Abstract
Extrahepatic biliary atresia is a severe neonatal liver disease resulting from a sclerosing cholangiopathy of unknown etiology. Although biliary obstruction may be surgically corrected by a “Kasai” hepatoportoenterostomy, most patients still develop progressive hepatic fibrosis, although the source of increased collagen deposition is unclear. This study examined the role of hepatic stellate cells (HSCs) and assessed the source of transforming growth factor-β (TGF-β) production in hepatic fibrogenesis in patients with biliary atresia. Liver biopsies from 18 biliary atresia patients (including 5 pre- and post-Kasai) were subjected to immunohistochemistry for α-smooth muscle actin and in situ hybridization for either procollagen α1 (I) mRNA or TGF-β1 mRNA. Sections were also subjected to immunohistochemistry for active TGF-β1 protein. The role of Kupffer cells in TGF-β1 production was assessed by immunohistochemistry for CD68. Procollagen α1 (I) mRNA was colocalized to α-smooth muscle actin-positive HSCs within the region of increased collagen protein deposition in fibrotic septa and surrounding hyperplastic bile ducts. The number of activated HSCs was decreased in only one post-Kasai biopsy. TGF-β1 mRNA expression was demonstrated in bile duct epithelial cells and activated HSCs and in hepatocytes in close proximity to fibrotic septa. Active TGF-β1 protein was demonstrated in bile duct epithelial cells and activated HSCs. This study provides evidence that activated HSCs are responsible for increased collagen production in patients with biliary atresia and therefore play a definitive role in the fibrogenic process. We have also shown that bile duct epithelial cells, HSCs, and hepatocytes are all involved in the production of the profibrogenic cytokine, TGF-β1.
Extrahepatic biliary atresia is a progressive, sclerosing, inflammatory process in neonates, causing atresia of all or part of the extrahepatic biliary system and rapidly extending to involve the major intrahepatic biliary ducts. 1,2 This bile duct obliteration may be relieved by hepatoportoenterostomy (HPE) or the “Kasai procedure,” 3-5 in which >80% of infants will develop some biliary flow, particularly if HPE is performed within 60 days of birth (reviewed in Ref. 6 ). However, the majority of patients still develop progressive hepatic fibrosis, with approximately one-third developing liver failure and requiring liver transplantation within 12 to 14 months and a further one-third by the teenage years, and the remainder will live with some form of liver disease, including mild transaminase elevations, recurrent cholangitis, or an inactive cirrhosis with portal hypertension. 7-9 Overall, biliary atresia accounts for up to 70% of all pediatric cases progressing to liver transplantation. 10,11 Therefore, despite surgical relief of the obstruction deposition of collagen, progressive hepatic fibrosis and portal hypertension usually occur. Indeed, the development of hepatic fibrosis in this disease is more rapid and aggressive than any other disorder in adults.
The mechanisms responsible for increased collagen production and hepatic fibrosis in neonatal liver diseases such as biliary atresia are unknown. A population of nonparenchymal cells known as hepatic stellate cells (HSCs) have been shown to be “activated” and therefore responsible for the increased production of type I collagen leading to hepatic fibrosis in pathological conditions of the adult human liver, 12-14 and in a number of experimental models of adult liver injury, 15-20 including cholestasis. 21-24 In liver injury, HSCs are transformed into myofibroblasts (activated HSCs), which produce increased levels of fibrillar collagen and express an intracellular microfilament protein, α-smooth muscle actin (SMA), which is traditionally used as a marker protein of the activated HSC phenotype (reviewed in Ref. 25 ). Activated HSCs also express a number of different cytokine receptors, including the transforming growth factor (TGF-β1) receptor. 26 TGF-β1 is an important profibrogenic cytokine and has been shown to increase collagen gene expression at the transcriptional level via binding of the transcription factors AP-1 and Sp-1. 27,28
This study was designed to evaluate whether activated HSCs are the cellular source of increased collagen production in infants with biliary atresia and to determine the role of hepatic parenchymal and nonparenchymal cells in the expression of the profibrogenic cytokine, TGF-β1, in this age group. We were particularly interested in bile duct epithelia in view of the unique bile ductule hyperplasia seen in this disorder.
Materials and Methods
Biopsy Collection and Patient Data
Eighteen patients (6 male and 12 female) with extrahepatic biliary atresia and failed HPE were studied. Diagnosis of extrahepatic biliary atresia was confirmed in all cases at the time of HPE by histopathological evaluation, which revealed characteristic observations of portal or perilobular fibrosis, ductular proliferation, and canalicular and cellular biliary stasis. 29 All patients were referred for liver transplantation assessment because of progressive liver disease, and orthotopic liver transplantation was performed at a mean age of 2.6 ± 0.63 years (range, 7 months to 11.75 years).
Twenty-three percutaneous liver biopsies, fixed in formalin and embedded in paraffin, were studied in these 18 patients. In 5 patients, both pre- and post-HPE biopsies were collected at a mean age of 1.8 ± 0.4 (mean ± standard error) and 8.2 ± 0.4 months, respectively. In the remaining 13 patients, liver biopsies were obtained at a mean age of 2.5 ± 0.8 years.
In Situ Hybridization
For detection of procollagen α1 (I) mRNA, a 1500-bp fragment of human procollagen α1 (I) cDNA was subcloned into pGEM 11Z vector. For detection of TGF-β1 mRNA, a 1000-bp fragment of human TGF-β1 cDNA was subcloned into pGEM-3zf(+) vector. Both fragments were then subjected to alkaline hydrolysis to produce a 300-bp fragment for use in in situ hybridization.
Digoxigenin-labeled riboprobes, for sense (control) and antisense, were produced for both procollagen α1 (I) and TGF-β1 by in vitro transcription with SP6 and T7 polymerases. In situ hybridization was performed on 5-μm human liver sections, deparaffinized by xylol, and rehydrated by gradient alcohol before exposure to hydrochloric acid (0.2 mol/L), as previously described. 30 Sections were permeabilized with 5 μg/ml proteinase K at 37°C for 15 minutes, followed by fixation in 4% paraformaldehyde for 20 minutes at room temperature. Prehybridization (50% formamide, 1% sodium dodecyl sulfate, 5× standard saline citrate, 500 μg/ml tRNA, and 50 μg/ml heparin) was performed at 70°C for 3 hours followed by hybridization for 16 hours at 70°C in a solution containing 1 μg/ml of digoxigenin-labeled riboprobe. Sections were then washed to remove unbound probe and incubated with alkaline phosphatase-conjugated anti-digoxigenin polyclonal sera (1:200) at room temperature for 2 hours. Unbound antibody was removed by washes, followed by visualization with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate in the dark at room temperature for 16 hours. Unbound complex was removed by washing, and sections were subjected to immunohistochemistry for SMA as previously described 31 to colocalize procollagen α1 (I) mRNA to activated HSCs (see Immunohistochemistry, below).
Immunohistochemistry
SMA
All liver sections were incubated with a mouse monoclonal anti-SMA primary antibody (1:400, clone 1A4; Sigma Chemical Co., St. Louis, MO), followed by a biotinylated rabbit anti-mouse immunoglobulin G as the secondary antibody, as previously described. 12 The detection system used was a DAKO (Glostrup, Denmark) streptavidin-biotin complex/horseradish peroxidase kit, with 3,3-diaminobenzidine tetrahydrochloride as the chromogenic substrate. Sections were counterstained with eosin.
Biopsies were graded histologically for SMA expression as previously described 24 using the following classification: 0, normal staining pattern for SMA with expression in smooth muscle cells within portal blood vessels only; 1+, mild perisinusoidal staining for SMA within activated HSCs; 2+, periportal staining for SMA, proliferation of SMA-expressing HSCs, and moderate SMA expression in perisinusoidal HSCs; 3+, septal and bridging SMA expression between portal tracts; and 4+, SMA expression within cirrhotic bands linking portal tracts.
TGF-β
All liver sections were subjected to antigen retrieval by heating in a microwave oven on high power for 8 minutes in 0.01 mol/L citrate buffer (ph 6.0) and then incubated with a mouse monoclonal anti-TGF-β1 -β2, and -β3 primary antibody to active TGF-β (150 μg/ml; Genzyme Diagnostics, Cambridge, MA) for the cellular localization of TGF-β protein. The sections were then subjected to the identical detection methodology as for SMA. 12
CD68
All liver sections were subjected to antigen retrieval by autoclaving in 0.01 mol/L citrate buffer (pH 6.0) at 121°C for 10 minutes. Immunohistochemistry for CD68, a specific marker for Kupffer cells, was performed by incubating sections with a mouse monoclonal anti-CD68 primary antibody (1:50, clone PG-M1; DAKO), followed by identical detection methodology as described for SMA. 12 This technique allowed assessment of Kupffer cells as a potential source of TGF-β1 mRNA in the livers of patients with biliary atresia.
The negative controls used for each immunohistochemical assessment used nonimmune normal mouse immunoglobulin G antisera (Santa Cruz, San Diego, CA) in place of the primary antibody for either SMA, TGF-β, or CD68 (results not shown).
Hematoxylin/Van Gieson Histology
All sections were subjected to hematoxylin/Van Gieson stain for the detection of collagen protein deposition.
Results
Hepatic Histopathological Assessment of Biliary Atresia
There was evidence of canalicular and cellular biliary stasis, variable inflammatory changes, bile duct hyperplasia with expanded portal tracts, periportal and bridging fibrosis, and mild to severe cirrhosis in all biopsies examined, including pre- and post-Kasai HPE livers.
Identification of Activated HSCs: Cellular Source of Procollagen α1 (I) mRNA Expression
Liver biopsies were subjected to immunohistochemistry for the intracellular microfilament protein, SMA, which has been shown to be an excellent marker for the activated HSC phenotype. Activated HSCs were demonstrated morphologically both by their stellate shape and by the expression of SMA (Figure 1A) ▶ in the extracellular matrix surrounding hyperplastic bile ducts and within fibrous septa bridging between portal tracts (Figure 1B) ▶ . Furthermore, procollagen α1 (I) mRNA expression was shown to colocalize to SMA-positive HSCs (Figure 1 ▶ , A and B), demonstrating that activated HSCs are the cellular source of increased collagen leading to hepatic fibrosis in biliary atresia. Procollagen α1 (I) mRNA expression was not seen in hepatocytes, bile duct epithelial cells, or smooth muscle cells of the portal tract vasculature. Procollagen α1 (I) mRNA signal specificity for the antisense probe was demonstrated by the absence of signal over SMA-expressing stellate cells using the sense probe (results not shown).
Figure 1.
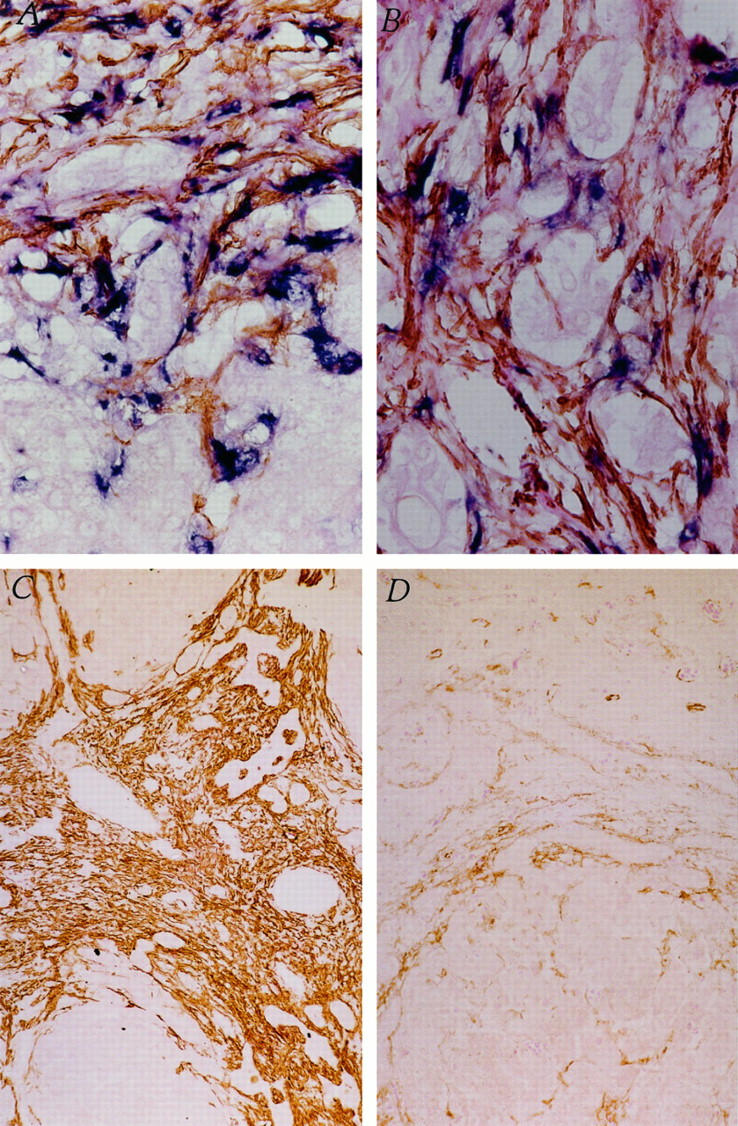
Identification of activated HSCs as cellular source of increased collagen production in biliary atresia. A: Colocalization of SMA (brown) and procollagen α1 (I) mRNA (blue) within stellate-shaped activated HSCs in a liver biopsy from an infant with biliary atresia, using immunohistochemistry and in situ hybridization, respectively. Original magnification, ×400. B: Bile duct hyperplasia within fibrotic bands in a liver biopsy from an infant with biliary atresia. Intense staining for SMA (brown) and procollagen α1 (I) mRNA (blue), colocalized in activated HSCs. Original magnification, ×400. C: Immunohistochemistry for SMA (brown) within activated HSCs surrounding hyperplastic bile ducts in a pre-Kasai HPE. D: A post-Kasai HPE liver biopsy from the same infant shown in C with biliary atresia. Original magnification, ×100.
Pre- and Post-Kasai HPE
Five patients were examined both pre- and post-Kasai HPE for evidence of HSC activation. Only one of five patients showed a decrease in the expression of SMA (grade 4+ to 2+) and hence in the number of activated HSCs surrounding hyperplastic bile ducts and within fibrous bridging septa after HPE (Figure 1 ▶ , C and D). All five of these patients subsequently progressed to liver transplantation.
Colocalization of Activated HSCs and Increased Collagen Protein Deposition
Liver biopsies were examined histologically for collagen protein deposition using hematoxylin/Van Gieson stain. Figure 2A ▶ demonstrates grossly enlarged bile ducts surrounded by excessive collagen protein deposition. Figure 2B ▶ demonstrates increased numbers of activated HSCs showing colocalization of SMA and procollagen α1 (I) mRNA in the identical region of increased collagen protein deposition. Elevated numbers of procollagen α1 (I) mRNA-expressing, activated HSCs (Figure 2D) ▶ were also demonstrated in the identical region of collagen protein deposition within fibrous tissue between portal tracts (Figure 2C) ▶ .
Figure 2.
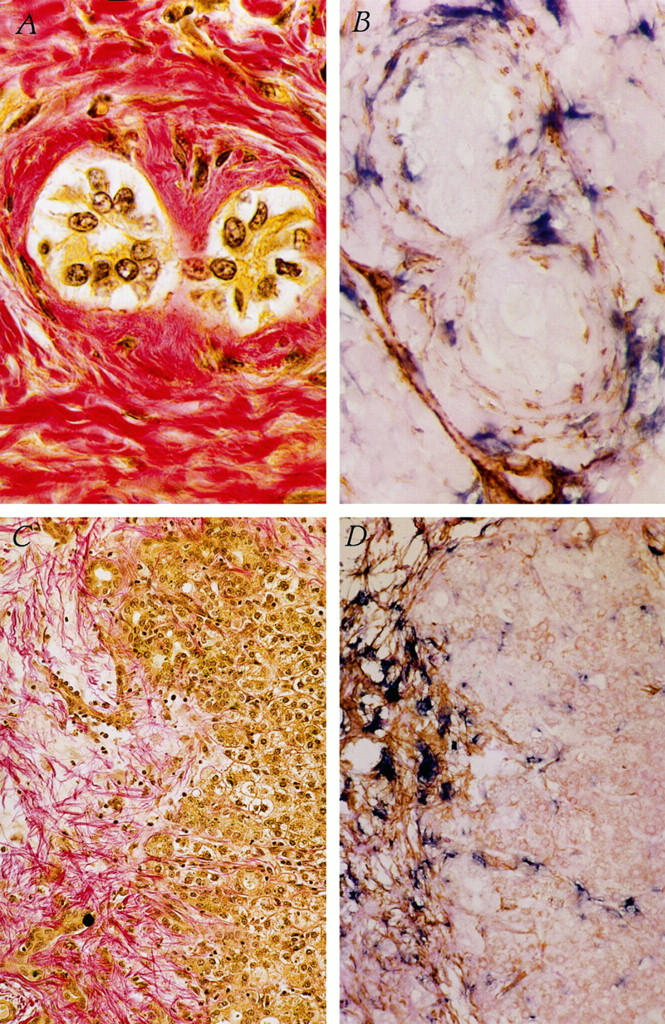
Colocalization of activated HSCs to collagen protein deposition in biliary atresia liver. A: Collagen protein deposition surrounding two bile ducts in liver biopsy from an infant with biliary atresia (pink). B: Activated HSCs surrounding bile ducts, showing colocalization of SMA (brown) and procollagen α1 (I) mRNA (blue). Original magnification, ×1000. C and D: Fibrotic region in liver biopsy from an infant with biliary atresia showing deposition of collagen protein fibrils (C, pink) and activated HSCs (D), as evidenced by colocalization of SMA (brown) and procollagen α1 (I) mRNA (blue). Original magnification, ×200.
Cellular Source of the Profibrogenic Cytokine, TGF-β
Immunohistochemistry for TGF-β
Immunohistochemistry for TGF-β protein revealed that TGF-β was predominantly expressed by bile duct epithelial cells within hyperplastic bile ducts and also by activated HSCs in the extracellular matrix of scar tissue (Figure 3A) ▶ . TGF-β was also expressed to a lesser extent in hepatocytes in close proximity to areas of fibrosis at the interface of the regenerative nodule (Figure 3B) ▶ . TGF-β expression was not evident in hepatocytes at a distance from scar tissue (results not shown).
Figure 3.
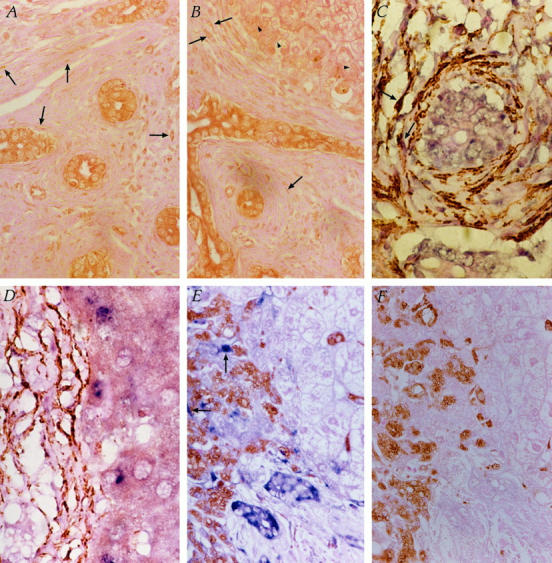
Identification of cellular source of TGF-β. A: Immunohistochemistry for TGF-β protein (brown) predominantly expressed within bile duct epithelial cells and also in activated HSCs in fibrotic extracellular matrix (arrows) and, to a lesser extent, in hepatocytes at the interface of fibrotic scar tissue (B, arrowheads). Original magnification, ×400. C: In situ hybridization for TGF-β1 mRNA (blue) expressed in bile duct epithelial cells and colocalized to SMA (brown) within activated HSCs (arrows). D: TGF-β1 mRNA (blue) was also demonstrated in hepatocytes at the interface of the regenerative nodule and fibrous scar tissue. Original magnification, ×1000. E: In situ hybridization for TGF-β1 mRNA (blue) expressed in bile duct epithelial cells and HSCs (arrows) within scar tissue. Immunohistochemistry for CD68, used as a marker of Kupffer cells (brown), demonstrated that Kupffer cells were present in the sinusoidal and perisinusoidal regions of the regenerative hepatocyte nodules, but they did not express TGF-β1 mRNA. In addition, CD68-positive macrophages within scar tissue did not contribute to TGF-β1 mRNA expression. Original magnification, ×400. F: TGF-β1 mRNA signal specificity for the antisense probe in E was demonstrated by the absence of signal over bile duct epithelial cells and HSCs using the sense probe. This serial section of E was also subjected to immunohistochemistry for CD68.
In Situ Hybridization for TGF-β
In situ hybridization for TGF-β1 mRNA demonstrated that TGF-β1 mRNA was expressed in bile duct epithelial cells within hyperplastic bile ducts (Figure 3 ▶ , C and E) and was also observed colocalized to SMA-positive HSCs (Figure 3C) ▶ . Increased expression of TGF-β1 mRNA was also demonstrated in hepatocytes along the interface of the regenerative nodules and fibrotic scar tissue (Figure 3D) ▶ . TGF-β1 mRNA was not detected in hepatocytes within the acinus distal from scar tissue (results not shown).
Role of Kupffer Cells in TGF-β Production
Figure 3E ▶ demonstrates the localization of increased numbers of Kupffer cells as assessed by CD68 immunohistochemistry, in sinusoidal and perisinusoidal regions of the regenerative hepatocyte nodule, and within scar tissue. TGF-β1 mRNA expression was not detected in CD68-positive Kupffer cells in close proximity to the interface of the fibrotic scar tissue, indicating that Kupffer cells may not contribute to the TGF-β1 mRNA expression seen in Figure 3D ▶ . In addition, CD68-positive cells within the scar tissue did not demonstrate colocalization of TGF-β1 mRNA, and therefore, these cells do not appear to play a role in collagen production by HSCs surrounding bile ducts. TGF-β1 mRNA signal specificity for the antisense probe was demonstrated by the absence of signal over bile duct epithelial cells, hepatocytes, and HSCs using the sense probe (Figure 3F) ▶ .
Discussion
This study has demonstrated that activated HSCs, identified by the colocalization of procollagen α1 (I) mRNA expression to cells expressing the HSC activation marker, SMA, are responsible for the production of increased levels of type I collagen leading to hepatic fibrosis in young patients with biliary atresia. In addition, this study has shown that the hyperplastic bile duct epithelium is the predominant source of the profibrogenic cytokine TGF-β1 within the portal tract and that hepatocytes produce increased levels of TGF-β1 along fibrous septa bridging portal tracts, which forms the fibrotic scar leading to cirrhosis. TGF-β1 was also produced by activated HSCs within the fibrous matrix but to a lesser degree than other cells. Finally, this study has demonstrated that the number of activated HSCs was decreased in only one of five patients after Kasai HPE.
Many different theories have been proposed to explain the pathogenesis of biliary atresia, including infectious, genetic, and immune-mediated etiologies, although convincing evidence to support these hypotheses is lacking (reviewed in Refs. 6 and 32 ). Furthermore, there is a paucity of knowledge concerning the mechanisms involved in the fibrogenesis associated with this condition. In a recent study, Malizia and colleagues 33 examined five patients with advanced biliary atresia and cirrhosis and showed increased expression of procollagen α1 (I) mRNA associated with “spindle-shaped fibroblast-like cells” in the fibrous tissue surrounding regenerative hepatocyte nodules and some proliferating bile ductules. The identification of the responsible cell type was not established in this study, although the cells were described as “vimentin-positive mesenchymal cells,” 33 which could describe either Kupffer cells, endothelial cells, 34 or ductal plate or biliary epithelial cells. 35 These authors also described the collagen-producing cells as desmin negative, suggesting that HSCs may not be the major cell type involved in fibrogenesis, based on a previous report that identified human HSCs as desmin-positive cells. 36 However, the literature on desmin reactivity of human HSCs is conflicting. Other studies have shown that the detection of desmin in human HSCs, either in vitro or in vivo, is quite variable and often unsuccessful. 12,37,38 Our study, however, clearly documents the identification of activated HSCs, as evidenced by both SMA expression and cell morphology, as the cellular source of increased procollagen α1 (I) mRNA in extrahepatic biliary atresia.
The hepatic histopathological presentation of biliary atresia is classically characterized by ductular proliferation, canalicular and cellular biliary stasis, swelling and vacuolization of biliary epithelial cells, portal tract edema and fibrosis, and monocytic inflammatory cell infiltration of portal tracts. 39 Although the mechanisms responsible for many of these phenomena are not known, portal fibrosis and cirrhosis are arguably the most damaging and have the greatest prognostic significance. It is now clear that activated HSCs are responsible for the increased production of type I collagen leading to hepatic fibrosis in biliary atresia similar to that of pathological conditions of the adult liver 12-14 and in experimental models of cholestatic liver injury. 21-24 The factors that are responsible for initiating the activation of HSCs are unclear, although it has been established that the profibrogenic cytokine, TGF-β1, and the proliferative cytokine, platelet-derived growth factor, are involved in perpetuating the activated HSC phenotype (reviewed in Ref. 14 ).
In the present study we have demonstrated that the bile duct epithelium is a major source of TGF-β1 in biliary atresia as evidenced by immunohistochemistry for active TGF-β protein and increased expression of TGF-β1 mRNA. We have also shown that activated HSCs surrounding hyperplastic bile ducts produce both TGF-β1 protein and mRNA. However, our results suggest that bile duct epithelial cells may be the predominant source of the TGF-β responsible for increased transcription of collagen type I genes in HSCs surrounding bile ductules leading to periductular fibrosis. Few previous studies have examined the cellular source of cytokine production in neonatal biliary obstruction. Milani and colleagues 40 demonstrated increased TGF-β2 mRNA in biliary epithelial cells and low levels of TGF-β1 transcripts in hepatocytes, mesenchymal cells, and some inflammatory cells in bile duct-ligated adult rats. Others have observed a significant increase in TGF-β1 immunohistochemistry in bile duct epithelium after ligation of the common bile duct. 41
In biliary atresia, Tan and colleagues 42 demonstrated increased TGF-β1 peptide immunoreactivity within bile duct structures at the porta hepatis and within intrahepatic portal tracts, whereas Malizia and colleagues 33 demonstrated TGF-β1 protein associated with the extracellular matrix in fibrous septa and in areas of periductular fibrosis. In addition, this group examined TGF-β1 mRNA expression and demonstrated increased message in scar tissue and associated with proliferating bile ductules at the interface of the regenerative nodules and the scar, although the precise cellular source of this TGF-β1 mRNA was not clearly defined. 33 They also found that bile ductules expressed increased levels of platelet-derived growth factor-A and -B mRNA.
We have also demonstrated increased expression of both TGF-β1 mRNA and active TGF-β protein by hepatocytes at the interface of the regenerative nodules and fibrous septa forming fibrotic and cirrhotic bands. We propose that the production of TGF-β at this interface may be intimately involved in the induction of collagen gene transcription by activated HSCs due to the close histological association between these two cell populations. Only one other study has previously observed TGF-β1 protein expression by hepatocytes in biliary atresia, although it is unclear whether this represented active TGF-β1. 42
Our study did not demonstrate a role for Kupffer cells in TGF-β1 production in biliary atresia. Although we demonstrated evidence of increased numbers of perisinusoidal CD68-positive macrophages within both regenerative hepatocyte nodules and scar tissue, Kupffer cells did not demonstrate TGF-β1 mRNA expression. Others have previously demonstrated hepatic Kupffer cell proliferation and monocyte migration to the liver in biliary atresia 43,44 and bile duct-ligated rats. 45 Tracy and colleagues have also shown increased numbers of resident CD68-positive Kupffer cells that also express CD14, which confers susceptibility to activation by low doses of lipopolysaccharide. 43 We did not show increased TGF-β1 expression by Kupffer cells, although they may take part in the local inflammatory response by releasing other cytokines such as tumor necrosis factor-α, interleukin-1, and interleukin-6. 43,46
Although the present study clearly implicates activated HSCs in the fibrogenic process and bile duct epithelial cells and hepatocytes in the production of the profibrogenic cytokine, TGF-β1, the mechanisms involved in HSC and bile duct proliferation and the induction of TGF-β1 remain elusive. Bile duct hyperplasia appears to be an early event in cholestatic liver injury, and some groups have suggested that in the bile duct-ligated rat, this may result from an increase in intraductal pressure. 47 Others suggest that circulating cholangiotrophic factors released from the liver in cholestasis may induce the bile duct proliferative response. 48 Supporting evidence is derived from studies that have demonstrated that the proliferation of biliary epithelial cells appears to accompany the increased hepatic expression of the growth-related proto-oncogenes, such as c-raf and c-erb-B2 48 and H-ras and c-myc 49 in bile duct-ligated rats.
More recent mechanistic hypotheses center on the injurious effect of hydrophobic bile acids on specific cell populations (reviewed in Ref. 6 ). Some groups have demonstrated increased levels of chenodeoxycholic acid in human cholestatic liver disease, 50 and others have reported the hepatotoxic effects of hydrophobic bile acids. 51,52 Varying the dose of chenodeoxycholic acid in vitro has been shown to induce either hepatocyte necrosis or apoptosis, 53,54 which may in turn alter mitochondrial function through the generation of oxygen free radicals. 51,55 Cholestatic hepatotoxicity may also be induced via the depletion of hepatic or mitochondrial antioxidants, such as vitamin E and glutathione. 56,57 Thus, it has been proposed that oxidant stress may play a major role in the induction of hepatocellular injury by bile acids such as chenodeoxycholic acid in cholestatic liver disease. 6,51,55 Hydrophobic bile acids and oxidant stress may also directly alter Kupffer cell or HSC viability and function. The result of any of these scenarios may be the induction of cytokine expression by injured hepatocytes or activated Kupffer cells, which may ultimately lead to the activation of HSCs and fibrogenesis. Additional investigations will be required to fully elucidate the association between increased biliary levels of hydrophobic bile acids, hepatocellular injury, and HSC activation in patients with biliary atresia.
The results of the present study suggest that important interactions exist between different hepatic cell populations, and these interactions are essential in the fibrogenic response in biliary atresia. In summary, this study has provided evidence that activated HSCs are responsible for increased collagen production in biliary atresia and are therefore involved in the development of hepatic fibrosis. This study has also shown that the profibrogenic cytokine, TGF-β1, is predominantly produced by bile duct epithelial cells and to a lesser extent by hepatocytes and activated HSCs. Although the results presented here have demonstrated the potential for bile duct epithelial cell-derived TGF-β to induce collagen production by periductular activated HSCs, the initiating stimulus to bile duct injury and the role of hydrophobic bile acid hepatotoxicity remains the subject of future investigation.
Footnotes
Address reprint requests to Dr. Grant A. Ramm, Laboratory Head, Hepatic Fibrosis Group, Clinical Sciences Unit, The Queensland Institute of Medical Research, 300 Herston Road, Brisbane, QLD 4029 Australia. E-mail: grantr@qimr.edu.au.
Supported by a Royal Children’s Hospital Foundation Research Award, Brisbane, Australia.
Darrell H. G. Crawford’s present address is Department of Gastroenterology and Hepatology, Princess Alexandra Hospital, Woolloongabba, Australia.
References
- 1.Balistreri WF: Neonatal cholestasis: medical progress. J Pediatr 1985, 106:171-184 [DOI] [PubMed] [Google Scholar]
- 2.Kasai M, Yakovac WC, Koop CE: Liver in congenital biliary atresia and neonatal hepatitis: a histopathological study. Arch Pathol 1962, 74:152-162 [PubMed] [Google Scholar]
- 3.Kasai M, Suzuki S: A new operation for “non-correctable” biliary atresia: hepatic portoenterostomy. Shujutsu 1959, 13:733-739 [Google Scholar]
- 4.Kasai M: Treatment of biliary atresia with special reference to hepatic portoenterostomy and its modifications. Prog Pediatr Surg 1974, 6:5-52 [PubMed] [Google Scholar]
- 5.Otte JB, de Ville de Goyet J, Reding R, Hausleithner V, Sokal E, Chardot C, Debande B: Sequential treatment of biliary atresia with Kasai portoenterostomy, and liver transplantation: a review. Hepatology 1994, 20(Suppl):41S-48S [DOI] [PubMed] [Google Scholar]
- 6.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ: Biliary atresia: current concepts and research directions. Hepatology 1996, 23:1682-1692 [DOI] [PubMed] [Google Scholar]
- 7.Kasai M, Mochizuki I, Ohkohchi N, Chiba T, Ohi R: Surgical limitations for biliary atresia: indications for liver transplantation. J Pediatr Surg 1989, 24:851-854 [DOI] [PubMed] [Google Scholar]
- 8.Laurent J, Gauthier F, Bernard O, Hadchouel M, Odievre M, Valayer J, Alagille D: Long-term outcome after surgery for biliary atresia: study of 40 patients surviving for more than 10 years. Gastroenterology 1990, 99:1793-1797 [DOI] [PubMed] [Google Scholar]
- 9.Stein JE, Vacanti JP: Biliary atresia and other disorders of the extrahepatic biliary tree. Suchy FJ eds. Liver Disease in Children. 1994, :pp 426-442 Mosby, St. Louis [Google Scholar]
- 10.Shepherd RW: Liver transplantation in children. Med J Aust 1990, 153:509-510 [PubMed] [Google Scholar]
- 11.Lynch SV, Akiyama T, Ong TH, Pillay SP, Balderson GA, Matsunami H, Shepherd RW, Cleghorn GJ, Patrick MK, Strong RW: Transplantation in children with biliary atresia. Transplant Proc 1992, 24:186-188 [PubMed] [Google Scholar]
- 12.Ramm GA, Crawford DHG, Powell LW, Walker NI, Fletcher LM, Halliday JW: Hepatic stellate cell activation in genetic hemochromatosis: lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol 1997, 26:584-592 [DOI] [PubMed] [Google Scholar]
- 13.Minato Y, Hasumura Y, Takeuchi J: The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology 1983, 3:559-566 [DOI] [PubMed] [Google Scholar]
- 14.Friedman SL: Hepatic stellate cells. Prog Liver Dis 1996, 14:101-130 [PubMed] [Google Scholar]
- 15.Ramm GA, Li SCY, Li L, Britton RS, O’Neill R, Kobayashi Y, Bacon BR: Chronic iron overload causes in vivo activation of rat lipocytes. Am J Physiol 1995, 268:G451-G458 [DOI] [PubMed] [Google Scholar]
- 16.Pietrangelo A, Gualdi R, Casalgrandi G, Geerts A, De Bleser P, Montosi G, Ventura E: Enhanced hepatic collagen type I mRNA expression into fat-storing cells in a rodent model of hemochromatosis. Hepatology 1994, 19:714-721 [DOI] [PubMed] [Google Scholar]
- 17.Rockey DC, Housset CN, Friedman SL: Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest 1993, 92:1795-1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiratori Y, Ichida T, Geerts A, Wisse E: Modulation of collagen synthesis by fat storing cells, isolated from CCl4- or vitamin A-treated rats. Dig Dis Sci 1987, 32:1281-1289 [DOI] [PubMed] [Google Scholar]
- 19.Takahara T, Kojima T, Miyabayashi C, Inoue K, Sasaki H, Muragaki Y, Ooshima A: Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat: immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest 1988, 59:509-521 [PubMed] [Google Scholar]
- 20.Matsuoka M, Zhang MY, Tsukamoto H: Sensitization of hepatic lipocytes by high-fat diet to stimulatory effects of Kupffer cell-derived factors: implication in alcoholic liver fibrogenesis. Hepatology 1990, 11:173-182 [DOI] [PubMed] [Google Scholar]
- 21.Maher JJ, McGuire RF: Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest 1990, 86:1641-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hines JE, Johnson SJ, Burt AD: In vivo responses of macrophages, and perisinusoidal cells to cholestatic liver injury. Am J Pathol 1993, 142:511-518 [PMC free article] [PubMed] [Google Scholar]
- 23.Ramm GA, Crawford DHG, Bridle KR, Britton RS, Bacon BR, Tracy TF, Jr: Biliary decompression results in rapid reversal of procollagen α1(I) mRNA gene expression in experimental biliary fibrosis. Hepatology 1996, 24:A463 [Google Scholar]
- 24.Olynyk JK, Yeoh GC, Ramm GA, Clarke SL, Hall PM, Britton RS, Bacon BR, Tracy TF: Gadolinium chloride suppresses hepatic oval cell proliferation in rats with biliary obstruction. Am J Pathol 1998, 152:347-352 [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman SL: The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 26.Friedman SL, Yamasaki G, Wong L: Modulation of transforming growth factor-β receptors of rat lipocytes during the hepatic wound healing response: enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem 1994, 269:10551-10558 [PubMed] [Google Scholar]
- 27.Armendariz-Borunda J, Simkevich CP, Roy N, Raghow R, Kang AH, Seyer JM: Activation of Ito cells involves regulation of AP-1 binding proteins and induction of type I collagen gene expression. Biochem J 1994, 304:817-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rippe RA, Almounajed G, Brenner DA: Sp-1 binding activity increases in activated Ito cells. Hepatology 1995, 251:241-251 [PubMed] [Google Scholar]
- 29.Koop CE: Biliary obstruction in the newborn. Surg Clin North Am 1976, 56:373-377 [DOI] [PubMed] [Google Scholar]
- 30.Rex M, Scotting PJ: Simultaneous detection of RNA and protein in tissue sections by nonradioactive in situ hybridization followed by immunohistochemistry. Biochemica 1994, 3:24-26 [Google Scholar]
- 31.Ooi LLPJ, Crawford DHG, Gotley DC, Clouston AD, Strong RW, Gobé GC, Halliday JW, Bridle KR, Ramm GA: Evidence that “myofibroblast-like” cells are the cellular source of capsular collagen in hepatocellular carcinoma. J Hepatol 1997, 26:798-807 [DOI] [PubMed] [Google Scholar]
- 32.Middlesworth W, Altman RP: Biliary atresia. Curr Opin Pediatr 1997, 9:265-269 [DOI] [PubMed] [Google Scholar]
- 33.Malizia G, Brunt EM, Peters MG, Rizzo A, Broekelmann TJ, McDonald JA: Growth factor and procollagen type I gene expression in human liver disease. Gastroenterology 1995, 108:145-156 [DOI] [PubMed] [Google Scholar]
- 34.Tsutsumi M, Takada A, Takase S: Characterization of desmin-positive rat liver sinusoidal cells. Hepatology 1987, 7:277-284 [DOI] [PubMed] [Google Scholar]
- 35.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA: Identification of bipotential progenitor cells in human liver development. Hepatology 1996, 23:476-481 [DOI] [PubMed] [Google Scholar]
- 36.Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G: Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology 1992, 15:234-243 [DOI] [PubMed] [Google Scholar]
- 37.Schmitt-Graff A, Kruger A, Bochard F, Gabbiani G, Denk H: Modulation of α smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human liver. Am J Pathol 1991, 138:1233-1242 [PMC free article] [PubMed] [Google Scholar]
- 38.Geerts A, De Bleser P, Hautekeete ML, Niki T, Wisse E: Fat-storing (Ito) cell biology. Arias IM Boyer JL Fausto N Jakoby WB Schachter D Shafritz DA eds. The Liver: Biology and Pathobiology. 1994, :pp 819-838 Raven, New York [Google Scholar]
- 39.McEvoy CF, Suchy FJ: Biliary tract disease in children. Pediatr Clin North Am 1996, 43:75-98 [DOI] [PubMed] [Google Scholar]
- 40.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C: Transforming growth factors β 1 and β 2 are differentially expressed in fibrotic liver disease. Am J Pathol 1991, 139:1221-1229 [PMC free article] [PubMed] [Google Scholar]
- 41.Saperstein LA, Jirtle RL, Farouk M, Thompson HJ, Chung KS, Meyers WC: Transforming growth factor-beta 1, and mannose 6-phosphate/insulin-like growth factor-II receptor expression during intrahepatic bile duct hyperplasia and biliary fibrosis in the rat. Hepatology 1994, 19:412-417 [PubMed] [Google Scholar]
- 42.Tan CEL, Chan VSW, Yong RYY, Vijayan V, Tan WL, Fook Chong SMC, Ho JMS, Cheng HH: Distortion of TGF-β1 peptide immunolocalization in biliary atresia: comparison with the normal pattern in the developing human intrahepatic bile duct system. Pathol Int 1995, 45:815-824 [DOI] [PubMed] [Google Scholar]
- 43.Tracy TF, Jr, Dillon P, Fox ES, Minnick K, Vogler C: The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg 1996, 31:121-126 [DOI] [PubMed] [Google Scholar]
- 44.Tracy TF, Jr, Fox ES: CD14-lipopolysaccharide receptor activation in hepatic macrophages following cholestatic liver injury. Surgery 1995, 118:371-377 [DOI] [PubMed] [Google Scholar]
- 45.Hines JE, Johnston SJ, Burt AD: In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am J Pathol 1993, 142:511-517 [PMC free article] [PubMed] [Google Scholar]
- 46.Roland CR, Goss JA, Mangino MJ, Hafenrichter D, Flye MW: Autoregulation by eicosanoids of human Kupffer cell secretory products: a study of interleukin-1, interleukin-6, tumor necrosis factor-α, transforming growth factor-β and nitric oxide. Ann Surg 1994, 219:389-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slott PA, Liu MH, Tavoloni N: Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology 1990, 99:466-477 [DOI] [PubMed] [Google Scholar]
- 48.Polimeno L, Azzarone A, Hua Zeng Q, Panella C, Subbotin V, Carr B, Bouzahzah B, Francavilla A, Starzl TE: Cell proliferation and oncogene expression after bile duct ligation in the rat: evidence of a specific growth effect on bile duct cells. Hepatology 1995, 21:1070-1078 [PMC free article] [PubMed] [Google Scholar]
- 49.Tracy TF, Jr, Goerke ME, Bailey PV, Sotelo-Avila C, Weber TR: Growth-related gene expression in early cholestatic liver injury. Surgery 1993, 114:532-537 [PubMed] [Google Scholar]
- 50.Greim H, Trulzsch D, Gzygan P, Rudick J, Hutterer F, Schaffner F, Popper H: Mechanisms of cholestasis: bile acids in human livers with or without biliary obstruction. Gastroenterology 1972, 63:846-850 [PubMed] [Google Scholar]
- 51.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM, Jr: Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology 1995, 109:1249-1256 [DOI] [PubMed] [Google Scholar]
- 52.Hofmann AF, Popper H: Ursodeoxycholic acid for primary biliary cirrhosis. Lancet 1987, 2:398-399 [DOI] [PubMed] [Google Scholar]
- 53.Sokol RJ, Devereaux M, Khandwala T, O’Brien K: Evidence for involvement of oxygen free radicals in bile acid toxicity to isolated rat hepatocytes. Hepatology 1993, 17:869-881 [PubMed] [Google Scholar]
- 54.Patel T, Bronk SF, Gores GJ: Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in hepatocytes. J Clin Invest 1994, 94:2183-2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spivey JR, Bronk SF, Gores GJ: Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes: role of ATP depletion and cytosolic free calcium. J Clin Invest 1993, 92:17-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krahenbuhl S, Talos C, Lauterburg BH, Reichen J: Reduced antioxidative capacity in liver mitochondria from bile duct-ligated rats. Hepatology 1995, 22:607-612 [DOI] [PubMed] [Google Scholar]
- 57.Sokol RJ, Devereaux M, Khandwala RA: Effect of dietary lipid and vitamin E on mitochondrial lipid peroxidation and hepatic injury in the bile duct-ligated rat. J Lipid Res 1991, 32:1349-1357 [PubMed] [Google Scholar]


