Abstract
Previous studies from our laboratory, using p53 transgenic mice, have suggested that ultraviolet (UV) light-induced keratinocyte apoptosis in the skin is not affected by overexpression of mutant p53 protein. To further elucidate a possible role for p53 in UV-induced keratinocyte cell death, we now examine apoptosis in skin and isolated keratinocytes from p53 null (−/−) mice and assess the influence of cell differentiation on this process. In vivo, using this knockout model, epidermal keratinocytes in p53−/− mice exhibited only a 5.2-fold increase in apoptosis after 2000 J/m2 UVB irradiation compared with a 26.3-fold increase in normal control animals. If this p53-dependent apoptosis is important in elimination of precancerous, UV-damaged keratinocytes, then it should be active in the undifferentiated cells of the epidermal basal layer. To test this hypothesis, we examined the effect of differentiation on UV-induced apoptosis in primary cultures of murine and human keratinocytes. Apoptosis was p53-independent in undifferentiated murine keratinocytes, which exhibited relative resistance to UVB-induced killing with only a 1.5-fold increase in apoptosis in p53+/+ cells and a 1.4-fold increase in p53−/− cells. Differentiated keratinocytes, in contrast, showed a 9.4-fold UVB induction of apoptosis in p53+/+ cells, almost three times the induction observed in p53−/− cells. This UV-induced difference in apoptosis was observed when keratinocytes were cultured on type IV collagen substrate, but not on plastic alone. Western blotting of UV-irradiated, differentiated keratinocytes did not support a role for either Bax or Bcl-2 in this process. In support of these findings in mice, cell death in human cultured keratinocytes also occurred in a differentiation-associated fashion. We conclude that p53-induced apoptosis eliminates damaged keratinocytes in the differentiated cell compartment, but this mechanism is not active in the basal, undifferentiated cells and is therefore of questionable significance in protection against skin cancer induction.
Keratinocytes are constantly exposed to the damaging and carcinogenic effects of ultraviolet (UV) radiation. Within the epidermis, keratinocytes are not a homogeneous population, but they differ in proliferative capacity and state of differentiation. 1 Basal keratinocytes, located along the basement membrane, include stem cells that are responsible for repopulating the epidermis. Above the basal layer, keratinocytes undergo a differentiation process characterized morphologically by an increase in cytoplasm and biochemically by expression of specific keratin subtypes. 1 Keratinocytes exposed to UV light undergo cell death, 2,3 but basal and differentiated keratinocytes differ in their response to this insult. UV-induced apoptotic cells (“sunburn cells”) appear within 12 hours and are predominately located in the suprabasal, differentiated keratinocyte compartment of human skin. 2 The molecular control of this process is poorly understood.
p53 is the most frequently mutated gene in cancer. 4 As a tumor suppressor gene, p53 protects the genome through cell cycle arrest, 5,6 by initiating cell death (apoptosis), 7-9 or in aiding DNA repair. 10-12 p53 is frequently mutated in cutaneous squamous cell carcinoma, 13-20 a common malignancy that arises from germinative keratinocytes located within the basal epidermis. The importance of UV radiation in the development of cutaneous squamous cell carcinoma and the frequency of p53 mutations in this form of cancer, implicates p53 in control of the keratinocyte protective response after UV damage. Using a murine transgenic model in which mice carry extra copies of a mutant p53 gene, we have recently demonstrated that UVB-induced keratinocyte apoptosis is not affected by overexpressed mutant p53 protein. 10 Others, using p53 null mice, have shown p53-dependent cell death within the epidermis, and authors have suggested that this apoptotic pathway serves to eliminate precancerous cells from the skin. 18 However, if this is true, p53-dependent apoptosis should be active in basal undifferentiated keratinocytes from which nonmelanoma skin cancer arises. 21,22 To date, the question of which keratinocyte population undergoes p53-dependent UV-induced apoptosis remains unanswered. In this study, we examine the significance of cell differentiation on UV-induced cell death in primary cultures of murine and human keratinocytes. Our results confirm that keratinocyte apoptosis is p53-dependent, but, more importantly, we demonstrate that this mechanism is restricted to the differentiated keratinocyte population.
Materials and Methods
Keratinocyte Culture
Keratinocytes were obtained from 6- to 8-week-old p53 wild-type and p53 null mice (Taconic, Germantown, NY) as previously described. 23 Briefly, the tail skin was dissected from the mice and treated with 0.25% dispase (Life Technologies, Inc., Mississauga, Ontario, Canada) for 16 to 18 hours. The epidermis was separated from the dermis with forceps. The epidermal sheet was then trypsinized for 5 minutes, and the cells were seeded-into 24 well plates coated with or without type IV collagen (Falcon, Becton-Dickinson, Franklin Lakes, NJ), in keratinocyte-serum-free medium (Life Technologies, Inc.) containing 100 units/ml penicillin G and 100 μg/ml streptomycin. Mouse keratinocytes were used as primary isolates and not passaged. Human keratinocytes were isolated from newborn foreskins and grown in keratinocyte-serum-free medium.
UV Irradiation
Shaved mouse skin and cultured keratinocytes were exposed to UVB (290 to 320 nm), using a bank of four unfiltered FS40 sun lamps (Westinghouse, Bloomfield, NJ). The intensity of the UV light was measured by an IL 700 radiometer fitted with a WN 320 filter and an A127 quartz diffuser (International Light, Newburyport, MA). Before UVB exposure, tissue culture medium was removed and the cells were rinsed twice in warm phosphate-buffered saline (PBS). For longer UV exposures, cells were covered with a thin film of PBS (100 μl) to prevent cell drying.
Induction of Keratinocyte Differentiation
Keratinocyte differentiation is calcium dependent and was induced in tissue culture by elevation of the calcium concentration in the growth medium to 1.0 mmol/L for 72 hours. This has previously been shown to induce differentiation-specific markers in murine keratinocytes. 24 After removing nonadherent cells, keratinocytes were exposed to varying doses of UVB and the level of apoptosis was assayed 24 hours later using methods described below.
Morphological Detection of Apoptosis in Vivo
Mice were exposed to 0, 1000, or 2000 J/m2 of UVB as previously described. 10 Animals were sacrificed 24 hours after UVB exposure, and skin samples were formalin fixed and paraffin embedded. Epidermal keratinocyte apoptosis in routine hematoxylin and eosin-stained sections was identified by cell shrinkage, nuclear condensation with fragmentation, and formation of apoptotic bodies. 10 Apoptotic cells in 100 random high-power fields of epidermis were counted and expressed as the number of apoptotic cells per linear cm.
In Vivo Terminal Deoxynucleotidyl Transferase-Mediated Nick End Labeling Assay
To confirm our apoptotic counts based on morphological criteria, an assay that end labels fragmented DNA in tissue was used. This protocol is a modification of a method described by Wijsman et al. 25 Twenty-four hours after UV irradiation, skin specimens were formalin fixed and paraffin embedded, and then 2-μm histological sections were deparaffinized and rehydrated in the usual manner. To improve incorporation of nucleotides, sections were treated with 0.5% pepsin in HCl (pH 2.0) for 15 minutes. Endogenous peroxidase was blocked with 0.1% H202 in PBS for 15 minutes. Tissue sections were then bathed in buffer A (50 mmol/L Tris-HCl pH 7.4, 5 mmol/L MgCl2, 10 mmol/L β-mercaptoethanol, and 0.005% bovine serum albumin) for 5 minutes at room temperature. They were then incubated at 37°C for 60 minutes with buffer A containing 0.01 mmol/L dATP, dCTP, and dGTP (Pharmacia, Uppsula, Sweden); 0.01 mmol/L biotin-21-dUTP (Clontech, Palo Alto, CA); and 20 U/ml DNA polymerase 1, Klenow fragment (Clontech). After rinsing in PBS for 5 minutes, slides were incubated in peroxidase-labeled streptavidin (Jackson ImmunoResearch Laboratories (West Grove, PA), 1:500) for 90 minutes at room temperature. The color reaction was developed by adding 3-amino-9-ethylcarbazole chromagen substrate for 5 to 10 minutes.
Detection of Apoptotic Nuclei in Culture
Cultured keratinocytes were exposed to UVB radiation at doses of 0, 1600, and 2000 J/m2 (when cultured on type IV collagen) and 0, 200, and 400 J/m2 when no collagen substratum was used. Apoptosis was assayed 24 hours after UVB exposure.
For detection of apoptosis in tissue culture, two methods were used. First, DNA fragmentation was detected using an end-labeling method with fluorescence detection (Fluorescent FragEL DNA Fragmentation Detection Kit, Oncogene Research Products, Cambridge, MA). In this assay, terminal deoxynucleotidyl transferase (TdT) binds to exposed 3′-OH ends of DNA fragments and promotes the addition of cyanine 3-conjugated deoxynucleotides. Labeling was done as per the manufacturer’s instructions. At least 200 cells were counted from randomly selected fields. Only those cells with characteristic morphological changes of apoptosis were scored as positive. Results were expressed as percentage apoptotic cells. Control samples, in which terminal deoxynucleotidyltransferase was omitted, demonstrated rare positive structures, but they did not have an apoptotic morphology.
The second apoptosis detection method used was an immunoassay that quantitates histone-associated DNA fragments (Cell Death Detection ELISA, Boehringer Mannheim, Indianopolis, IN). Experiments were performed according to the manufacturer’s instructions. Results were expressed as absorbance.
Western Blotting
Keratinocytes were harvested using a triple-detergent lysis buffer (PBS, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, and 0.5% sodium deoxycholate). Protein was boiled in loading buffer (1× loading buffer, 30 mmol/L Tris, pH 6.8, 1% sodium dodecyl sulfate, 10% mercaptoethanol, 10% glycerol, and 0.01% bromphenol blue) for 3 minutes and then electrophoresed in a 12% sodium dodecyl sulfate-polyacrylamide gel and electroblotted onto a nitrocellulose filter. The filter was blocked with 5% milk in Tris-buffered saline (10 mmol/L Tris, pH 7.4, and 150 mmol/L NaCl) overnight and incubated with anti-Bax antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-Bcl-2 (Santa Cruz Biotechnology) for 2 hours. After three washes with 0.1% Tween-20 in Tris-buffered saline, the filter was then incubated with horseradish peroxidase-labeled secondary goat anti-rabbit immunoglobulin G (1:10,000 dilution, Life Technologies, Inc.) for 1 hour at room temperature. The signals were detected by enhanced chemiluminescence detection (Amersham, Arlington Heights, IL).
Immunohistochemistry
Mice were exposed to 0, 1000, or 2000 J/m2 of UVB. Animals were sacrificed 24 hours later and skin samples were formalin fixed and paraffin embedded. Sections were then immunostained using an anti-Bax rabbit polyclonal antibody as previously described. 26 A section from each paraffin block was subjected to the same staining procedure using antiserum that was preabsorbed with competing Bax peptide to confirm the specificity of staining.
Results
p53 Regulates Apoptosis in Murine Epidermis
To assess the role of p53 in regulating apoptosis in the epidermis, shaved mouse skin was irradiated with UVB and apoptotic keratinocytes counted 24 hours later. In p53 wild-type skin, there was a 12.9- and 26.3-fold increase in apoptotic keratinocytes, at 1000 and 2000 J/m2, respectively, whereas p53 null mice demonstrated only a 2.2- and 5.2-fold increase at the same UV doses (Table 1) ▶ . To confirm these counts, a terminal deoxynucleotidyl transferase-mediated nick end labeling assay was performed on selected cases (data not shown). A strong correlation (r = 0.98, P = 0.02) between the terminal deoxynucleotidyl transferase-mediated nick end labeling assay and histological cell counts was observed.
Table 1.
p53-Regulated UV-Induced Apoptosis of Mouse Keratinocytes in Vivo
| Apoptotic cells per linear cm | |||
|---|---|---|---|
| 0 J | 1000 J | 2000 J | |
| p53+/+ | 1.1 ± 0.9 | 14.2 ± 0.8 (12.9) | 28.9 ± 7.1 (26.3) |
| p53−/− | 2.3 ± 2.1 | 5.1 ± 4.1 (2.2) | 12.0 ± 7.2 (5.2) |
Values stated are means ± SD (n = 3 in each group). Values in parentheses represent the ratio of UV-irradiated means over controls.
Apoptosis Is p53 Dependent in Differentiated Murine Keratinocytes
Skin biopsies from human subjects have shown that UV-induced apoptosis (“sunburn cells”) is observed within suprabasal, differentiated keratinocytes. We therefore sought to determine whether p53-dependent, UV-induced apoptosis was differentiation associated.
Baseline epidermal apoptosis in cultured unirradiated, undifferentiated keratinocytes was mildly increased, twofold, in p53+/+ animals (13.3 ± 6.4%) compared with p53−/− mice (6.1 ± 2.1%) as assessed using the FragEL method (Table 2) ▶ . A similar 1.7-fold increase was noted using the enzyme-linked immunosorbent assay method (p53+/+, 1.57 ± 0.31 versus p53−/−, 0.94 ± 0.14). Exposure to UVB did not markedly increase apoptosis in undifferentiated keratinocytes in culture, with only a 1.5-fold increase in p53+/+ cells and a 1.4-fold increase in p53−/− cells irradiated with 2000 J/m2 (Table 2) ▶ .
Table 2.
p53-Independent UV-Induced Apoptosis of Basal Mouse Keratinocytes in Vitro
| Percentage apoptotic cells | |||
|---|---|---|---|
| 0 J | 1600 J | 2000 J | |
| p53+/+ | 13.3 ± 6.4 | 14.7 ± 3.4 (1.1) | 19.7 ± 0.5 (1.5) |
| p53−/− | 6.06 ± 2.1 | 8.5 ± 1.3 (1.4) | 8.2 ± 2.8 (1.4) |
Values stated are means ± SD (n = 3 in each group). Values in parentheses represent the ratio of UV-irradiated means over controls.
In cultures of differentiated murine keratinocytes, baseline apoptosis for unirradiated cells was two- to threefold lower than in undifferentiated keratinocytes (Tables 2 and 3) ▶ ▶ and was similar in p53+/+ (3.1 ± 1.9) and p53−/− cells (3.6 ± 0.6). This observation was confirmed using the enzyme-linked immunosorbent assay method (p53+/+, 0.64 ± 0.13 versus p53−/−, 0.65 ± 0.08). In marked contrast to undifferentiated cells, differentiated keratinocytes exposed to UVB in vitro demonstrated a strong induction of apoptosis. At doses of 1600 and 2000 J/m2 in cultures of p53+/+ cells, a 5.8- and 9.4-fold increase, respectively, in apoptosis was observed compared with baseline unirradiated cells (Table 3) ▶ . However, a much weaker augmentation was noted in p53−/− cells (1.7- and 3.7-fold increase in apoptosis at 1600 and 2000 J/m2, respectively) (Table 3) ▶ .
Table 3.
p53-Dependent UV-Induced Apoptosis of Differentiated Mouse Keratinocytes in Vitro
| Percentage apoptotic cells | |||
|---|---|---|---|
| 0 J | 1600 J | 2000 J | |
| p53+/+ | 3.1 ± 1.9 | 18.2 ± 2.8 (5.8) | 29.3 ± 6.9 (9.4) |
| p53−/− | 3.6 ± 0.6 | 6.0 ± 0.7 (1.7) | 13.2 ± 2.8 (3.7) |
Values stated are means ± SD (n = 4 in each group). Values in parentheses represent the ratio of UV-irradiated means over controls.
p53-Dependent Apoptosis Is Extracellular Matrix Dependent
In human keratinocytes, p53-dependent apoptosis was shown to be regulated through substrate attachment. 27 As has been previously described, the UVB dose required for apoptosis induction was much lower when keratinocytes were cultured directly on plastic without a type IV collagen coating. For example, an eightfold increase in apoptosis was observed at a UVB dose of 400 J/m2 in differentiating keratinocytes cultured on plastic, whereas when cells were grown on type IV collagen, 2000 J/m2 was required to achieve a similar level of cell killing. When murine keratinocytes were grown on tissue culture dishes without type IV collagen, p53 dependence of UVB-induced apoptosis was lost (Table 4) ▶ . Both p53+/+ and p53−/− keratinocytes showed similar levels of apoptosis after UVB exposure, with the most marked increase in apoptosis observed in differentiated cells.
Table 4.
p53-Independent UV-Induced Apoptosis of Mouse Keratinocytes Grown without Type IV Collagen
| Apoptosis (absorbance) | |||
|---|---|---|---|
| 0 J | 200 J | 400 J | |
| Basal | |||
| p53+/+ | 0.42 ± 0.14 | 0.75 ± 0.19 (1.8) | 1.24 ± 0.02 (3.0) |
| p53−/− | 0.61 ± 0.32 | 1.19 ± 0.11 (2.0) | 1.35 ± 0.07 (2.2) |
| Differentiated | |||
| p53+/+ | 0.04 ± 0.02 | 0.07 ± 0.03 (1.8) | 0.34 ± 0.10 (8.5) |
| p53−/− | 0.08 ± 0.04 | 0.16 ± 0.03 (2.0) | 0.76 ± 0.14 (8.5) |
Values stated are means ± SD (n = 4 in each group). Values in parentheses represent the ratio of UV-irradiated means over controls.
UVB-Induced Apoptosis in Cultured Human Keratinocytes Is Also Differentiation Dependent
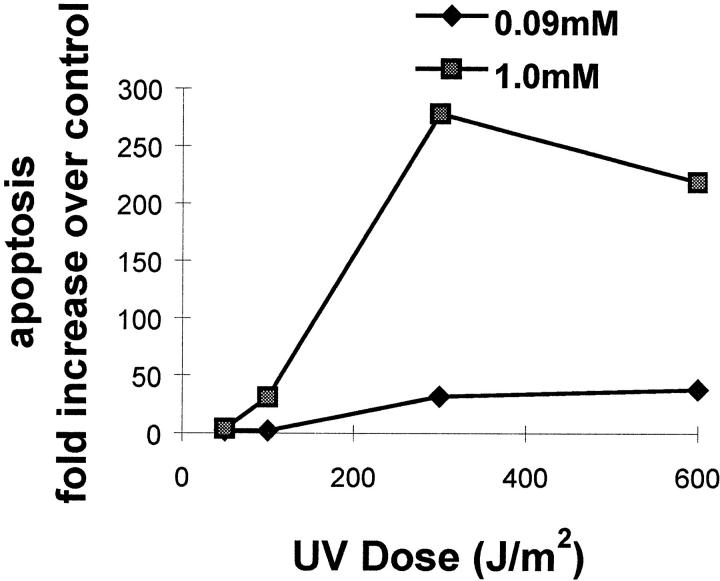
As noted in mice, the keratinocyte population most susceptible to UV-induced cell death resides within the differentiated compartment. To confirm this observation, human keratinocytes were induced to undergo differentiation by increasing calcium in the medium to 1.0 mmol/L. After UVB exposure, apoptosis was much more pronounced in differentiated keratinocytes compared with undifferentiated cells (Figure 1) ▶ .
Figure 1.
UV-induced apoptosis in undifferentiated (0.09 mmol/L Ca+2) and differentiated (1.0 mmol/L Ca+2) human cultured keratinocytes. Data are expressed as fold increase over unirradiated cells.
p53-Dependent Murine Keratinocyte Apoptosis Is Not Bax or Bcl-2-Mediated
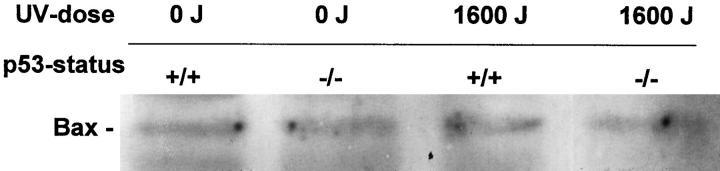
Wild-type p53 is known to be an upstream regulator of the Bax gene promoter, which contains p53 binding sites and can be directly activated by wild-type but not mutant p53. 28 On the contrary, Bcl-2 is known to be negatively regulated by p53. 29 We examined Bax and Bcl-2 expression in UV-irradiated cultured mouse keratinocytes with Western blotting. These data show no UV induction of Bax in p53 wild-type or null mice (Figure 2) ▶ . Immunohistochemistry with an anti-Bax antibody confirmed these data in vitro (data not shown). Bcl-2 levels fell slightly after exposure to UV, both in p53 wild-type and null mice (data not shown).
Figure 2.
Western blot analysis for Bax protein expression in differentiated mouse keratinocytes. Differentiated (1.0 mmol/L Ca+2) mouse cultured keratinocytes were either sham irradiated or exposed to 1600 J UVB and harvested 24 h later. Bax levels remained unchanged.
Discussion
Using a mutant p53 transgenic mouse model, we have previously observed that UV-induced cell death in keratinocytes was unaltered by the presence of overexpressed mutant p53 protein. 10 This result was somewhat unexpected, because abundant evidence supports a role for p53 in apoptosis. In our prior work, we could not exclude apoptosis induced by a functionally active mutant p53, and therefore, in this present study, we used p53 null mice lacking any p53 protein expression. 23 In this murine model, our results show a definite increase in the number of UVB-induced apoptotic (sunburn) cells in normal mice as compared with p53−/− animals. We conclude that UVB-induced apoptosis in murine epidermis is p53-dependent.
The precise location of apoptosis in the epidermis is an important question. We hypothesized that p53-dependent apoptosis occurred in the differentiated compartment. Unfortunately, we were unable to address this question in vivo, because in normal mouse epidermis, only two cell layers at most are present, and a clear distinction between basal and differentiated cells cannot be made. Therefore, we relied on a cell culture system in which keratinocytes were induced to differentiate by elevation of calcium concentration in the medium. Under these conditions, we observed p53-regulated, UVB-induced apoptosis only in differentiated keratinocytes and not in undifferentiated, basal cells.
In general, it is assumed that differentiated cells are more resistant to UV radiation. However, in human skin, UV-induced cell death seems to occur predominately in the differentiated compartment of the epidermis. 2,3 Our study confirms this finding, both in murine and human keratinocytes (Table 3, 4 ▶ ▶ and Figure 1 ▶ ). Furthermore, using human skin organ culture exposed to UV, we were able to demonstrate numerous dead keratinocytes located in the mid-epidermis by 24 hours (data not shown). In summary, although a small number of basal keratinocytes undergo UV-induced apoptosis, it appears that those cells committed to differentiate are most sensitive to the UV light.
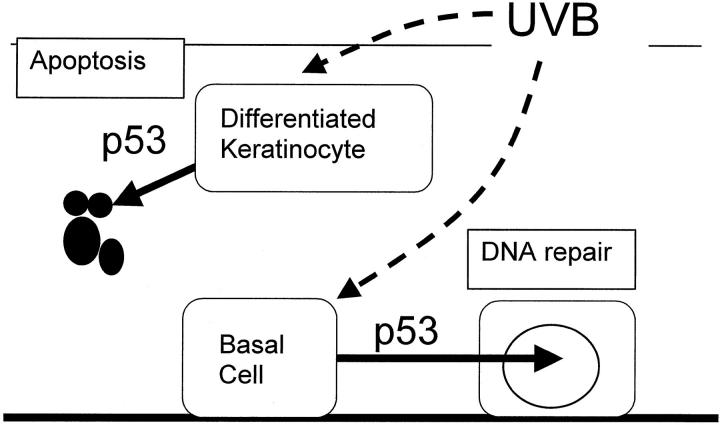
In a previous study, Ziegler et al 18 proposed that p53-dependent apoptosis is an important response to DNA damage, allowing the selective removal of precancerous cells. These authors examined p53-induced keratinocyte cell death using mouse skin in vivo, but they did not comment on the susceptible cell type. 18 However, because nonmelanoma skin cancer is thought to arise from basal keratinocytes, our present study, in which p53-dependent apoptosis is confined to differentiated cells, argues against a role for this process in removing premalignant keratinocytes. This does not mean that p53 is unimportant in skin cancer pathogenesis. We have recently shown that p53 or p53-regulated proteins enhance DNA repair in basal keratinocytes but not in differentiated cells, and we now believe that this is the important mechanism preventing formation of early cancerous cells. 30 We propose the following “dual-role” model for the p53-regulated response to UV damage in the epidermis (Figure 3) ▶ . After exposure to UV irradiation, basal keratinocytes repair damaged DNA, whereas differentiating keratinocytes undergo cell death; both of these processes are regulated by p53.
Figure 3.
Proposed dual-role model for the p53-regulated response to UV damage in the epidermis. After exposure to UV irradiation, basal keratinocytes repair damaged DNA, whereas differentiating keratinocytes undergo apoptosis, both processes regulated by p53. Although p53-induced apoptosis eliminates damaged keratinocytes in the differentiated cell compartment, this mechanism is inactive in the basal, undifferentiated cells and is therefore of questionable significance in protection against skin cancer induction.
Molecular evidence in support of our model comes from studies on the alternate splice variant of p53 (p53as). p53as has 17 amino acid substitutions and 9 amino acids truncated at the carboxy terminus compared with normal p53. 31 Functionally, p53as has augmented specific DNA-binding activity when compared with normal p53. 32 Within the skin, basal keratinocytes preferentially express normal p53, whereas differentiated keratinocytes preferentially express p53as. 33,34 Because most studies suggest that p53 induces apoptosis via its function as a transcriptional regulator, it would follow that p53as promotes cell death in differentiated keratinocytes. Indeed, differentiation-dependent differences in the level of transcriptional activity of p53 protein have been documented. Weinberg et al 24 compared the state of p53 activation in differentiated versus basal murine keratinocytes. They found that basal keratinocytes express high levels of p53 protein, but this protein is not transcriptionally active. 24 Conversely, differentiated cells, located suprabasally within the epidermis, have much less p53 protein, but it is in an “activated” state. 24
With respect to DNA nucleotide excision repair, the carboxy terminus of p53 is important for binding to important repair proteins, including XPB/D, 35,36 and also binds to single-stranded DNA. 37 These two observations, that differentiated keratinocytes express p53as, and that the carboxy terminus of p53 is important in nucleotide excision repair, are compatible with our finding that undifferentiated, basal keratinocytes show p53-dependent DNA repair, whereas differentiated keratinocytes (expressing p53as) have reduced nucleotide excision repair that is p53-independent. 30 Consistent with this model is a study showing that latent p53 (inactive) is primarily responsible for rapid non-sequence-specific binding to sites of DNA damage, whereas p53as is responsible for sustained regulation of transcriptionally mediated processes. 38
As a further piece of the puzzle, we found that p53-dependent UV-induced apoptosis is in some way regulated via association with extracellular matrix. Such an observation suggests a cell surface effect, possibly via expression of a differentiation-associated integrin. Integrin expression has been shown to be crucial in p53-induced apoptosis in human keratinocytes. 27 More studies are required to substantiate the role of integrins in UV-induced apoptosis.
The mechanism by which p53 induces apoptosis in differentiated keratinocytes remains unknown. We hypothesized that Bax or Bcl-2, both known downstream effector molecules of p53, 28,29,39 might mediate the cell death in differentiating keratinocytes. Using both immunohistochemistry of irradiated mouse skin and Western blotting of cultured keratinocytes, we failed to demonstrate induction of Bax protein. Bcl-2 protein levels fell after exposure to UV, but in both p53 wild-type and null keratinocytes. Our conclusion is consistent with a study showing no Bax mRNA induction, but reduced Bcl-2 mRNA expression in rat skin post UV irradiation. 40
Why is this somewhat complex mechanism necessary in the epidermis? We postulate that this design protects the epidermal stem cell population at all costs. Epidermal integrity is crucial to maintain homeostasis of most organisms. In fact, loss of the epidermis would resemble a third-degree burn, resulting in death. Cell death may be an acceptable form of eliminating damaged cells in the differentiating compartment, but DNA repair is the preferable method of managing damaged DNA in basal keratinocytes, which includes the stem cell population.
Acknowledgments
We thank Hong Ying Li for her superb technical support.
Footnotes
Address reprint requests to Dr. Victor A. Tron, Department of Pathology, Vancouver Hospital and Health Sciences Centre, 910 West 10th Avenue, Vancouver, BC, Canada V5Z 1L8. E-mail: vtron@vanhosp.bc.ca.
Supported by the Medical Research Council of Canada.
References
- 1.Murphy GF: Histology of the skin. Elder D eds. Histopathology of the skin. 1997, :pp 5-11 Lippincott-Raven, Philadelphia, [Google Scholar]
- 2.Gilchrest BA, Soter NA, Stoff JS, Mihm MC, Jr: The human sunburn reaction: histologic and biochemical studies. J Am Acad Dermatol 1981, 5:411-422 [DOI] [PubMed] [Google Scholar]
- 3.Daniels F: Acute cutaneous effects of light. Ben-Hur E Rosenthal I eds. Photomedicine. 1990, :pp 45-61 CRC Press, Inc., Boca Raton, FL, [Google Scholar]
- 4.Hollstein M, Sidransky D, Vogelstein B, Harris CC: p53 mutations in human cancers. Science 1991, 253:49-53 [DOI] [PubMed] [Google Scholar]
- 5.Kastan MB, Onyine O, Sidransky D, Vogelstein B, Craig RW: Participation of p53 protein in the cellular response to DNA damage. Cancer Res 1991, 51:6304-6311 [PubMed] [Google Scholar]
- 6.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ, Jr: A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992, 71:587-597 [DOI] [PubMed] [Google Scholar]
- 7.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T: p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993, 362:847-849 [DOI] [PubMed] [Google Scholar]
- 8.Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jacks T, Van Dyke T: p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell 1994, 78:703-711 [DOI] [PubMed] [Google Scholar]
- 9.Midgley CA, Owens B, Briscoe CV, Thomas DB, Lane DP, Hall PA: Coupling between γ irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Science 1995, 108:1843-1848 [DOI] [PubMed] [Google Scholar]
- 10.Li G, Mitchell DL, Ho VC, Reed JC, Tron VA: Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol 1996, 148:1113-1123 [PMC free article] [PubMed] [Google Scholar]
- 11.Smith ML, Chen IT, Zhan Q, O’Connor PM, Fornace AJ, Jr: Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene 1995, 10:1053-1059 [PubMed] [Google Scholar]
- 12.Ford JM, Hanawalt PC: Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair, and enhanced UV resistance. Proc Natl Acad Sci USA 1995, 92:8876-8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Pontén JA: A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 1991, 88:10124-10128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns JE, Baird MC, Clark LJ, Burns PA, Edington K, Chapman C, Mitchell R, Robertson G, Soutar D, Parkinson EK: Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br J Cancer 1993, 67:1274-1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulter LK, Wolber R, Tron VA: Site-specific comparison of p53 immunostaining in squamous cell carcinomas. Hum Pathol 1995, 26:531-533 [DOI] [PubMed] [Google Scholar]
- 16.Dumaz N, Drougard C, Sarasin A, Daya-Grosjean L: Specific UV-induced mutation spectrum in the p53 gene of skin tumors from DNA-repair-deficient xeroderma pigmentosum patients. Proc Natl Acad Sci USA 1993, 90:10529-10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Ho VC, Berean K, Tron VA: Ultraviolet radiation induction of squamous cell carcinomas in p53 transgenic mice. Cancer Res 1995, 55:2070-2074 [PubMed] [Google Scholar]
- 18.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE: Sunburn and p53 in the onset of skin cancer. Nature 1994, 372:773-776 [DOI] [PubMed] [Google Scholar]
- 19.Ren ZP, Hedrum A, Pontén F, Nistér M, Ahmadian A, Lundberg J, Uhlén M, Pontén J: Human epidermal cancer and accompanying precursors have identical p53 mutations different from p53 mutations in adjacent areas of clonally expanded non-neoplastic keratinocytes. Oncogene 1996, 12:765-773 [PubMed] [Google Scholar]
- 20.Sim CS, Slater S, McKee PH: Mutant p53 expression in solar keratosis: an immunohistochemical study. J Cutan Pathol 1992, :302-308 [DOI] [PubMed] [Google Scholar]
- 21.Morris RJ, Fischer SM, Slaga TJ: Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res 1986, 46:3061-3066 [PubMed] [Google Scholar]
- 22.Morris RJ, Coulter K, Tryson K, Steinberg SR: Evidence that cutaneous carcinogen-initiated epithelial cells from mice are quiescent rather than actively cycling. Cancer Res 1997, 57:3436-3443 [PubMed] [Google Scholar]
- 23.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A: Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992, 356:215-221 [DOI] [PubMed] [Google Scholar]
- 24.Weinberg WC, Azzoli CG, Chapman K, Levine AJ, Yuspa SH: p53-mediated transcriptional activity increases in differentiating epidermal keratinocytes in association with decreased p53 protein. Oncogene 1995, 10:2271-2279 [PubMed] [Google Scholar]
- 25.Wijsman JH, Jonker RR, Keijzer R, Van De Velde CJH, Cornelisse CJ, Van Dierendonck JH: A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem 1993, 41:7-12 [DOI] [PubMed] [Google Scholar]
- 26.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC: Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 1994, 145:1323-1336 [PMC free article] [PubMed] [Google Scholar]
- 27.Gniadecki R, Hansen M, Wulf HC: Two pathways for induction of apoptosis by ultraviolet radiation in cultured human keratinocytes. J Invest Dermatol 1997, 109:163-169 [DOI] [PubMed] [Google Scholar]
- 28.Miyashita T, Reed JC: Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80:293-299 [DOI] [PubMed] [Google Scholar]
- 29.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC: Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994, 9:1799-1805 [PubMed] [Google Scholar]
- 30.Li G, Ho VC, Mitchell DL, Trotter MJ, Tron VA: Differentiation-dependent p53 regulation of nucleotide excision repair in keratinocytes. Am J Pathol 1997, 150:1457-1464 [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf D, Harris N, Goldfinger N, Rotter V: Isolation of full-length mouse cDNA clone coding for an immunologically distinct p53 molecule. Mol Cell Biol 1985, 5:127-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayle JH, Elenbaas B, Levine AJ: The carboxy-terminal domain of the p53 protein regulates sequence-specific DNA binding through its nonspecific nucleic acid-binding activity. Proc Natl Acad Sci USA 1995, 92:5729-5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulesz-Martin MF, Lisafeld B, Huang H, Kisiel ND, Lee L: Endogenous p53 protein generated form wild-type alternatively spliced p53 in mouse epidermal cells. Mol Cell Biol 1994, 14:1698-1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehberger PA, Richter KH, Schwartz D, Goldfinger N, Oskato R, Almog N, Marks F, Rotter V: Differential expression of the regularly spliced wild-type p53 and its COOH-terminal alternatively spliced form during epidermal differentiation. Cell Growth Differ 1997, 8:851-860 [PubMed] [Google Scholar]
- 35.Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC: Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA 1994, 91:2230-2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang XW, Yeh H, Schaeffer L, Roy R, Moncollin V, Egly JM, Wang Z, Freidberg EC, Evans MK, Taffe BG, Bohr VA, Weeda G, Hoeijmakers JHJ, Forrester Keratinocytes, Harris CC: p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet 1995, 10:188-195 [DOI] [PubMed] [Google Scholar]
- 37.Wu L, Bayle JH, Elenbaas B, Pavletich NP, Levine AJ: Alternatively spliced forms in the carboxy-terminal domain of the p53 protein regulate its ability of promote annealing of complementary single strands of nucleic acids. Mol Cell Biol 1995, 15:497-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Huang H, Miner Z, Kulesz-Martin M: Activities and response to DNA damage of latent and active sequence-specific DNA binding forms of mouse p53. Proc Natl Acad Sci USA 1997, 94:8982-8987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan O, Fan S, Bae I, Guillouf C, Liebermann DA, O’Connor PM, Fornace AJ, Jr: Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene 1994, 9:3743-3751 [PubMed] [Google Scholar]
- 40.Gillardon F, Eschenfelder C, Uhlmann E, Hartschuh W, Zimmerman M: Differential regulation of c-fos, fosB, c-jun, junB, bcl-2 and bax expression in rat skin following single or chronic ultraviolet irradiation and in vivo modulation by antisense oligonucleotide superfusion. Oncogene 1994, 9:3219-3225 [PubMed] [Google Scholar]