostaining was intense in the epithelia of regressing VPs and DLPs (4+ intensity; Figure 5B ▶ , from a 7-day castrate). Strong staining for this protein was also consistently observed in dysplastic lesions of the DLPs of rats that had been treated with T+E2 for 16 weeks (3+ intensity; Figure 5C ▶ ). In these cases, staining was seen either as dense reaction product within vacuoles or fine cytoplasmic granules. Staining was also present in morphologically nondysplastic epithelia in the DLPs of T+E2-treated rats, but it was considerably less intense than that observed in the lesions. Thus, the localization and extent of clusterin immunostaining in dysplastic foci and regressing prostate closely mirrored that observed for TRPM-2/clusterin transcript expression, as detected in these same tissues by in situ hybridization (see above). In all tissues studied, omission of the primary antibody resulted in the absence of staining (Figure 5D) ▶ .
Figure 5.
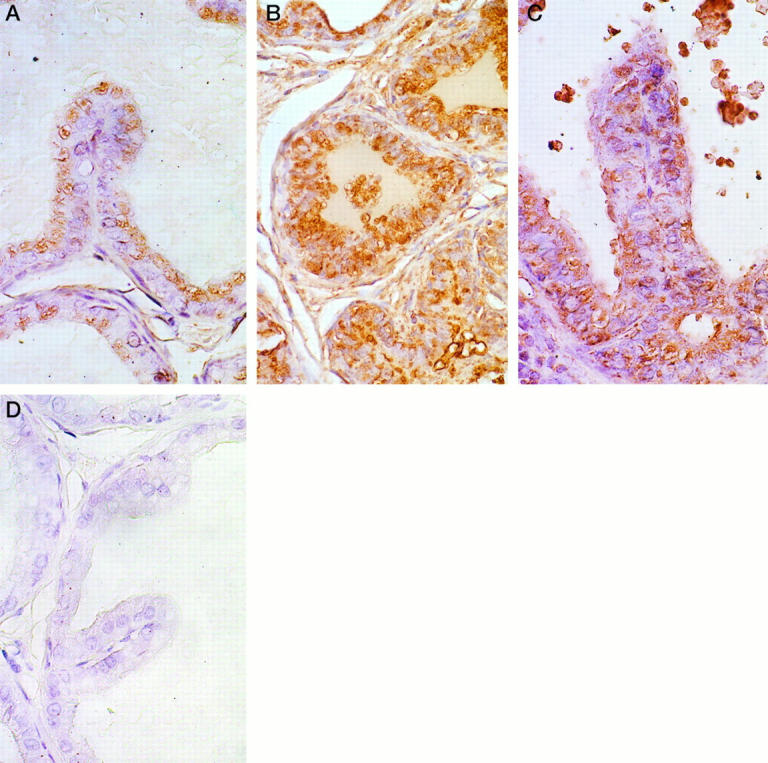
Immunohistological detection of clusterin expression in prostatic epithelia. A: Clusterin immunostaining in the DLP of an intact, untreated rat. Light positive immunostaining is present within apical vacuoles of secretory cells. Magnification, ×420. B: Clusterin immunostaining in the DLP of a rat castrated for 7 days. Note the intense expression of clusterin in the atrophic acinar lining cells. Although not strikingly evident in this photomicrograph, positive staining was also evident in stromal cells in the DLPs of castrated rats. Magnification, ×160. C: Clusterin immunostaining in a dysplastic lesion in the DLP of a rat treated with T+E2 for 16 weeks. Strong immunostaining is evident in this lesion. Clusterin expression in this dysplastic acinus is comparable to that observed in the regressing ducts/acini of the castrated rat (illustrated in B). Within any DLP section from a T+E2-treated rat, expression of clusterin was always stronger in dysplastic foci than in unaltered glands. Magnification, ×500. D: Omission negative control. No immunostaining was detected in this section of DLP from an intact rat. The same negative results were observed in omission controls from the VPs and DLPs of castrated and T+E2-treated rats. Magnification, ×500. All sections were counterstained with Harris hematoxylin.
Detection of Apoptotic Cells in Prostatic Sections
Tissue apoptotic activity was estimated using the ApopTag procedure. Only cells with strong staining of nuclei and/or chromatin fragments were considered positive (Figure 6A) ▶ .
Figure 6.
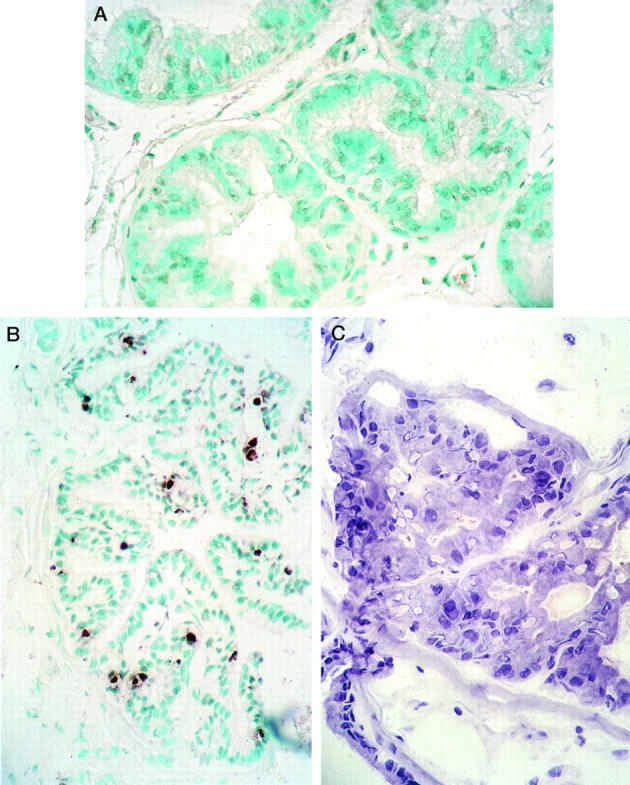
In situ detection of epithelial cells undergoing apoptosis by the ApopTag kit (Oncor). A: A representative section of the DLP from an untreated intact rat (mean AI value for the group, 0.9 ± 0.3%; n = 3 animals per group). No apoptotic cells are present in this section of DLP. In other sections and in glands from intact untreated rats, occasional staining of one or more glandular cells was found. Methyl green counterstain; magnification, ×220. B: DLP section from a rat castrated 7 days before its death. Numerous epithelial cells contain positively stained brown nuclei in these atrophic glands (mean AI value for the group, 11.0 ± 3.0%; n = 3 animals per group). There is considerable agreement here between the apoptotic activity and degree of TRPM-2/clusterin expression in this tissue. Methyl green counterstain; magnification, ×150. C: A DLP dysplastic lesion from a rat treated with T+E2 for 16 weeks. This section is from the same ductal lesion illustrated in Figures 4, A and B, and 5C ▶ ▶ . Note the piling up of dysplastic epithelial cells, some of which have formed pseudoacini, and the variations in size and shape of nuclei. No positively stained cells were detected in these dysplastic lesions. Compare the total absence of apoptosis in this section with the intense expression of TRPM-2/clusterin message and protein, illustrated in Figures 4, A and B, and 5C ▶ ▶ , respectively. Harris hematoxylin counterstain; magnification, ×400.
Apoptotic activity (group mean ± SD; n = 3 animals per group) in the prostatic epithelia of untreated intact rats was low (DLP epithelia, 0.9 ± 0.3%; Figure 6A ▶ ) whereas those in the DLPs (Figure 6B ▶ , 7-day castrates, 11 ± 3%; n = 3) or VPs (3-day castrates, 32 ± 5%, not illustrated) of orchiectomized rats were high. These findings were therefore consistent with the levels of TRPM-2/clusterin expression (see above) and histological evidence of marked apoptosis in these epithelia. In contrast, our findings of enhanced expression of TRPM-2/clusterin by in situ hybridization immunohistochemistry in dysplastic foci was not correlated with parallel increases in apoptotic activity in these epithelia (Figure 6C) ▶ . Thus, ApopTag analyses revealed a very low percentage of apoptotic cells in the DLP of T+E2-treated rats (1.5 ± 1.0%; n = 3), which was statistically not different from values found in prostatic epithelia of untreated intact animals (P > 0.05). It should be noted that the vast majority of ApopTag-positive cells in the DLPs of T+E2-treated rats were in nondysplastic glands. Only in rare instances were a few positive cells found in dysplastic lesions. In sum, dissociation of TRPM gene expression and apoptotic activity occurred in the DLPs of T+E2-treated rats.
Discussion
In the present study, we have demonstrated that castration readily induced enhanced TRPM-2/clusterin expression that was associated with increased apoptotic activity in the epithelia of VP and DLP. Past reports have shown parallel increases in TRPM-2/clusterin expression and apoptosis in the regressing VP, 1,13 but the current study has also compared the changes that occur in the VP and DLP after castration. The increase in TRPM-2/clusterin expression appeared earlier and attained higher levels in the VP than in the DLP. This result is consistent with past reports that demonstrated that the VP undergoes involution more rapidly and to a greater extent than does the DLP after castration. 39 Treatment of rats with T+E2 for 16 weeks also augmented TRPM-2/clusterin expression, but this occurred selectively in the dysplastic lesions of the DLP and not in the lesion-free areas of this lobe or in the unaltered VP. Despite the high level of TRPM-2/clusterin expression, apoptotic activity in the dysplastic lesions of sex-hormone-treated rats, however, remained low and appeared unchanged from basal levels seen in the DLPs of untreated rats. Our findings demonstrate, for the first time, that up-regulation of TRPM-2/clusterin is associated with a premalignant prostatic lesion. We suggest that overexpression of the gene product may indicate that cells within dysplastic foci have undergone neoplastic transformation.
We previously demonstrated that T+E2-treated animals had normal physiological levels of T in their circulation, 25,26 and therefore, the observed up-regulation of TRPM-2/clusterin expression in the dysplastic lesions could not be attributed to depletion of the hormone. This finding suggests that TRPM-2/clusterin expression is not suppressed by androgen and may be constitutively expressed in these lesions. A similar phenomenon was reported in the Shionogi mouse mammary cancer cell line, where it was shown that, as the androgen-dependent cancer cells adapted to an androgen deprived environment, several androgen-repressed genes, including TRPM-2/clusterin, became constitutively expressed. 40 Alternatively, the estrogen component of the dual hormone treatment may have counteracted the repressive action of androgen on TRPM-2/clusterin expression. In this regard, Russo et al 14 reported that the administration of diethylstilbestrol, a long-acting estrogen, to rats elevated prostatic TRPM-2/clusterin expression more markedly and over a longer duration than did castration. Their findings suggest that the estrogen may act as a positive regulator of TRPM-2/clusterin expression. 14
Although the functional role of TRPM-2/clusterin in the prostate remains controversial, it has been postulated that transcriptional activation of the TRPM-2/clusterin gene is an important mediator of castration-induced prostatic involution. 1,11-13 The gene product is believed to affect the apoptotic process by playing an important role in cell membrane turnover, lipid transport, suppression of local immune response, or inhibition of complement-mediated cytolysis. 8,10 Our finding of marked TRPM-2/clusterin expression in dysplastic lesions in the virtual absence of apoptosis suggests that the dysplastic cells may have developed resistance to this mode of cell death. In this regard, Sensibar et al 41 have recently suggested that TRPM-2/clusterin may serve as a survival factor for prostatic cells. In their study on tumor-necrosis-factor-induced cell death in LNCaP cells, TRPM-2/clusterin was shown to be transiently elevated before the apoptosis. This increase was followed by a period of TRPM-2/clusterin depletion, which preceded cell death. Moreover, these workers reported that introduction of TRPM-2/clusterin antisense oligonucleotide into LNCaP cells resulted in a significant increase in apoptotic activity. Although the function of TRPM-2/clusterin in the dysplastic DLP lesions remains unknown, we speculate that its overexpression in the dysplastic foci may represent a phenotype of early neoplastic transformation. Alternatively, its expression in the absence of apoptosis may reflect deregulation of cell death signaling in the dysplastic DLP epithelium. Finally, it remains possible that enhanced expression of TRPM-2/clusterin may confer apoptotic resistance to this premalignant tissue in a manner similar to that described for the LNCaP cells. 41 It is widely believed that deregulation/dysfunction of apoptotic signaling disrupts cell division/cell death balance, an action that could favor accumulation of transformed cells in a premalignant tissue that eventually may turn cancerous. 42-44
The derangement of apoptosis, specifically the development of resistance to inductive signals, has also been reported in many human neoplasms. 42-46 Apropos to our current finding, Kyprianou et al 47 have observed down-regulation of apoptotic activity in primary-site prostatic adenocarcinomas as compared with adjacent normal tissue. In these early localized neoplasms, expression of Bcl-2, an intracellular antagonist of apoptosis, was markedly elevated. Similarly, we 48 and others 49 have reported the up-regulation of MKP-1, a putative apoptotic inhibitor, in dysplastic lesions in rat and human prostates. Taken together, these reports and our present findings support the hypothesis that blocking of apoptosis, via the enhanced expression of endogenous inhibitors or possible survival factors, such as TRPM-2/clusterin, may be an important step in early carcinogenesis.
Acknowledgments
We thank Dr. C. Yan Cheng of the Population Council, Center for Biomedical Research, New York, NY, for his generous gift of the anti-rat clusterin antibody. Likewise, we are in debt to Dr. Martin Tenniswood, Department of Biochemistry, University of Ottawa, Canada, for pG21–04 containing a TRPM-2 cDNA. We are in debt to Mr. Kin-Mang Lau who assisted in the preparation of the figures.
Footnotes
Address reprint requests to Dr. Shuk-mei Ho, Department of Biology, Tufts University, Medford, MA 02155.
Supported in part by the National Cancer Institute, NIH grants CA15776, AG13965, and CA62269.
References
- 1.Montpetit ML, Lawless KR, Tenniswood MP: Evidence for an androgen repressed mRNA in rat ventral prostate. Prostate 1995, 8:25-36 [DOI] [PubMed] [Google Scholar]
- 2.Collard MW, Griswold MD: Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry 1987, 26:3297-3303 [DOI] [PubMed] [Google Scholar]
- 3.Griswold MD, Roberts K, Bishop P: Purification and characterization of a sulfated glycoprotein secreted by Sertoli cells. Biochemistry 1986, 25:7265-7270 [DOI] [PubMed] [Google Scholar]
- 4.Blaschuk O, Burdzy K, Fritz IB: Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem 1983, 258:7714-7720 [PubMed] [Google Scholar]
- 5.Palmer DJ, Christie DL: The primary structure of glycoprotein III from bovine adrenal medullary chromaffin granules: sequence similarity with human serum protein-40,40 and rat Sertoli cell glycoprotein. J Biol Chem 1990, 265:6617-6623 [PubMed] [Google Scholar]
- 6.de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J: Apolipoprotein J: structure and tissue distribution. Biochemistry 1990, 29:5380-5389 [DOI] [PubMed] [Google Scholar]
- 7.Jenne DE, Tschopp J: Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci USA 1989, 86:7123-7127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenniswood MP, Guenette RS, Lakins J, Mooibroek M, Wong P, Welsh JE: Active cell death in hormone-dependent tissue. Cancer Metastasis Rev 1992, 11:197-220 [DOI] [PubMed] [Google Scholar]
- 9.Buttyan R, Olsson CA, Pintar J, Chang C, Bandyk M, Ng PY, Sawczuk IS: Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol 1989, 9:3473-3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May PC, Finch CE: Sulfated glycoprotein 2: new relationships of this multifunctional protein to neurodegeneration. Trends Neurosci 1992, 15:391-396 [DOI] [PubMed] [Google Scholar]
- 11.Leger JG, Montpetit ML, Tenniswood MP: Characterization and cloning of androgen repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun 1987, 147:196-203 [DOI] [PubMed] [Google Scholar]
- 12.Kyprianou N, Isaacs JT: Expression of transforming growth factor-β in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol 1989, 3:1515-1522 [DOI] [PubMed] [Google Scholar]
- 13.Sensibar JA, Griswold MD, Sylvester SR, Buttyan R, Bardin CW, Cheng CY, Dudek S, Lee C: Prostatic ductal system in rats: regional variation in localization of an androgen-repressed gene product, sulfated glycoprotein-2. Endocrinology 1991, 128:2091-2102 [DOI] [PubMed] [Google Scholar]
- 14.Russo P, Warner JA, Huryk R, Perez G, Heston WD: TRPM-2 gene expression in normal rat ventral prostate following castration and exposure to diethylstilbestrol, flutamide, MK-906 (finasteride), and coumarin. Prostate 1994, 24:237-243 [DOI] [PubMed] [Google Scholar]
- 15.Norman DJ, Feng L, Cheng SS, Gubbay J, Chan E, Heintz N: The lurcher gene induces apoptotic death in cerebellar Purkinje cells. Development 1995, 121:1183-1193 [DOI] [PubMed] [Google Scholar]
- 16.Conner J, Buttyan R, Olsson CA, D’Agati V, O’Toole K, Sawczuk IS: SGP-2 expression as a genetic marker of progressive cellular pathology in experimental hydronephrosis. Kidney Int 1991, 39:1098-1103 [DOI] [PubMed] [Google Scholar]
- 17.May PC, Lampert-Etchells M, Johnson SA, Poirier J, Masters JN, Finch CE: Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer’s disease and in response to experimental lesions in rat. Neuron 1990, 5:831-839 [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Jow GM, Moulton BC, Lee C, Sensibar JA, Park-Sarge OK, Chen TJ, Gibori G: Apoptosis in decidual tissue regression and reorganization. Endocrinology 1994, 135:1272-1279 [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz A, Ruutiainen-Altman K, Marzella L, Botero L, Dushnik M, Adashi EY: Follicular atresia as an apoptotic process: atresia-associated increase in the ovarian expression of the putative apoptotic marker sulfated glycoprotein-2. J Soc Gynecol Invest 1996, 3:199-208 [PubMed] [Google Scholar]
- 20.Fleshner NE, Trachtenberg J: Sequential androgen blockade: a biological study in the inhibition of prostatic growth. J Urol 1992, 148:1928-1931 [DOI] [PubMed] [Google Scholar]
- 21.Brandstrom A, Westin P, Bergh A, Cajander S, Damber JE: Castration induces apoptosis in the ventral prostate but not in an androgen-sensitive prostatic adenocarcinoma in the rat. Cancer Res 1994, 54:3594-5601 [PubMed] [Google Scholar]
- 22.Akakura K, Bruchovsky N, Rennie PS, Coldman AJ, Goldenberg SL, Tenniswood M, Fox K: Effects of intermittent androgen suppression on the stem cell composition and the expression of the TRPM-2 (clusterin) gene in the Shionogi carcinoma. J Steroid Biochem Mol Biol 1996, 59:501-511 [DOI] [PubMed] [Google Scholar]
- 23.Rennie PS, Bruchovsky N, Akakura K, Goldenberg SL, Otal N, Akakura S, Wong P, Tenniswood M: Effect of tumor progression on the androgenic regulation of the androgen receptor, TRPM-2 and YPT1 genes in the Shionogi carcinoma. J Steroid Biochem Mol Biol 1994, 50:31-40 [DOI] [PubMed] [Google Scholar]
- 24.Marinelli M, Quaglino D, Bettuzzi S, Strocchi P, Davalli P, Corti A: Increased levels of clusterin mRNA in the ventral prostate of the aging rat are associated to increases in cuboidal (atrophic) cell population and not to changes in apoptotic activity. Biochem Cell Biol 1994, 72:512-521 [DOI] [PubMed] [Google Scholar]
- 25.Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D: Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst 1988, 80:1045-1053 [DOI] [PubMed] [Google Scholar]
- 26.Leav I, Merk FB, Kwan PW, Ho SM: Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate 1989, 15:23-40 [DOI] [PubMed] [Google Scholar]
- 27.McNeal JE, Bostwick DG: Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol 1986, 17:64-71 [DOI] [PubMed] [Google Scholar]
- 28.Bostwick DG, Pacelli A, Lopqez-Beltran A: Molecular biology of prostatic intraepithelial neoplasia. Prostate 1996, 29:117-134 [DOI] [PubMed] [Google Scholar]
- 29.Drago JR: The induction of NB rat prostatic carcinomas. Anticancer Res 1984, 4:255-256 [PubMed] [Google Scholar]
- 30.Bosland MC, Ford H, Horton L: Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd: SD rats treated with a combination of testosterone and estradiol 17β or diethylstilbestrol. Carcinogenesis 1994, 16:1311-1317 [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 32.Chomczynski P: One-hour downward alkaline capillary transfer for blotting of DNA and RNA. Anal Biochem 1992, 201:134-139 [DOI] [PubMed] [Google Scholar]
- 33.Clements JA, Matheson BA, Wines DR, Brady JM, MacDonald RJ, Funder JW: Androgen dependence of specific kallikrein gene family members expressed in rat prostate. J Biol Chem 1988, 263:16132-16137 [PubMed] [Google Scholar]
- 34.Höfler H, Childers H, Montminy MR, Lechan RM, Goodman RH, Wolfe HJ: In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J 1986, 18:597-604 [DOI] [PubMed] [Google Scholar]
- 35.Komminoth P, Merk FB, Leav I, Wolfe HJ, Roth J: Comparison of 35S- and digoxigenin-labeled RNA and oligonucleotide probes for in situ hybridization: expression of mRNA of the seminal vesicle protein II and androgen-receptor genes in the rat prostate. Histochemistry 1992, 98:217-218 [DOI] [PubMed] [Google Scholar]
- 36.Hendy GN, Kronenberg HM, Potts JT, Jr, Rich A: Nucleotide sequence of cloned cDNAs encoding human preproparathyroid. Proc Natl Acad Sci USA 1981, 78:408-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grima J, Zwain I, Lockshin RA, Bardin CW, Cheng CY: Diverse secretory of clusterin by epididymis and prostate/seminal vessels undergoing cell repression following orchiectomy. Endocrinology 1990, 126:2989-2997 [DOI] [PubMed] [Google Scholar]
- 38.Ahuja HS, Tenniswood M, Lockshin R, Zakeri ZF: Expression of clusterin in cell differentiation and cell death. Biochem Cell Biol 1994, 72:523-529 [DOI] [PubMed] [Google Scholar]
- 39.Banerjee PP, Banerjee S, Tilly KI, Tilly JL, Brown TR, Zirkin BR: Lobe-specific apoptotic cell death in rat prostate after androgen ablation by castration. Endocrinology 1995, 136:4368-4376 [DOI] [PubMed] [Google Scholar]
- 40.Rennie PS, Bruchovsky N, Coldman AJ: Loss of androgen dependence is associated with an increase in tumorigenic stem cells and resistance to cell-death genes. J Steroid Biochem Mol Biol 1990, 37:843-847 [DOI] [PubMed] [Google Scholar]
- 41.Sensibar JA, Sutkowski DM, Raffo A, Buttyan R, Griswold MD, Sylvester SR, Kozlowski JM, Lee C: Prevention of cell death induced by tumor necrosis factor α in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin). Cancer Res 1995, 55:2431-2437 [PubMed] [Google Scholar]
- 42.Dixon SC, Soriano BJ, Lush RM, Borner MM, Figg WD: Apoptosis: its role in the development of malignancies and its potential as a novel therapeutic target. Ann Pharmacol 1997, 31:76-82 [DOI] [PubMed] [Google Scholar]
- 43.Bergman PJ, Harris D: Radioresistance, chemoresistance, and apoptosis resistance: the past, present, and future. Vet Clin North Am Small Anim Pract 1997, 27:47-57 [DOI] [PubMed] [Google Scholar]
- 44.McConkey DJ, Zhivotovsky B, Orrenius S: Apoptosis: molecular mechanisms and biomedical implications. Mol Aspects Med 1996, 17:1-110 [DOI] [PubMed] [Google Scholar]
- 45.Schott AF, Apel IJ, Nunez G, Clarke MF: Bcl-XL protects cancer cells from p53-mediated apoptosis. Oncogene 1995, 11:1389-1394 [PubMed] [Google Scholar]
- 46.Reed JC, Miyashita T, Takayama S, Wang HG, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M: BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem 1996, 60:23-32 [DOI] [PubMed] [Google Scholar]
- 47.Tu H, Jacobs SC, Borkowski A, Kyprianou N: Incidence of apoptosis and cell proliferation in prostate cancer: relationship with TGF-β1 and bcl-2 expression. Int J Cancer 1996, 69:357-363 [DOI] [PubMed] [Google Scholar]
- 48.Leav I, Galluzzi CM, Ziar J, Stork PJ, Ho SM, Loda M: Mitogen-activated protein kinase and mitogen-activated kinase phosphatase-1 expression in the Noble rat model of sex hormone-induced prostatic dysplasia and carcinoma. Lab Invest 1996, 75:361-370 [PubMed] [Google Scholar]
- 49.Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ, Loda M: Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab Invest 1997, 76:37-51 [PubMed] [Google Scholar]


