Abstract
Cytokines interact not only with membrane anchored receptors, but also with specific soluble receptors which circulate in the bloodstream. In general, soluble cytokine receptors such as soluble tumor necrosis factor receptor, soluble interleukin 1 receptor, and soluble interleukin 4 receptor compete with their membrane-bound counterparts for the ligands and therefore act as antagonists. In contrast, soluble receptors for cytokines of the interleukin-6 (IL-6) family complex with their ligands act agonistically. Interestingly, the complex of IL-6 and the soluble interleukin 6 receptor (sIL-6R) activates target cells that do not express the membrane-bound IL-6R and therefore cannot respond to IL-6. To identify cellular responses that are due to IL-6/sIL-6R but not to IL-6 alone, IL-6/sIL-6R double-transgenic mice were generated and compared with IL-6 single-transgenic mice. IL-6/sIL-6R transgenic mice develop a severe phenotype showing 1) marked hepatocellular hyperplasia frequently surrounded by peliosis and necrosis, 2) significant acceleration and aggravation of plasmacytoma formation, and 3) excessive activation of extramedullary hematopoiesis in spleen and liver followed by a subsequent increase of all cellular components in the peripheral blood. These in vivo data suggest that the sIL-6R recruits primarily unresponsive cell populations such as hematopoietic progenitor cells and hepatocytes to IL-6-induced proliferation, but also enhances the known mitogenic effect of IL-6 on plasma cells and thereby contributes to plasmacytoma formation.
Interleukin (IL)-6 is a pleiotropic cytokine (for reviews see Refs. 1 and 2 ) stimulating a variety of cell types, including hematopoietic and neuronal cells, keratinocytes, osteoclasts, and vascular endothelial cells. IL-6 also modulates the hepatic expression of acute-phase response genes during inflammation. 3
IL-6 and the closely related cytokines IL-11, ciliary neurotrophic factor, leukemia inhibitory factor, oncostatin M, and cardiotrophin-1 interact with a multisubunit receptor complex 4,5 that induces intracellular signaling via the Janus kinase-signal transducer and activator of transcription and ras-mitogen-activated protein-kinase pathway. 6,7 This receptor complex contains the transmembranous signal transducer gp130 as a common component. For IL-6, IL-11, and ciliary neurotrophic factor, the existence of specific soluble receptors (sRs) has been demonstrated. 8 sRs are generated from membrane-bound precursors by limited proteolysis. 9-11 Soluble IL-6 receptor (sIL-6R) binds to IL-6 before interaction with gp130 and modifies IL-6-induced signal transduction, a process called transsignaling. 8 In contrast to sRs for tumor necrosis factor-α (TNFα), IL-1, and IL-4 that act antagonistically, sIL-6R is an agonistic receptor that stimulates signal transduction and sensitizes cells to IL-6 stimulation. 8,12 sIL-6R is required to mediate the IL-6 stimulatory effects on neuronal and hematopoietic cells in cell culture systems 13,14 and is found in elevated concentrations during inflammation in patients. 15 The specific in vivo effects of the IL-6/sIL-6R interaction have not been defined so far. Transgenic mice constitutively overexpressing human IL-6 develop plasma cell proliferation with progression to plasmacytomas. 16,17 Transgenic mice expressing the human sIL-6R do not develop any specific morphological changes, because murine IL-6 does not bind to the human IL-6R. 12,18 Upon injection of human IL-6, however, sIL-6R transgenic mice are hypersensitized toward IL-6, and the plasma half-life of IL-6 is drastically prolonged. 12
Here we describe the phenotype of mice constitutively and simultaneously overexpressing human IL-6 and sIL-6R and show that these mice are different from IL-6 transgenic mice: 1) IL-6/sIL-6R coexpression leads to hepatocellular hyperplasia as well as to secondary liver pathology, including extensive necrosis and peliosis; 2) sIL-6R enhances the stimulatory effect of IL-6 on plasma cells and accelerates significantly plasmacytoma development; and 3) sIL-6R recruits extramedullary hematopoietic cells to the stimulatory effect of IL-6 and thus mediates excessive hematopoiesis in spleen and liver. Our results demonstrate that, in addition to the sensitizing effect of sIL-6R on plasma cells, cell populations that are not responsive to IL-6 alone become activated by the complex of IL-6/sIL-6R in vivo.
Materials and Methods
Generation of Transgenic Mice
The generation of transgenic mice expressing human sIL-6R or human IL-6 has been described. 12,19 The expression of the sIL-6R transgene was under control of the phosphoenolpyruvate carboxykinase promoter, which is active in liver and kidney. 20,21 The promoter is known to be silent prenatally and is induced from day 3 after birth. The IL-6 transgene was under control of the murine metallothionein-1 promoter. Double-transgenic mice were generated by crossing homozygous sIL-6R and IL-6 transgenic mice. The transgene status was assayed by polymerase chain reaction as described previously. 12,19 Overall, 86 transgenic mice between the ages of 3 and 16 weeks were investigated; 22 mice were transgenic for IL-6, 31 for sIL-6R, and 36 mice were double transgenic for both IL-6 and sIL-6R. The presence of the IL-6 transgene led to serum concentrations of IL-6 between 10 and 20 ng/ml in IL-6 single-transgenic and IL-6/sIL-6R double-transgenic mice; sIL-6R single-transgenic mice and IL-6/sIL-6R double-transgenic mice showed sIL-6R serum concentrations between 4 and 8 μg/ml. 12,22
Peripheral Blood Cell Counts and Plasma Enzyme Activities
Peripheral blood was taken under general anesthesia by cardiac puncture. The cellular composition of 100 μl heparinized blood was analyzed in a Cell Dyn 3500 hemocounter (Abbott, Delkenheim, Germany). Plasma enzyme activities of alanine aminotransferase and aspartate aminotransferase (AST) were determined according to standard methods using an automated procedure.
Organ Weight and Histomorphology
Upon sacrifice, total body weight and organ weights were recorded. Tissues were fixed in 4% formaldehyde/phosphate-buffered saline and embedded in paraffin; bone sections were decalcified in ethylenediaminetetraacetic acid. Sections 1 to 2 μm were stained with hematoxylin/eosin. Stained sections were viewed and photomicrographs were taken with a Diaplan microscope (Leica, Bensheim, Germany). When a significant focal or diffuse accumulation of plasma cells was detected only in a single location, eg, spleen, the lesion was termed plasma cell proliferation. When plasma cell proliferation exhibited a focal, mostly nodular pattern frequently showing significant nuclear atypia and involving two or more different organs, the lesions were judged to constitute a plasmacytoma. Extramedullary hematopoiesis was evaluated histologically and scored as follows: 0, absent; +, mild; ++, moderate; and +++, severe/maximal; in the spleen, where extramedullary hematopoiesis persists in normal mice, the presence of physiological hematopoiesis equaled a score of +. The extent of liver pathology, consisting of hepatocellular hyperplasia, areactive necrosis and peliosis, was scored as follows: 0, absent; +, mild; ++, moderate; and +++, severe.
Analysis of Hepatocellular Proliferation
Hepatocellular proliferation was analyzed by immunohistochemistry for proliferating cell nuclear antigen (PCNA) on sections from formalin-fixed and paraffin-embedded liver tissues. Immunodetection was performed using a monoclonal antibody that specifically reacts with the PCNA p36 protein expressed at high levels in proliferating cells (Santa Cruz Biotechnology, Santa Cruz, CA). Specific immunoreactivity was detected by the indirect avidin-biotin complex method using diaminobenzidine as the chromogen. The percentage of positive hepatocytes was determined by evaluating at least 500 hepatocytes per animal on different parts of the liver section. Only those hepatocytes showing distinct nuclear staining were judged as positive.
To exclude secondary effects induced by liver necrosis or activated hepatic hematopoiesis, only animals between 6 and 7 weeks of age were analyzed. In IL-6/sIL-6R double-transgenic mice, only those animals were studied that showed neither hepatocellular necrosis nor activated hepatic hematopoiesis by careful histological analysis.
Results
General Phenotype of IL-6/sIL-6R Double-Transgenic Mice
IL-6/sIL-6R mice displayed a significant phenotype. They were smaller than control or single-transgenic mice and had a reduced body weight. Moreover, they developed distended abdominal regions caused by two- to fourfold increases of liver and spleen weight relative to the total body weight. Livers appeared dark in color and had a rugged and humpy surface caused by round, whitish, and (with respect to the liver surface) elevated foci. The spleen was diffusely and symmetrically enlarged. Weight and macroscopic appearance of the other organs and behavior of the mice were in general not suspicious, with the exception of plasmacytoma development (see below).
sIL-6R Enhances the Proliferative Effect of IL-6 on Plasma Cells
IL-6 transgenic mice are known to frequently develop plasma cell proliferations and plasmacytomas. 16,17 In our study, plasma cell proliferations were first observed in the spleen and further progressed to multifocal plasmacytomas detectable in different organs, such as liver, spleen, lung, kidney, and bone marrow (Table 1 ▶ ; Figure 1 ▶ , C and D). None of 13 IL-6 transgenic mice younger than 11 weeks of age developed plasmacytoma, whereas 7 of 9 mice that were 11 weeks or older developed plasmacytomas. None of 31 sIL-6R transgenic mice between 3 and 16 weeks of age showed plasma cell proliferation or plasmacytoma (Table 1) ▶ .
Table 1.
Plasmacytoma Development in Transgenic Mice
| Age (weeks) | IL-6 (n = 22) | sIL-6R (n = 31) | IL-6/sIL-6R (n = 36) |
|---|---|---|---|
| ≤4 | 0 of 6 | 0 of 9 | 0 of 8 |
| 5–10 | 0 of 7 | 0 of 17 | 5 of 13 (PCP) |
| 2 of 13 (P) | |||
| ≥11 | 7 of 9 (P) | 0 of 5 | 15 of 15 (P) |
P, plasmacytoma; PCP, plasma cell proliferation.
Figure 1.
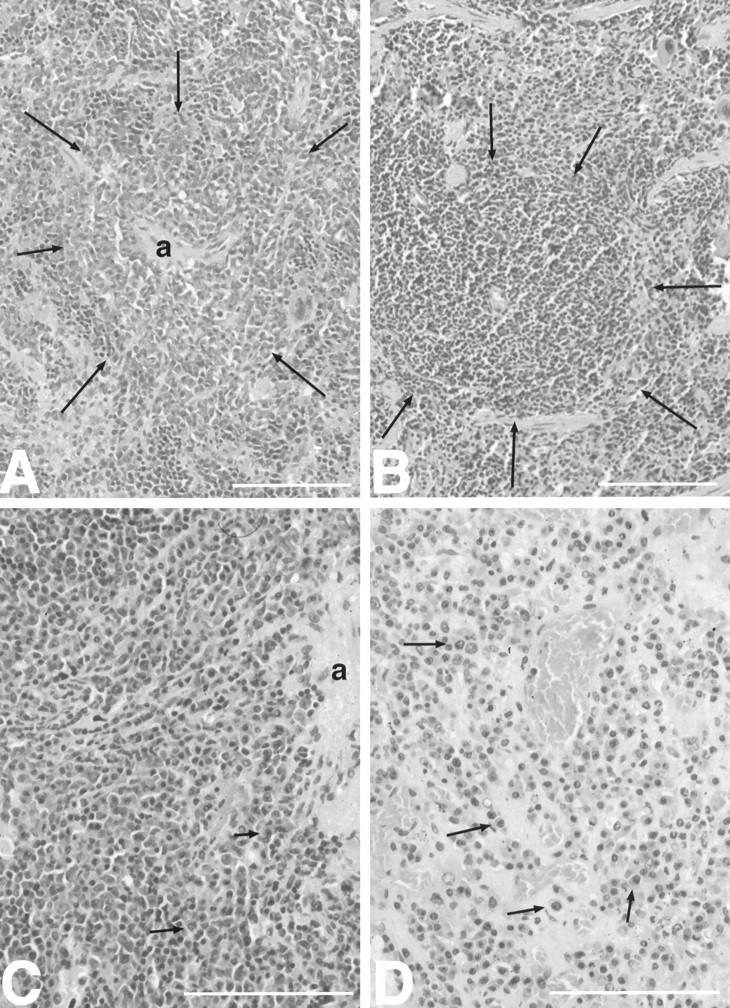
Accelerated development of plasmacytomas in IL-6/sIL-6R double-transgenic mice compared with IL-6 single transgenic mice. A: Focal periarteriolar plasma cell proliferation (arrows) in the spleen of an IL-6/sIL-6R double-transgenic mouse (7 weeks). Note the extensive activation of extramedullary hematopoiesis in the surrounding parenchyma. B: Normal spleen without plasma cell proliferation in an IL-6 transgenic mouse (8 weeks). Normal follicle (white pulp) is denoted by arrows. C: Plasmacytoma in the spleen of an IL-6/sIL-6R double-transgenic mouse (16 weeks). Note atypical plasma cells with eccentric round-to-ovoid pleomorphic and hyperchromatic nuclei (arrows). D: Excessive plasmacytoma in the spleen of an IL-6 single-transgenic mouse (16 weeks). Atypical plasma cells are marked by arrows. a in A and C denotes arterioles in the center of the white pulp. Scale bars, 100 μm.
In IL-6/sIL-6R double-transgenic mice, the development of plasma cell proliferation and plasmacytoma was significantly accelerated when compared with IL-6 single-transgenic mice (Figure 1 ▶ ; Table 1 ▶ ). Between 5 and 11 weeks of age, 7 of 13 animals showed plasma cell proliferations (5 animals) or plasmacytomas (2 animals). All 15 animals beyond the age of 11 weeks had plasmacytomas. These data demonstrate that the presence of the sIL-6R significantly accelerates the development of IL-6-induced plasmacytomas. Also, in IL-6/sIL-6R double-transgenic mice, plasmacytoma development was seen more frequently as compared with IL-6 single-transgenic mice (Figure 1 ▶ , A and B).
Extramedullary Hematopoiesis in IL-6/sIL-6R Double-Transgenic Mice
The development of extramedullary hematopoiesis in transgenic and nontransgenic mice is demonstrated in Table 2 ▶ . In nontransgenic mice or single-transgenic mice, mild extramedullary hematopoiesis was present in the spleen but absent in the liver (not shown).
Table 2.
Extramedullary Hematopoiesis in Transgenic Mice
| Age (weeks) | IL-6 (n = 22) | sIL-6R (n = 31) | IL-6/sIL-6R (n = 36) |
|---|---|---|---|
| ≤4 | |||
| Liver | 0 of 6 | 0 of 9 | + : 1 of 8 |
| Spleen | ++ : 3 of 6 | 0 of 9 | ++ : 4 of 7 |
| +++ : 2 of 7 | |||
| 5–10 | |||
| Liver | + : 1 of 7 | 0 of 17 | + : 4 of 13 |
| ++ : 3 of 13 | |||
| +++ : 2 of 13 | |||
| Spleen | ++ : 4 of 7 | 0 of 17 | ++ : 3 of 13 |
| +++ : 8 of 13 | |||
| ≥11 | |||
| Liver | ++ : 1 of 9 | 0 of 5 | + : 1 of 15 |
| ++ : 4 of 15 | |||
| +++ : 9 of 15 | |||
| Spleen | ++ : 8 of 9 | 0 of 5 | +++ : 15 of 15 |
Extramedullary hematopoiesis was not induced in the livers of IL-6 or sIL-6R single-transgenic mice and was only moderately activated in the spleens of some IL-6 single-transgenic mice (Table 2) ▶ . Bone marrow hematopoiesis examined in femoral sections was not significantly different between single- and double-transgenic mice (data not shown). In IL-6/sIL-6R double-transgenic mice, however, extramedullary hematopoiesis was extensively activated (Table 2) ▶ . Proliferating hematopoietic foci that increased in size with the age of the mice were observed in spleens and livers from the age of 4 weeks on. In mice between 3 and 4 weeks of age, 6 of 7 animals had developed moderate or severe hematopoiesis as compared with 0 of 9 in sIL-6R mice; 3 of 6 IL-6 mice had moderate hematopoiesis. Fifteen of 15 mice 11 weeks or older displayed severe hematopoiesis compared with 0 of 5 sIL-6R mice and 8 of 9 IL-6 mice, in which hematopoiesis was only moderate and only present in the spleen.
Interestingly, no significant extramedullary hematopoiesis was observed in organs other than spleen and liver. Histology showed that splenic and hepatic hematopoiesis were different. In the spleen, the number of erythro- and granulopoietic cells as well as megakaryocytes appeared to be elevated to an equal extent (Figure 2 ▶ , A and C). However, hepatic hematopoiesis was predominantly granulopoietic (Figure 2D) ▶ . Splenic hematopoiesis generally preceded hepatic hematopoiesis and was more severe within the same animal (Figure 2 ▶ , A and B). Furthermore, splenic hematopoiesis diffusely involved the whole organ and dissolved the definition of the red and white pulp (Figure 2 ▶ , A and C). In the liver, the number and size of hematopoietic foci increased with time but remained localized (Figure 2D) ▶ . Frequently, hepatic hematopoietic foci were accompanied by significant fibrosis (see below).
Figure 2.
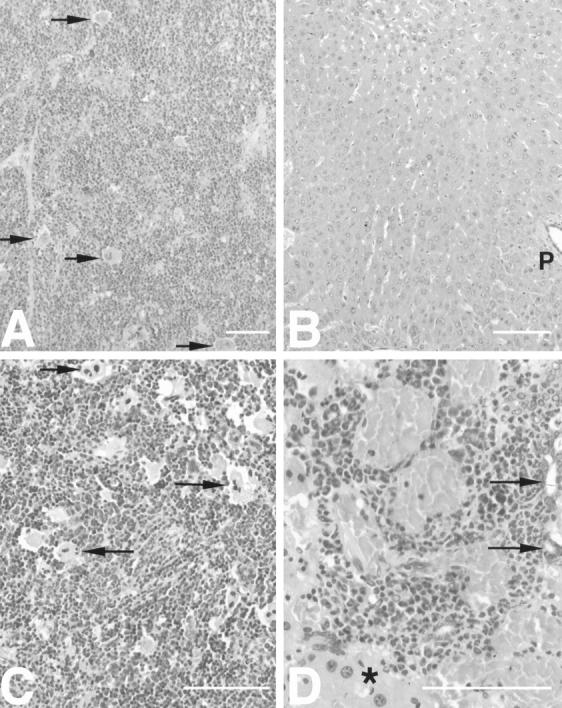
Extramedullary hematopoiesis in IL-6/sIL-6R double transgenic mice with splenic hematopoiesis preceding hepatic hematopoiesis. A: Highly activated diffuse extramedullary hematopoiesis in the spleen of IL-6/sIL-6R double-transgenic mice (6 weeks). Note the lack of definition between the red and white pulp (megakaryocytes indicated by arrows). B: Lack of extramedullary hematopoiesis in the liver of the same IL-6/sIL-6R double-transgenic mouse as shown in A (6 weeks) (p, portal tract). C: Excessive diffuse extramedullary hematopoiesis in the spleen of an IL-6/sIL-6R double-transgenic mouse (13 weeks). Some megakaryocytes are indicated by arrows. D: Extramedullary hematopoietic focus in a portal tract in the liver of the same IL-6/sIL-6R double-transgenic mouse as depicted in C (13 weeks). Bile ducts are labeled by arrows; bordering hepatic parenchyma is denoted by asterisk. Scale bars, 100 μm.
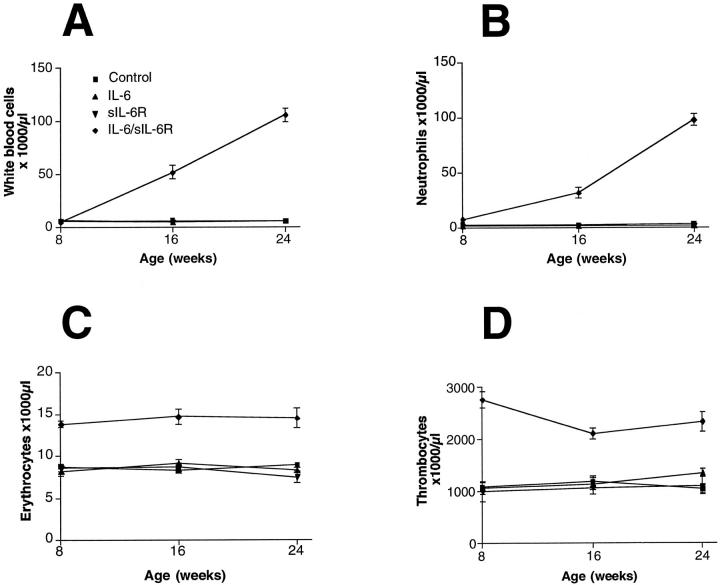
The time course of extramedullary hematopoiesis (see Table 2 ▶ ) matched well to the time course of the cellular blood components. A significant rise of white blood cells (Figure 3A) ▶ consisting mainly of neutrophilic granulocytes (Figure 3B) ▶ occurred in IL-6/sIL-6R double-transgenic mice from the age of 8 weeks on but not in the IL-6 or sIL-6R single-transgenic mice, nor in control mice. In double-transgenic mice, erythrocyte counts (Figure 3C) ▶ and thrombocyte counts (Figure 3E) ▶ already increased between the ages of 2 and 4 weeks and remained high throughout the study period.
Figure 3.
Time course of cellular blood composition in double- and single-transgenic mice as compared with control animals. A: White blood cell counts. B: Neutrophilic granulocyte counts. C: Thrombocyte counts. D: Red blood cell counts.
Severe Liver Pathology in IL-6/sIL-6R Double-Transgenic Mice
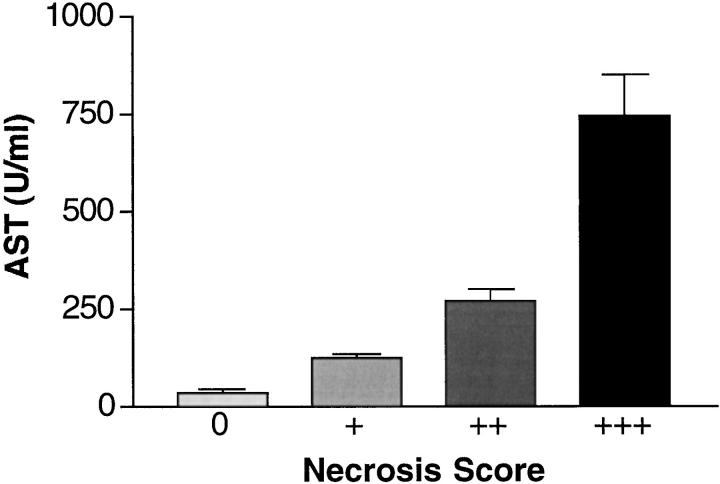
IL-6/sIL-6R double-transgenic mice developed significant liver pathology that was not observed in IL-6 or sIL-6R single-transgenic mice, except plasmacytoma infiltrates in IL-6 transgenic animals (Table 3) ▶ . Normal liver morphology was seen in all single-transgenic mice or control animals (Figure 4A) ▶ . In IL-6/sIL-6R double-transgenic mice, the development of multifocal hepatocellular hyperplasia, frequently surrounded by areactive necrosis and peliosis was observed from the age of 4 weeks on (Figure 4 ▶ , B and C). Necrosis and peliosis were predominantly found in the perivenular (zone III) parenchyma, whereas hepatocellular hyperplasia extended multifocally from the periportal parenchyma (Figure 4B) ▶ . In a few animals that showed extensive necrosis and peliosis, focal coagulation was infrequently found within the distended sinusoids. Liver pathology, especially the extent of necrosis and peliosis, was quite variable although demonstrable in the majority of IL-6/sIL-6R double-transgenic mice. In 5 to 10-week-old double-transgenic mice, four animals showed mild or moderate hepatocellular hyperplasia, respectively. Two animals showed severe hyperplasia. In mice 11 weeks old or older, three animals showed mild, five animals moderate, and three animals severe hyperplasia (Table 3) ▶ . The severity of necroses as demonstrated by the histological score (see Materials and Methods) was reflected by the elevation of liver transaminases (Figure 5) ▶ . Whereas single-transgenic mice and nontransgenic littermates had normal serum transaminases (data not shown), in double-transgenic mice there was an elevation of transaminases in strict correlation with the histological score. In mice with mild necrosis, the mean AST level was 124 ± 13 U/ml; in those with moderate necrosis, the mean AST level was 273 ± 30 U/ml; and in mice with severe necroses, AST levels were 746 ± 105 U/ml (Figure 5) ▶ . One IL-6/sIL-6R double-transgenic and one IL-6 single-transgenic mouse, both with multiorgan involvement of plasmacytoma, exhibited plasmacytoma kidneys with significant hyaline deposition.
Table 3.
Liver Pathology in Transgenic Mice
| Age (weeks) | IL-6 (n = 22) | sIL-6R (n = 31) | IL-6/sIL-6R (n = 36) |
|---|---|---|---|
| ≤4 | 0 of 6 | 0 of 9 | 0 of 9 |
| 5–10 | 0 of 7 | 0 of 17 | + : 4 of 13 |
| ++ : 4 of 13 | |||
| +++ : 2 of 13 | |||
| ≥11 | 0 of 8 | 0 of 5 | + : 3 of 15 |
| ++ : 5 of 15 | |||
| +++ : 3 of 15 |
Figure 4.
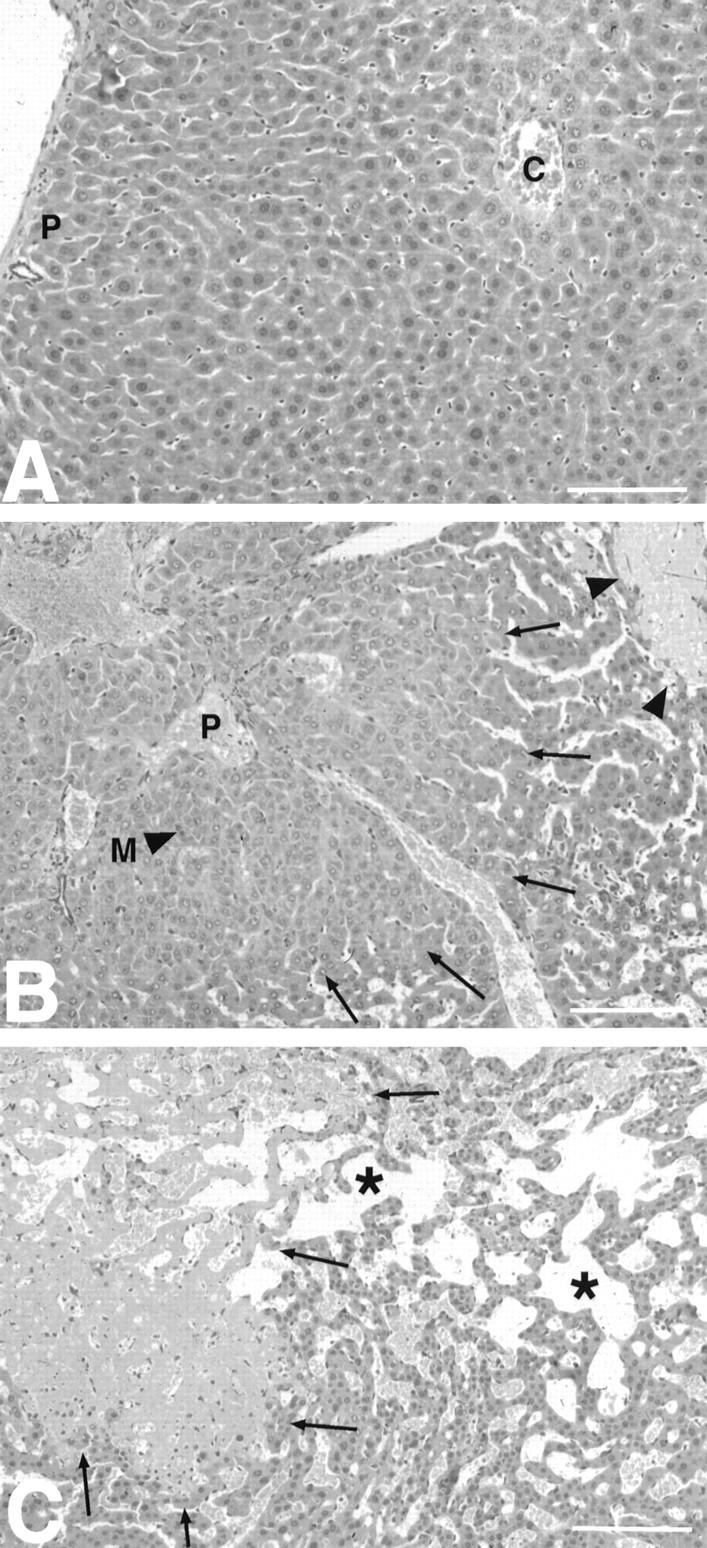
Severe liver pathology in IL-6/sIL-6R double-transgenic mice. A: Normal liver with regular trabecular arrangement of hepatocytes and lack of extramedullary hematopoiesis in an IL-6 single-transgenic animal (16 weeks). B: Overview of hepatic pathology in a 6-week-old IL-6/sIL-6R transgenic animal. Hepatocellular hyperplasia (marked by arrows) peripherally surrounded by peliosis and nonreactive necrosis (arrowheads). C: Extensive hepatic peliosis (*) in the vicinity of an areactive necrosis (arrows). Note the increased cellularity of liver cell plates as a sign of additional hepatocellular hyperplasia. (p, portal tracts; c, central vein). Scale bars, 100 μm.
Figure 5.
Elevation of serum AST levels in IL-6/sIL-6R double-transgenic mice. Note the correlation of the necrosis score (+, mild; ++, moderate; +++, severe) with the increase of serum AST levels in double-transgenic mice.
Hepatocellular Proliferation in Double-Transgenic Mice
To determine whether hepatocellular proliferation was reactive to hepatic necrosis or to activated hepatic hematopoiesis, double-transgenic mice, as well as IL-6 and sIL-6R single-transgenic mice, all without hepatocellular necrosis or hepatic hematopoiesis, were selected for proliferation analysis. We used a monoclonal antibody recognizing the PCNA p36 protein, also known as cyclin or polymerase D-associated protein, which is synthesized in early G1 and S phases of the cell cycle and serves as an excellent marker for proliferating cells. Semiquantitative evaluation of PCNA immunohistology demonstrated that hepatocellular proliferation in IL-6/sIL-6R double-transgenic mice (Figure 6C) ▶ is highly increased compared with IL-6 (Figure 6A) ▶ and sIL-6R (Figure 6B) ▶ single-transgenic animals. It turned out that in the double-transgenic animals, 92% of the hepatocytes stained positive for PCNA, whereas in IL-6 and sIL-6R single-transgenic mice, only 5% and 7%, respectively, of the hepatocytes were PCNA positive (Figure 6D) ▶ . These results demonstrate that hepatocellular proliferation is activated in hepatocytes independently of hepatic necrosis and hematopoiesis (Figure 7) ▶ .
Figure 6.
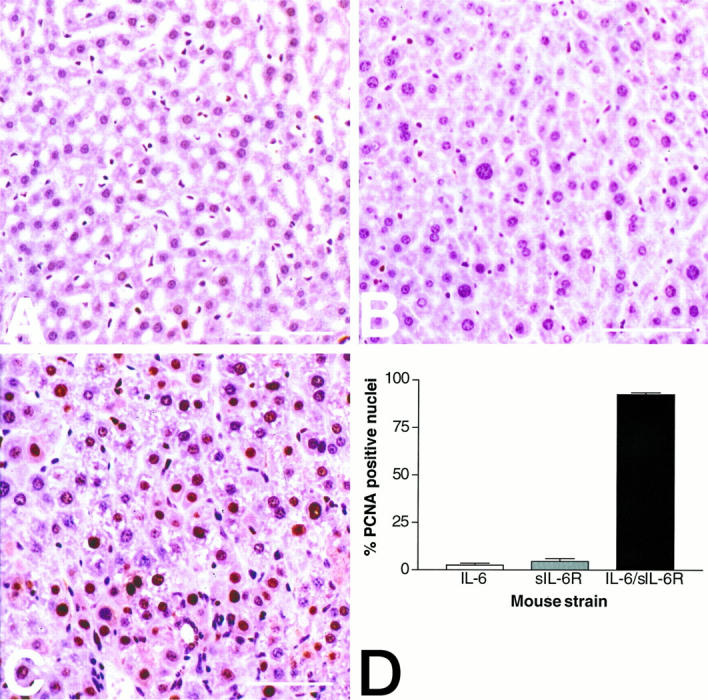
Increased hepatocellular proliferation in IL-6/sIL-6R double-transgenic mice. C: Highly activated hepatocellular proliferation in an IL-6/sIL-6R double-transgenic mouse (7 weeks of age) with numerous PCNA-positive hepatocellular nuclei. By contrast, the number of PCNA-positive hepatocytes is low in a 7-week-old IL-6 single-transgenic mouse (A) and in a 7-week-old sIL-6R single-transgenic mouse (B). D: Graphic representation of the semiquantitative evaluation of hepatocellular proliferation in IL-6 single-transgenic mice (white bar), in sIL-6R single-transgenic mice (gray bar), and in IL-6/sIL-6R double-transgenic mice (black bar). Two animals were analyzed per strain. The percentage of PCNA-positive hepatocellular nuclei determined by evaluating 500 hepatocytes per animal ± standard deviation is presented in the figure.
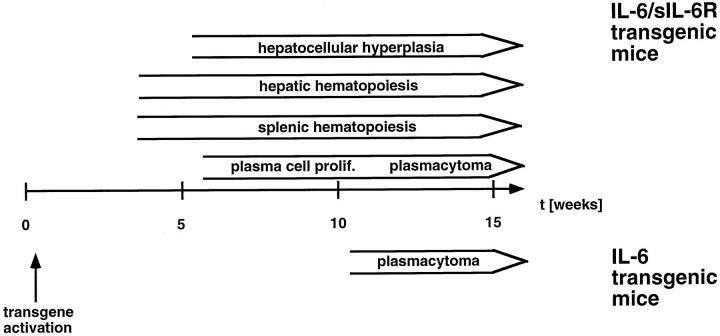
Figure 7.
Schematic chronology of pathology in IL-6/sIL-6R double-transgenic mice compared with IL-6 transgenic mice.
Discussion
IL-6/sIL-6R double-transgenic mice differed from sIL-6R and IL-6 single-transgenic mice in three main aspects: 1) plasmacytoma development was significantly accelerated and aggravated compared with IL-6 single-transgenic mice; 2) extramedullary hematopoiesis was strongly activated first in the spleen and later in the liver, and subsequently all peripheral blood cell counts were highly increased; and 3) significant hepatocellular hyperplasia and secondary liver pathology, including peliosis and areactive necroses, were induced (Figure 7) ▶ .
The severe extramedullary hematopoiesis observed in IL-6/sIL-6R double-transgenic mice was not a compensatory event secondary to altered medullary hematopoiesis, given that analysis of femoral bone marrow sections in IL-6/sIL-6R mice showed neither a reduction nor altered composition of cellular components nor significant fibrosis. 22 Plasmacytoma infiltrates in the bone marrow were only found in a few mice and much later in life compared with the onset of extramedullary hematopoiesis. Thus, activated extramedullary hematopoiesis appears to be a direct consequence of combined IL-6/sIL-6R overexpression. This notion is supported by recent observations obtained in vitro that the expansion of human hematopoietic progenitor cells is enhanced by IL-6/sIL-6R but not by IL-6 alone. 14,23 The extramedullary hematopoiesis observed in IL-6/sIL-6R transgenic mice indicates that IL-6/sIL-6R complexes are potent stimulators of hematopoietic progenitor cells in vivo and that these cells do not express IL-6R and therefore are not responsive to IL-6 alone.
Interestingly, significant extramedullary hematopoiesis was only detectable in the primary hematopoietic organs, spleen and liver, but not elsewhere in the body. Thus, it can be hypothesized that activated hematopoiesis may arise from persisting hematopoietic progenitor cells in these organs. In keeping with that, activation of splenic hematopoiesis preceded and predominated hepatic hematopoiesis, reflecting significant pre-existing splenic hematopoiesis and the absence or rarity of hematopoietic progenitor cells in normal adult liver. Furthermore, activated and pre-existing hematopoiesis reflected the differential composition of hepatic and splenic hematopoiesis in that splenic hematopoiesis showed a balanced composition of all three lines (granulopoiesis, erythropoiesis, megakaryocytes), whereas hepatic hematopoiesis was predominantly granulopoietic. Nevertheless, it cannot be excluded that IL-6/sIL-6R signaling acts preferentially on circulating hematopoietic stem cells and that specific homing factors recruit these cells either to the liver or to the spleen and regulate their differentiation.
It has recently been demonstrated that the presence of sIL-6R on the plasma membrane of hepatocytes, which are responsive toward IL-6, leads to their sensitization with regard to the IL-6 stimulation. 12 Moreover, the time course of the IL-6-induced acute-phase response in the liver was drastically prolonged. 12 In this respect, the earlier onset and increased occurrence rate of plasmacytomas in IL-6/sIL-6R transgenic mice in comparison with IL-6 transgenic mice 16,17 can be explained by the presence of the sIL-6R. This raises the question as to whether the sIL-6R or soluble cytokine receptors of other IL-6 family members contribute to pathophysiological manifestations caused by this cytokine family. 24
IL-6 is known to be involved in bone diseases and hematologic disorders, such as Castleman’s disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) syndrome, and multiple myeloma. 24 So far, however, little information is available as to whether in vivo concentrations of soluble cytokine receptors correlate with the course of these diseases. In three recent clinical studies, the presence of elevated levels of the sIL-6R was associated with reduced therapeutic response, 25 increased disease activity, 26 and poor survival 27 in patients with plasmacytomas. However, further clinical studies will have to address the question as to whether serum sIL-6R concentrations in patients suffering from multiple myeloma correlate with clinical stage and disease progression and thus influence therapeutic aggressiveness.
It has recently been shown that the sIL-6R acts predominantly in a paracrine rather than in a systemic fashion. 28 Therefore, local concentrations of soluble cytokine receptors appear to be even more relevant than serum concentrations. Highly elevated levels of sIL-6R have been detected in patients with chronic lymphocytic leukemia and leukemic centrocytic lymphoma. 29 From the in vivo effect of coordinate IL-6/sIL-6R overexpression on plasmacytoma development, it can be hypothesized that elevated levels of soluble cytokine receptors of the IL-6 family lead to increased severeness of IL-6-mediated diseases.
In contrast to IL-6 and sIL-6R single-transgenic mice, severe liver pathology was observed only in IL-6/sIL-6R double-transgenic mice from the age of 4 weeks on. Peliosis and necrosis, although sometimes significant, appear to be rather secondary phenomena due to disturbed blood flow caused either by altered blood composition or hepatocellular hyperplasia. Detailed proliferation assays have clearly shown that increased hepatocellular proliferation occurs independently of hepatic necrosis and hematopoiesis. These results demonstrate that the highly increased hepatocellular proliferation is a direct consequence of the constitutive overexpression of IL-6 and the sIL-6R, leading to a permanent stimulation of gp130 molecules on hepatocytes. At present, however, it is unclear as to whether this effect is mediated directly by IL-6/sIL-6R or indirectly, eg, by activation of hepatocellular growth-stimulating factors in other cell types such as extramedullary hematopoietic cells.
Two recent studies have suggested a role of IL-6 in liver cell proliferation. First, Cressman et al 30 have shown that IL-6-deficient mice have a severely impaired capacity for liver regeneration after partial hepatectomy. Secondly, after partial hepatectomy in rats, there is a marked rise of TNF-α followed by an increase of IL-6-serum levels, suggesting an active role of TNF-α and IL-6 in liver regeneration. 31 DNA synthesis as well as STAT3 and nuclear factor-κB activation is severely compromised in TNF-α type 1 receptor-deficient mice after partial hepatectomy. 32 These findings can be corrected after the injection of IL-6. 32 These data suggest that TNF-α acts upstream of IL-6 and that one of its major roles could be the regulation of IL-6 secretion.
We have now for the first time demonstrated that the combination of IL-6 and the soluble IL-6R synergize to promote liver cell hyperplasia in vivo. After hepatectomy and perhaps in clinical situations associated with acute liver failure, there might be a significant production of IL-6 and the soluble IL-6R from different cells (Kupffer cells, hepatocytes) in the remaining liver. This could be an intrinsic mechanism of the organ to initiate and drive liver regeneration. The presented in vivo data now provide direct evidence that liver cells proliferate in vivo in response to the combination of IL-6 and sIL-6R, but not in response to IL-6 alone.
Acknowledgments
The authors thank K. Petmecky and B. Gilberg for excellent technical assistance and S. Hunger for help with typing the manuscript.
Footnotes
Address reprint requests to Dr. Malte Peters, I. Department of Medicine, Division of Pathophysiology, University of Mainz, Obere Zahlbacher Str. 63, D-55101 Mainz, Germany. E-mail: mpeters@mail.uni-mainz.de.
Supported by grants from the Deutsche Forschungsgemeinschaft, Bonn, Germany (to MP, SR-J) and from the Stiftung Rheinland-Pfalz für Innovation (PS, SR-J). MB is supported by the Boehringer Ingelheim Foundation.
Peter Schirmacher and Malte Peters contributed equally to this work.
References
- 1.Akira S, Taga T, Kishimoto T: Interleukin-6 in biology and medicine. Adv Immunol 1993, 54:1-78 [DOI] [PubMed] [Google Scholar]
- 2.Bauer J, Herrmann F: Interleukin-6 in clinical medicine. Ann Hematol 1991, 62:203-210 [DOI] [PubMed] [Google Scholar]
- 3.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H: Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA 1987, 84:7251-7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grötzinger J, Kurapkat G, Wollmer A, Kalai M, Rose-John S: The family of the IL-6-type cytokines: specificity and promiscuity of the receptor complexes. Proteins Struct Funct Genet 1997, 27:96-109 [PubMed] [Google Scholar]
- 5.Hibi M, Nakajima K, Hirano T: IL-6 cytokine family and signal transduction: a model of the cytokine system. J Mol Med 1996, 74:1-12 [DOI] [PubMed] [Google Scholar]
- 6.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T: Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 1996, 5:449-460 [DOI] [PubMed] [Google Scholar]
- 7.Schindler C, Darnell JE: Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem 1995, 64:621-651 [DOI] [PubMed] [Google Scholar]
- 8.Rose-John S, Heinrich PC: Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J 1994, 300:281-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müllberg J, Dittrich E, Graeve L, Gerhartz C, Yasukawa K, Taga T, Kishimoto T, Heinrich PC, Rose-John S: Differential shedding of the two subunits of the interleukin-6 receptor. FEBS Lett 1993, 332:174-178 [DOI] [PubMed] [Google Scholar]
- 10.Müllberg J, Durie FH, Otten Evans C, Alderson MR, Rose-John S, Cosman D, Black RA, Mohler KM: A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol 1995, 155:5198-5205 [PubMed] [Google Scholar]
- 11.Müllberg J, Schooltink H, Stoyan T, Gunther M, Graeve L, Buse G, Mackiewicz A, Heinrich PC, Rose-John S: The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol 1993, 23:473-480 [DOI] [PubMed] [Google Scholar]
- 12.Peters M, Jacobs S, Ehlers M, Vollmer P, Müllberg J, Wolf E, Brem G, Meyer zum Büschenfelde KH, Rose-John S: The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med 1996, 183:1399-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.März P, Herget T, Lang E, Otten U, Rose-John S: Activation of gp130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur J Neurosci 1997, 9:2765-2773 [DOI] [PubMed] [Google Scholar]
- 14.Sui X, Tsuji K, Tanaka R, Tajima S, Muraoka K, Ebihara Y, Ikebuchi K, Yasukawa K, Taga T, Kishimoto T, Nagahata T: gp130 and c-Kit signalings synergize for ex vivo expansion of human primitive hemopoietic progenitor cells. Proc Natl Acad Sci USA 1995, 92:2859-2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBenedetti F, Massa M, Pignatti P, Albani S, Novick D, Martini A: Serum soluble interleukin-6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest 1994, 93:2114-2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T: IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA 1989, 86:7547-7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J-I, Yamamura K-I, Hirano T, Kishimoto T: Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA 1992, 89:232-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dam M, Müllberg J, Schooltink H, Stoyan T, Brakenhoff JP, Graeve L, Heinrich PC, Rose-John S: Structure-function analysis of interleukin-6 utilizing human/murine chimeric molecules: involvement of two separate domains in receptor binding. J Biol Chem 1993, 268:15285-15290 [PubMed] [Google Scholar]
- 19.Fattori E, Della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, Ciliberto G: Development of progressive kidney damage and myeloma kidney in interleukin-6 transgenic mice. Blood 1994, 83:2570-2579 [PubMed] [Google Scholar]
- 20.Bartels H, Herbort H, Jungermann K: Predominant periportal expression of the phosphoenolpyrovate carboxykinase and tyrosine aminotransferase genes in rat liver. Histochemistry 1990, 99:303-309 [DOI] [PubMed] [Google Scholar]
- 21.McGrane MM, deVente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman RM, Hanson RW: Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem 1988, 263:11443-11451 [PubMed] [Google Scholar]
- 22.Peters M, Schirmacher P, Goldschmitt J, Odenthal M, Peschel C, Dienes HP, Fattori E, Ciliberto G, Meyer zum Büschenfelde KH, Rose-John S: Extramedullary expansion of hematopoietic progenitor cells in IL-6/sIL-6R double transgenic mice. J Exp Med 1997, 185:755-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajima S, Tsuji K, Ebihara Y, Sui X, Tanaka R, Muraoka K, Yoshida M, Yamada K, Yasukawa K, Taga T, Kishimoto T, Nakahata T: Analysis of interleukin-6 receptor and gp130 expressions and proliferative capability of human CD34+ cells. J Exp Med 1996, 184:1357-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallen K-J, Meyer zum Büschenfelde K-H, Rose-John S: The therapeutic potential of interleukin-6 hyperagonists and antagonists. Exp Opin Invest Drugs 1997, 6:237-266 [DOI] [PubMed] [Google Scholar]
- 25.Kyrtsonis MC, Dedoussis G, Zervas C, Perifanis V, Baxevanis C, Stamatelou M, Maniatis A: Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. Br J Haematol 1996, 93:398-400 [DOI] [PubMed] [Google Scholar]
- 26.Papadaki H, Kyriakou D, Foudoulakis A, Markidou F, Alexandrakis M, Eliopoulos GD: Serum levels of soluble IL-6 receptor in multiple myeloma as indicator of disease activity. Acta Haematol 1997, 97:191-195 [DOI] [PubMed] [Google Scholar]
- 27.Pulkki K, Pelliniemi TT, Rajamaki A, Tienhaara A, Laakso M, Lahtinen R: Soluble interleukin-6 receptor as a prognostic factor in multiple myeloma: Finnish leukaemia group. Br J Haematol 1996, 92:370-374 [DOI] [PubMed] [Google Scholar]
- 28.Peters M, Odenthal M, Schirmacher P, Blessing M, Ciliberto G, Meyer zum Büschenfelde KH, Rose-John S: Soluble IL-6 receptor leads to a paracrine modulation of the hepatic acute phase response in double transgenic mice. J Immunol 1997, 159:1474-1481 [PubMed] [Google Scholar]
- 29.Lavabre Bertrand T, Exbrayat C, Liautard J, Gaillard JP, Baskevitch PP, Poujol N, Duperray C, Bourquard P, Brochier J: Detection of membrane and soluble interleukin-6 receptor in lymphoid malignancies. Br J Haematol 1995, 91:871-877 [DOI] [PubMed] [Google Scholar]
- 30.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R: Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996, 274:1379-1383 [DOI] [PubMed] [Google Scholar]
- 31.Trautwein C, Rakemann T, Niehof M, Rose-John S, Manns MP: Acute-phase response factor, increased binding, and target gene transcription during liver regeneration. Gastroenterology 1996, 110:1854-1862 [DOI] [PubMed] [Google Scholar]
- 32.Yamada Y, Kirillova I, Peschon JJ, Fausto N: Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 1997, 94:1441-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]