Abstract
Organs used for transplantation undergo varying degrees of cold ischemia and reperfusion injury after transplantation. In renal transplantation, prolonged cold ischemia is strongly associated with delayed graft function, an event that contributes to inferior graft survival. At present, the pathophysiological changes associated with ischemia/reperfusion injury in clinical renal transplantation are poorly understood. We have performed an immunohistochemical analysis of pre- and postreperfusion biopsies obtained from cadaver (n = 55) and living/related donor (LRD) (n = 11) renal allografts using antibodies to adhesion molecules and leukocyte markers to investigate the intragraft changes after cold preservation and reperfusion. Neutrophil infiltration and P-selectin expression were detected after reperfusion in 29 of 55 (53%) and 24 of 55 (44%) cadaver renal allografts, respectively. In marked contrast, neutrophil infiltration was not observed in LRD allografts, and only 1 of 11 (9%) had an increased level of P-selectin after reperfusion. Immunofluorescent double-staining demonstrated that P-selectin expression resulted from platelet deposition and not from endothelial activation. No statistically significant association was observed between neutrophil infiltration and P-selectin expression in the glomeruli or intertubular capillaries despite the large number of cadaver renal allografts with postreperfusion changes. Neutrophil infiltration into the glomeruli was significantly associated with long cold ischemia times and delayed graft function. Elevated serum creatinine levels at 3 and 6 months after transplantation were also associated with the presence of neutrophils and platelets after reperfusion. Our results suggest that graft function may be influenced by early inflammatory events after reperfusion, which can be targeted for future therapeutic intervention.
Organs that are used for transplantation require effective ex vivo preservation from the moment the organ is retrieved to the time of transplantation. Hypothermic preservation solutions have been developed to maintain tissue viability by reducing metabolic activity and the accumulation of toxic substances during the cold ischemic period. Organs used for transplantation can undergo lengthy periods of cold ischemic storage after devascularization and cold perfusion, resulting in an increased susceptibility to damage upon reperfusion.
In clinical renal transplantation, prolonged cold storage has been demonstrated in many studies to be strongly associated with delayed graft function (DGF). 1-8 DGF is broadly defined as the requirement for dialysis within the first week after transplantation and results in complications in the immunosuppressive management of the transplant patient, prolonged hospitalization, and potentially detrimental effects to subsequent graft function and survival. 9,10 Some studies have suggested that DGF has little or no effect on graft survival, especially when the compounding effects of acute rejection are taken into account. 4,11-13 In marked contrast, other studies show a profound effect of DGF on subsequent short- and long-term graft survival. 1,2,5-8 This effect has recently been highlighted in a multivariate analysis of 37,216 primary cadaver renal allografts from the U.S. Renal Data System, in which DGF was shown to be an independent factor in determining poor short- and long-term graft survival, regardless of both the incidence of early rejection episodes and the degree of human leukocyte antigen (HLA) matching. 8
Although it is widely accepted that prolonged cold storage has a detrimental effect upon graft function, the precise mechanisms by which this occurs are not completely understood. During cold ischemic storage of organs before transplantation, biochemical events occur within the tissue leading to free radical-mediated damage upon reperfusion of the vascularized graft (Reviewed in Refs. 14-17 ). Free radicals appear to mediate tissue injury through lipid peroxidation and the activation of endothelial cells, resulting in functional and structural cell damage.
In vitro experiments on human umbilical vein endothelial cells have shown that reactive oxygen species induce adhesion molecule expression, resulting in activation and increased binding of neutrophils. 18-23 Furthermore, in experimental animal models, in situ cold ischemia followed by reperfusion of the kidney has been shown to lead to increased expression of adhesion molecules and neutrophil infiltration within hours of reperfusion, followed by mononuclear cell infiltration and up-regulation of major histocompatibility complex (MHC) class II expression several days later. 24,25 This increase in immunogenicity resulting from the early nonspecific inflammatory events may intensify subsequent alloimmune responses and play a major role in determining the quality of graft function in the long term. 26-28
Studies investigating the effects of ischemia/reperfusion injury in clinical renal transplantation are limited and have relied mainly on the measurement of a marker of lipid peroxidation, malondialdehyde. 29-31 Although these studies showed elevated levels of malondialdehyde in plasma after reperfusion of the graft, potential correlations with subsequent graft function were not examined. A more informative method of investigating reperfusion injury of renal allografts would be to analyze biopsies obtained immediately after transplantation.
During the early era of transplantation, biopsies were frequently obtained an hour after revascularization, when hyperacute rejection was suspected. A neutrophil infiltration in the glomeruli was an indicator of hyperacute rejection of the allograft, 32,33 but subsequent reports did not show a direct correlation between neutrophil infiltration and either hyperacute rejection or acute rejection episodes. 34-36 In the modern era of transplantation, hyperacute rejection has been virtually eliminated because of improvements in antibody screening, crossmatching, and immunosuppression, and thus there have been few studies of postreperfusion biopsies. In one recent study of postreperfusion biopsies, polymorphonuclear leukocytes were detected in biopsies from cadaver renal allografts, and this was found to be associated with long cold-storage times. 37 Interpretation of these results is complicated by the presence of hyperacute rejection in 4 of 57 allografts studied and by the fact that prereperfusion biopsies were not available for comparison; thus, it is not clear whether these cells entered upon reperfusion or were already present within the donor kidney.
To investigate the potential effects of cold ischemic damage and reperfusion injury in renal transplantation, we have performed an immunohistochemical study on renal allograft biopsies obtained immediately after transplantation and, for comparison, on biopsies from the same kidney before transplantation. This has enabled us to analyze changes resulting from reperfusion of the allograft, while excluding pre-existing factors associated with the donor kidneys. Furthermore, biopsies from living/related donor (LRD) renal allografts with minimal cold ischemia times have been obtained for comparison. The results from the analysis have been related to relevant donor parameters and factors relating to graft function and rejection.
Materials and Methods
Patients and Biopsy Material
Biopsy material was obtained from transplants of cadaveric (n = 55) and living/related (n = 11) kidney allografts performed at the Oxford Transplant Centre. Wedge biopsies were obtained from all transplanted kidneys at two time points: 1) prereperfusion, after nephrectomy, flushing with ice-cold hypertonic citrate (Marshall’s solution) and storage, but before implantation, and 2) postreperfusion, approximately 20 to 40 minutes after reperfusion of the kidney, immediately before wound closure. In five of the cadaver kidneys, additional biopsies were obtained at the time of nephrectomy, immediately after flushing, and before the period of cold storage. All biopsies were snap frozen in liquid nitrogen and stored at −80°C.
After transplantation, all patients received standard triple-therapy immunosuppression (cyclosporine, azathioprine, and steroids). 38 Details relating to important clinical parameters and outcome indicators are given in Table 1 ▶ . There were no significant differences observed between cadaver and LRD renal allografts in donor age; HLA-A, -B, and -DR mismatches; number of retransplants; anastomosis time; and recipient sex.
Table 1.
Clinical Factors and Graft Outcome Indicators after Transplantation
| Clinical Details | Cadaver (n = 55) | LRD (n = 11) |
|---|---|---|
| Donor age (±SD) | 41 ± 14.8 | 41 ± 11.3 |
| Cold ischemia time (hours)± SD* | 24.7 ± 9 | 1.8 ± 0.5 |
| Positive crossmatch† | 7 of 55 | 0 of 11 |
| DGF‡ | 8 of 55 | 1 of 11 |
| Recipient age (±SD)* | 47.8 ± 12 | 29.4 ± 12 |
| Serum creatinine, μmol/L (3 months)± SD | 154.7 ± 52 | 142.3 ± 37 |
| Serum creatinine, μmol/L (6 months)± SD | 154.2 ± 51 | 143.7 ± 31 |
| No. of rejection episodes (0:1:2:3) | 29:16:5:5 | 4:5:2:0 |
*Significant difference between cadaver and LRD groups; P < 0.01.
†Positive crossmatch resulting from non-HLA, autoreactive IgM antibodies.
‡DGF is defined as the requirement for dialysis in the first week after transplantation.
Immunohistochemistry
Cryostat tissue sections (7 μm) from wedge biopsies were stained with monoclonal antibodies (mAbs) using an indirect immunoperoxide technique as previously described. 39 The sections were stained with the following mAbs: 5D11 (anti-E-selectin (CD62E)), 4B2 (anti-vascular cellular adhesion molecule-1 (VCAM-1) (CD106)), and 14C11 (anti-intercellular adhesion molecule-1 (ICAM-1) (CD54)), all obtained from British Biotechnology Ltd (Oxford, UK); F10.89.4 (anti-CD45 leukocyte common marker 40 ); UCHT-1 (anti-CD3 T cell marker 41 ); UCHM-1 (anti-CD14 macrophage/monocyte marker 42 ); EBM/11 (anti-CD68 macrophage/monocyte marker 43 ); G1 (anti-P-selectin (CD62P) 44 ); 1G10 (anti-CD15s neutrophil marker 45 ); 5B12 (anti-CD41 platelet-specific marker) and anti-neutrophil elastase, (DAKO Ltd., High Wycombe, Bucks, UK). An anti-dog Thy-1 (F3.20.7 46 ) mAb was used as a negative control.
Briefly, mAb bound to the sections was detected using a peroxidase-conjugated rabbit anti-mouse immunoglobulin (Ig) (DAKO Ltd.) preincubated with human AB serum to prevent nonspecific binding. The reaction was developed using 3,3′-diaminobenzidine tetrachloride (Sigma Ltd., Poole, Dorset, UK) and H2O2, counterstained with Harris’ Haematoxylin (Merck Ltd., Atherstone, UK), dehydrated, and mounted in dextropropoxyphene mountant (Merck Ltd.). The signal for E-selectin and P-selectin was enhanced by incubating with a further antibody, a peroxidase-conjugated swine anti-rabbit Ig (DAKO Ltd.) preincubated with human AB serum.
To determine the origin of leukocyte infiltration into the postreperfusion biopsies, pre- and postreperfusion biopsies from transplants mismatched for HLA-A2 or B17 were stained with an anti-HLA-A2/B17 antibody (MA2.1 47 and a monomorphic anti-HLA-A, -B, and -C antibody (PA2.6 48 ), as a control for the presence of HLA class I antigens.
Double-Immunofluorescent Staining
Double-immunofluorescent staining was performed to clarify the origin of the increased expression of P-selectin detected in the postreperfusion biopsies. All incubations were performed for 30 minutes at room temperature in the dark. Acetone-fixed cryosections of pre- and postreperfusion biopsies were first incubated with antibodies to either P-selectin (IgG1) or ICAM-1 (IgG2a), and after washing, bound antibody was detected with the appropriate Texas Red-conjugated isotype-specific goat anti-mouse Ig antibodies (Southern Biotechnology Associates, Birmingham, AL) preincubated with human AB serum. Sections were then incubated with a fluorescein isothiocyanate (FITC)-conjugated anti-CD41 (platelet-specific) antibody (5B12, DAKO Ltd). After a final washing, slides were mounted in Vectashield (Vector Laboratories, Peterborough, UK) and analyzed by fluorescent microscopy. The specificity of the staining procedure was confirmed by including isotype control antibodies to ensure that there was no nonspecific binding of the secondary antibodies. All antibodies were used in isolation to check that the binding was in no way altered by the double-staining protocol.
Assessment of Staining
Staining of endothelial and leukocyte markers was scored by two independent observers (SVF and DDHK) without knowledge of the clinical status of the patients. Minor differences in the scoring were resolved by conference. The semiquantitative grades given for E-selectin, P-selectin, and CD41 detected on endothelium were scored as follows: 0, negative; 1, predominantly negative, with an isolated positive vessel; 2, focus of positive vessels/occasional positive vessels; and 3, multiple foci/positive vessels throughout biopsy. A significant increase in the level of expression of adhesion molecules after reperfusion was considered as an increase in grades of ≥1. Changes between grades 0 and 1 were not considered significant.
Leukocytes were quantified and expressed as 1) mean number of positive cells/glomerulus per section, with a minimum of three glomeruli required for inclusion in the analysis, and 2) mean number of positive cells in the intertubular areas per field of view (×10 objective). A significant increase in glomerular infiltration after reperfusion was taken as an increase in mean glomerular count of ≥1.5, and an increase in intertubular infiltration was scored as an increase of ≥10 positive cells.
Statistical Analyses
Statistical analyses of the immunohistochemical results and the clinical data were performed using the Student’s t-test, Fisher’s exact, and χ2 tests.
Results
Immunohistochemical Changes after Reperfusion
Biopsies obtained from donor kidneys before and after reperfusion were stained with mAbs to leukocyte subpopulations and endothelial adhesion molecules, to provide information about the changes that may occur immediately after reperfusion in cadaveric and LRD transplants. In addition, five biopsies were obtained at the time of donor nephrectomy and compared with prereperfusion biopsies from the same kidney to determine changes arising from cold storage.
Leukocyte Subpopulations
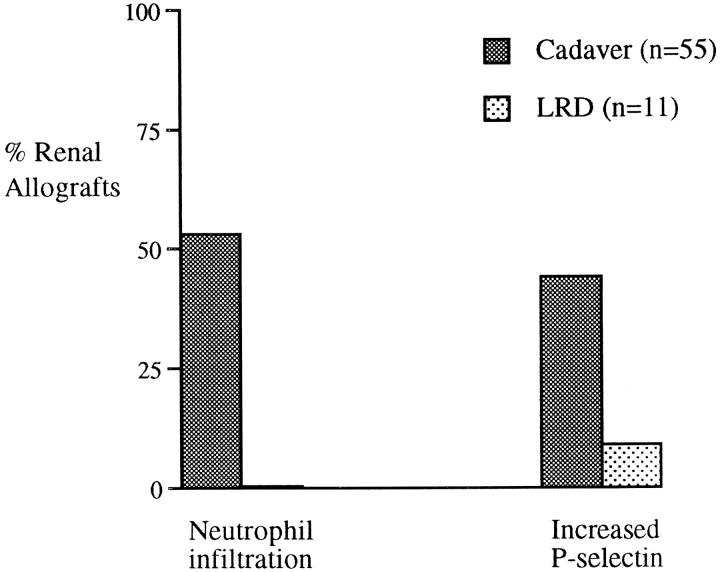
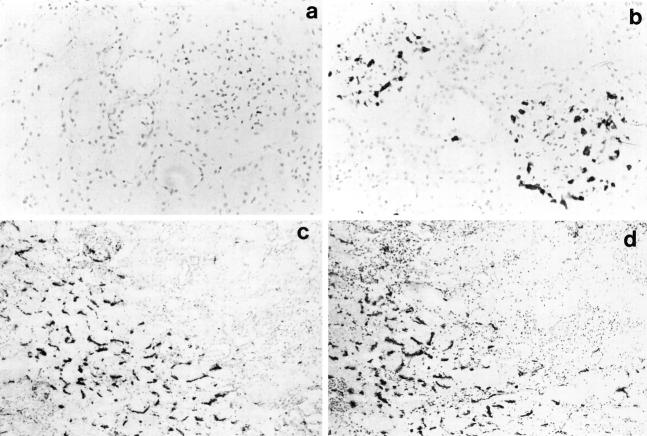
The major leukocyte population in the prereperfusion biopsies comprised CD14/CD68+ macrophages localized in the interstitial areas, but occasional CD3+ T lymphocytes were detected. There were no quantifiable differences in either of these cell populations immediately after reperfusion. In contrast, an increase in neutrophil infiltration, as determined by staining with antibodies to CD15s and neutrophil elastase, was observed in 29 of 55 (53%) cadaver renal allografts after reperfusion (Figure 1) ▶ . In the 29 cadaver allografts in which a neutrophil infiltration was detected, the infiltration was localized in the glomeruli of 16 of 29 (55%) (Figure 2 ▶ , a and b) and in the intertubular regions of 25 of 29 (86%) cadaver allografts (Table 2) ▶ . The increase in neutrophil infiltration after reperfusion was restricted to cadaver allografts; there was no neutrophil infiltration detected in the postreperfusion biopsies of LRD renal allografts (Table 2) ▶ . Furthermore, no changes occurred during the period of cold storage as determined by comparison between the five biopsies obtained at donor nephrectomy and the corresponding prereperfusion biopsies taken after cold storage.
Figure 1.
Percentage of cadaver (n = 55) and LRD (n = 11) renal allografts with an increase in neutrophil infiltration and P-selectin expression after reperfusion.
Figure 2.
Immunohistological changes observed after reperfusion in cadaver renal allografts. a: Indirect immunoperoxidase staining of a prereperfusion biopsy with an antineutrophil elastase mAb demonstrating the absence of neutrophils. b: The subsequent postreperfusion biopsy stained with the same antibody showing neutrophils infiltrating into the glomeruli (a and b, magnification, ×200). c: Postreperfusion biopsy stained by the indirect immunoperoxidase method using an anti-P-selectin antibody, showing a positive signal on the intertubular capillaries. d: A consecutive section from the same postreperfusion biopsy stained with the anti-CD41 platelet-specific antibody showing a pattern of staining similar to that detected for P-selectin (c and d, magnification, ×100).
Table 2.
Comparison of Immunohistochemical Changes Detected after Reperfusion of Cadaver and LRD Renal Allografts
| Changes detected between pre- and postreperfusion biopsies | Cadaver (n = 55) | LRD (n = 11) | P value |
|---|---|---|---|
| Neutrophil infiltration (glomerular and/or ITC) | 29 of 55 (53%) | 0 of 11 (0%) | <0.01 |
| Glomerular neutrophil infiltration | 16 of 29 (55%) | 0 of 11 (0%) | <0.01 |
| Intertubular neutrophil infiltration | 25 of 29 (86%) | 0 of 11 (0%) | <0.01 |
| P-selectin expression (glomerular and/or ITC) | 24 of 55 (44%) | 1 of 11 (9%) | <0.03 |
| Glomerular P-selectin expression | 13 of 24 (54%) | 0 of 11 (0%) | <0.01 |
| ITC P-selectin expression | 20 of 24 (83%) | 1 of 1 (100%) | NS |
ITC, intertubular capillary; NS, not significant.
Adhesion Molecule Expression
There was no difference in the levels of ICAM-1, VCAM-1, and E-selectin between pre- and postreperfusion biopsies from any of the transplants studied, as may be anticipated, given that all of these molecules require protein synthesis for surface expression. Nevertheless, there was considerable variation between kidneys in the extent of endothelial E-selectin expression and tubular ICAM-1 and VCAM-1, consistent with the findings of our earlier study 49 (data not shown). In marked contrast, P-selectin, which is expressed on the surface of endothelium and platelets within minutes of stimulation, was noticeably increased after reperfusion in 24 of 55 (44%) cadaver renal allografts (Figure 1) ▶ . In 13 of 24 (54%) of these transplants, P-selectin localized to the glomeruli, and in 20 of 24 (83%) it was detected within the intertubular areas (Table 2) ▶ . An increase in P-selectin after reperfusion occurred in only 1 of 11 LRD renal allografts and was localized in the intertubular areas. Comparison of five biopsies obtained at donor nephrectomy with prereperfusion biopsies taken after cold storage showed identical staining patterns, suggesting that changes in adhesion molecule expression did not occur during the period of cold storage.
Relationship between P-selectin Expression and Neutrophil Infiltration
An analysis of cadaver renal allografts with a neutrophil infiltration and/or increased P-selectin expression after reperfusion was performed to assess whether there were significant correlations between these postreperfusion changes. Of the 24 cadaver renal allografts with an increase in P-selectin expression after reperfusion, 14 had a corresponding increase in neutrophil infiltration. When considering changes detected within the glomeruli, 5 of the 16 cadaver allografts with a glomerular neutrophil infiltration had increased P-selectin expression in the glomeruli. With respect to the intertubular capillaries, in 12 of the 25 cadaver renal allografts with a neutrophil infiltration, an increase in P-selectin expression was also detected. Therefore, although a proportion of cadaver renal allografts had corresponding postreperfusion changes in the glomeruli and intertubular capillaries, there were no statistically significant correlations observed between neutrophil infiltration and P-selectin expression.
Characterization of P-Selectin Expression
To investigate whether the P-selectin expression resulted from endothelial stimulation and thus release of P-selectin from Weibel-Palade bodies or from deposition of activated platelets on the endothelium, consecutive sections were stained with an antibody to P-selectin and with the platelet-specific marker CD41. The patterns of staining with the two antibodies appeared to be identical, indicating that P-selectin may, at least in part, be of platelet origin (Figure 2 ▶ , c and d).
To confirm that the P-selectin was attributable to platelets, double staining was performed. The specificity of the staining protocol was confirmed with negative, isotype control antibodies. Biopsy sections were double stained with an FITC-conjugated anti-CD41 (platelet-specific) antibody in combination with an antibody against ICAM-1 (expressed constitutively on endothelium). The results showed that platelets detected after reperfusion were localized on the endothelium of the graft after reperfusion. FITC-labeled platelets were clearly identified on Texas Red-stained endothelium (Figure 3 ▶ , a to c). Double staining was then performed using antibodies against P-selectin and CD41. All stained structures were double positive for CD41 and P-selectin, indicating that the P-selectin detected in the postreperfusion biopsies was present as a result of activated platelets attached onto the microvascular endothelium (Figure 3 ▶ , d to f). Furthermore, P-selectin was not observed on vessels in the absence of CD41 signal, suggesting that endothelial P-selectin was not expressed in these biopsies.
Figure 3.
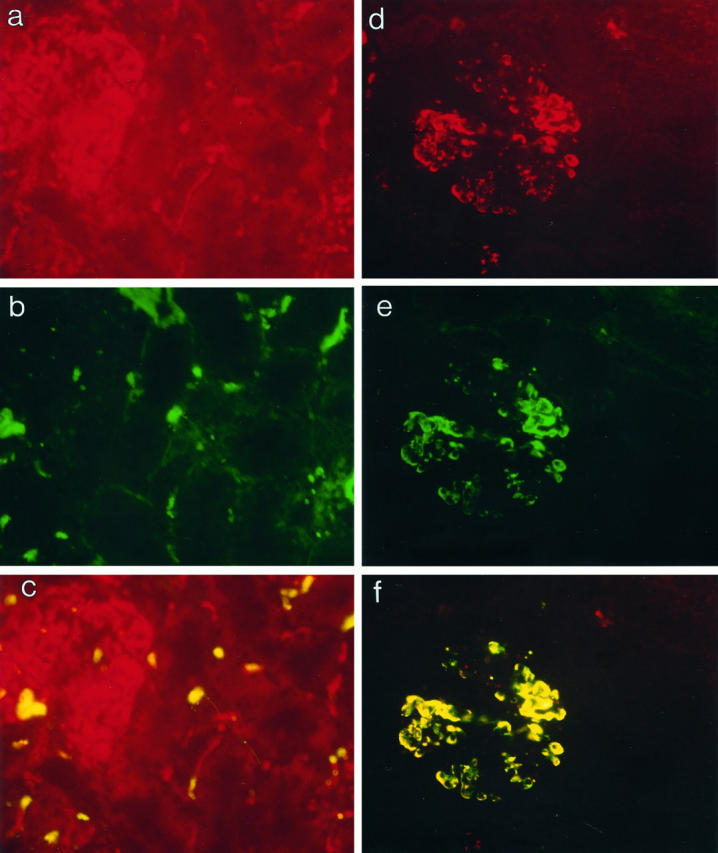
Double-immunofluorescent staining to identify the origin of the P-selectin expression in postreperfusion biopsies. a to c: Postreperfusion biopsy double-stained with a directly conjugated anti-CD41FITC mAb and an anti-ICAM-1 antibody detected with a Texas Red-conjugated secondary antibody. a: ICAM-1 staining (red) of glomerular and intertubular capillaries on the kidney. b: CD41+ platelets (green) detected after reperfusion. c: CD41-positive staining of platelets overlaid on ICAM-1+ endothelial structures, suggesting that platelet deposition on the microvascular endothelium is detectable after reperfusion. (a to c, magnification, ×200.) d to f: Double-immunofluorescent staining of a postreperfusion biopsy with an anti-CD41FITC mAb and an anti-P-selectin mAb developed with a Texas Red-conjugated secondary antibody. d: P-selectin expression (red) detected in glomeruli after reperfusion. e: CD41+ platelets (green) detected on identical structures in the glomerulus. f: Double-positive (yellow) structures within the glomerulus for CD41- and P-selectin-positive staining indicating that P-selectin expression occurs on CD41+ platelets. (d to f, magnification, ×200.)
Origin of Infiltrating Cells
To determine whether or not the postreperfusion infiltration was attributable to recipient cells infiltrating into the allograft, selected transplants, in which donor and recipient were mismatched for HLA-A2 or B17, were stained with a polymorphic antibody specific for these antigens (MA2.1). The results demonstrated that recipient cells were present after reperfusion (Figure 4) ▶ . Furthermore, in biopsies in which platelet deposition and neutrophil infiltration were detected, it was evident that these cells were positive for recipient antigen.
Figure 4.
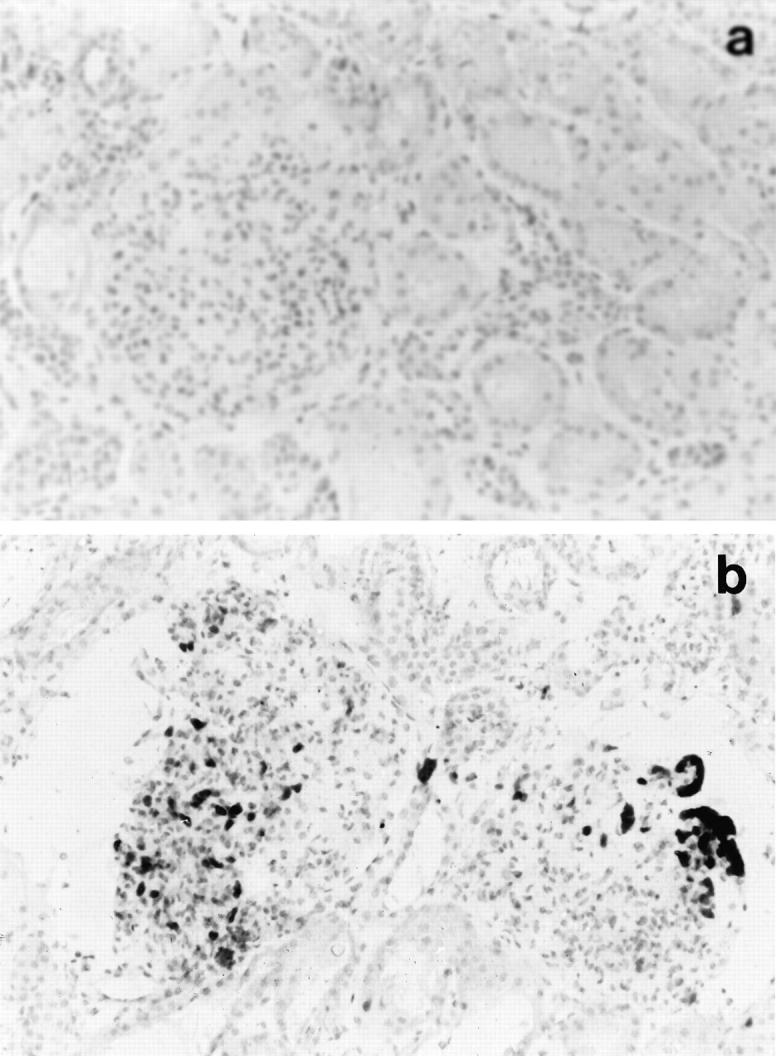
Detection of recipient-derived cells in cadaver renal allografts after reperfusion. Indirect immunoperoxidase staining of prereperfusion biopsy from an HLA-A2/B17 negative donor kidney showing the absence of positive staining with MA2.1, an anti-HLA-A2/B17 antibody (a), and postreperfusion biopsy stained with the same antibody showing the presence of recipient-derived HLA-A2/B17-positive cells within the graft after reperfusion (b). Magnification, ×200.
Clinical Significance of Immunohistological Changes Detected after Reperfusion in Cadaver Renal Allografts
To determine the possible factors that may be associated with the changes detected after reperfusion, relevant parameters were examined for the cadaver renal allografts. No significant associations were observed between intertubular neutrophil infiltration and any of the clinical parameters analyzed. However, in kidneys with a glomerular neutrophil infiltration after reperfusion, the mean cold ischemia time was 29.9 hours, significantly higher than in cadaver allografts without a neutrophil infiltration, in which the mean cold ischemia time was 22.7 hours (P = 0.0067) (Table 3) ▶ . This strongly suggests that a long cold ischemia time plays an important role in causing neutrophil infiltration immediately after transplantation.
Table 3.
Clinical Significance of Glomerular Neutrophil Infiltration and P-Selectin Expression after Reperfusion in Cadaver Renal Allografts
| Clinical parameter | Neutrophil infiltration | No infiltration | P value | P-selectin expression | No P-selectin | P value |
|---|---|---|---|---|---|---|
| Donor age | 41.9 ± 12 | 41.3 ± 14 | NS | 44.9 ± 15 | 39.8 ± 14 | NS |
| Mean cold ischemia time (hours) | 29.9 ± 13.7 | 22.7 ± 7.0 | 0.0067 | 27.7 ± 15 | 23.7 ± 8 | NS |
| CIT >30 hours | 8 of 16 (50%) | 5 of 39 (13%) | 0.011 | 4 of 13 (31%) | 9 of 42 (25%) | NS |
| Glomerular neutrophil influx | NA | NA | NA | 5 of 13 (38%) | 11 of 42 (26%) | NS |
| Glomerular P-selectin | 5 of 16 (31%) | 8 of 39 (21%) | NS | NA | NA | NA |
| DGF | 5 of 16 (31%) | 3 of 39 (8%) | 0.041 | 3 of 13 (23%) | 5 of 42 (12%) | NS |
| 3-month serum creatinine (μmol/L) | 188.6 ± 63 | 141.1 ± 40 | 0.0015 | 185.6 ± 52 | 144.6 ± 48 | 0.008 |
| 6-month serum creatinine (μmol/L) | 177.1 ± 59 | 144.6 ± 45 | 0.024 | 178.5 ± 58 | 145.9 ± 46 | 0.023 |
| No. of rejection episodes (0:1:2:3) | 10:4:0:2 | 19:12:5:3 | NS | 5:5:2:1 | 24:11:3:4 | NS |
NA, not applicable; NS, not significant; CIT, cold ischemia time.
The effect of glomerular neutrophil infiltration upon graft function after transplantation was also analyzed. Interestingly, delayed graft function was observed in 5 of 16 (31%) kidneys with a glomerular neutrophil infiltration, but in only 3 of 38 (8%) cadaver allografts with no infiltration (P = 0.041). Similarly, the mean serum creatinine levels at both 3 months and 6 months posttransplant for kidneys with a glomerular neutrophil infiltrate were significantly higher than in those kidneys without an increase in neutrophils postreperfusion (P = 0.002 and 0.024, respectively) (Table 3) ▶ . There was no significant association between allografts with neutrophil infiltration postreperfusion and increases in the number of acute rejection episodes or early rejection episodes.
The increase in platelet deposition detected after reperfusion was also assessed for associations with pre- and posttransplant factors. No significant associations were observed between platelet deposition on the intertubular capillaries and any clinical parameters analyzed. In cadaver allografts with glomerular platelet deposition after reperfusion, a significantly higher serum creatinine level was observed than in allografts without an increase in platelets at both 3 months and 6 months after transplantation (P = 0.008 and 0.023, respectively) (Table 3) ▶ . Platelet deposition was only observed in 1 of 11 LRD allografts postreperfusion. It is possible that the presence of both neutrophils and platelets immediately after reperfusion is indicative of an antibody-mediated event, but all patients were transplanted in the absence of a positive crossmatch. There were no significant associations in the seven patients transplanted with autoreactive antibodies.
Discussion
All solid organs used for transplantation undergo varying degrees of ischemic damage and reperfusion injury after retrieval, storage, and transplantation into the recipient. Hence, the immune response against a transplanted organ may not solely involve a MHC-specific alloimmune response, but in addition, an immediate nonspecific inflammatory response caused by ischemia/reperfusion injury.
In this study, we have investigated the events that occur after reperfusion of LRD and cadaver renal allografts, by comparing biopsies obtained from the same kidneys at two time points: prereperfusion (after cold storage but before transplantation) and postreperfusion biopsies (20 to 40 minutes after revascularization). The most striking observations of this study were the clear differences between living/related and cadaver renal allografts in the immediate posttransplant period.
Our results demonstrate that a neutrophil infiltration was observed after reperfusion in 29 of 55 (53%) cadaver allografts, whereas no increase in neutrophil infiltration was detected in any of the living donor renal allografts analyzed. An increase in the level of P-selectin expression after reperfusion was also detected in 24 of 55 (44%) cadaver renal allografts but in only 1 of 11 (9%) LRD allografts. No direct correlations were observed between P-selectin expression and neutrophil infiltration in the intertubular and/or glomerular regions of cadaver renal allografts after reperfusion. The absence of any significant association between neutrophil infiltration and P-selectin expression after reperfusion may in part result from the time frame in which the postreperfusion biopsies were taken.
Another pathway by which neutrophils can attach to endothelium and infiltrate into the graft is through interactions with E-selectin. 50,51 Although the level of E-selectin expression on the endothelium did not increase after reperfusion, there were high levels of E-selectin detected on the intertubular capillaries of 28 of 55 (51%) cadaver renal allografts, but there was no significant association between high levels of E-selectin and neutrophil infiltration. In addition to E-selectin expression, high levels of tubular ICAM-1 and VCAM-1 were detected in cadaver kidneys, but not in LRD kidneys. The expression of high levels of adhesion molecules in cadaver but not LRD kidneys suggests that injury to the organs as a result of trauma or brain death may be partly responsible for these detectable differences. 52,53 However, no significant associations were found between these pre-existing differing levels of adhesion molecule expression and any of the donor and clinical parameters analyzed.
The neutrophil infiltration in cadaver renal allografts was significantly associated with prolonged cold storage times, suggesting that cold ischemia and reperfusion may, in part, be responsible for initiating the early inflammatory response against the graft. Furthermore, we have demonstrated that the presence of neutrophils in the glomeruli of cadaver allografts was significantly associated with DGF, suggesting a possible effect of neutrophil-mediated damage upon early graft function.
Although no significant association was found between neutrophil infiltration and P-selectin expression, the presence of P-selectin as a contributing factor to the initial inflammatory response may be important. P-selectin has been shown to be mobilized from intracellular Weibel-Palade bodies in endothelial cells, or from α-granules in activated platelets and expressed on the cell surface within minutes of initial stimulation. 54-57 Therefore, it was necessary to determine the source of P-selectin expression. Double-immunofluorescent staining using an anti-CD41 platelet-specific antibody, a nonplatelet endothelial antibody against ICAM-1, and an anti-P-selectin mAb enabled us to conclude that P-selectin detected was of platelet, and not endothelial, origin. It is surprising that endothelial P-selectin was not detected, because there is evidence showing that endothelial P-selectin is expressed rapidly after oxygen free radical-mediated damage in vitro. 20-23,58 The transient nature by which P-selectin is expressed and reinternalized on the endothelium could provide a possible explanation for the lack of endothelial P-selectin observed, if this event occurred before the postreperfusion biopsies were obtained.
Platelets have been shown to attach to damaged blood vessels by binding directly to components of the subendothelial matrix, such as collagen, via the CD41/CD61 integrin complex (reviewed in Ref. 57 ). Activation of platelets leads to the up-regulation and increased binding avidity of the CD41/CD61 integrin, and also of the β2-integrins, thus resulting in localized platelet accumulation. 57,59 Surface-adherent activated platelets have been shown to support neutrophil rolling, arrest, and transmigration in a manner similar to that of activated endothelium. 60-64 Furthermore, the expression of P-selectin by activated platelets enables them to bind to neutrophils, causing aggregation and modifications in the activity of both cell types. 65-67 Therefore, although no direct association was found between platelet deposition and neutrophil infiltration in the time frame in which the biopsies were obtained for this study, it is possible that platelet-neutrophil interactions at subsequent time points may result in further recruitment and accumulation of both cell types at a site of damage.
In transplantation, platelets have been implicated in ischemia/reperfusion injury of human liver allografts 68 and rat syngeneic lung transplants, in which the level of platelet accumulation was shown to be proportional to the preservation time. 69 To our knowledge, the presence of platelets after reperfusion in clinical renal transplantation has not been previously reported.
Our results indicate that the presence of platelets and/or neutrophils in the glomeruli postreperfusion is significantly associated with higher serum creatinine levels at 3 and 6 months after transplantation. It is possible that reperfusion injury to the glomerular capillaries may result in partial endothelial denudation and thus platelet attachment to the underlying extracellular matrix. We have demonstrated that neutrophils and platelets detected after reperfusion are of recipient origin and are not pre-existing cells within the donor kidney. Their presence immediately after transplantation of a renal allograft is indicative of an early nonspecific, inflammatory event that is potentially detrimental to long-term graft function.
The immunohistological results from our study may in part, explain the success of clinical trials in which treatment with anti-ICAM-1 and anti-leukocyte function-associated antigen-1 (LFA-1) antibodies resulted in a reduction in the incidence of DGF after renal transplantation. 70,71 It is possible that anti-adhesion molecule therapy may limit the damage resulting from ischemia/reperfusion injury by inhibiting the infiltration of neutrophils into the kidney. Further evidence of the significant effect of ischemia/reperfusion injury in clinical renal transplantation has been demonstrated by the increased 1-year and 4-year graft survival observed in patients treated with superoxide dismutase, (a free radical scavenger) given intravenously just before reperfusion. 72 Although investigations were not performed to analyze the biological effects of administering superoxide dismutase at reperfusion, the results from the clinical outcome were promising and indicate that reperfusion injury may have a significant impact on chronic changes to the graft.
We have demonstrated that immunohistological changes after reperfusion of renal allografts are significantly associated with short- and long-term graft function. Improved methods of preservation and therapeutic strategies directed at reducing the detrimental effects of ischemia/reperfusion injury require further investigation. Furthermore, immunohistological analyses performed in conjunction with clinical studies would provide a more effective approach to understanding the mechanisms involved in this process.
Footnotes
Address reprint requests to Dr. S. V. Fuggle, Nuffield Department of Surgery, University of Oxford, John Radcliffe Hospital, Headington, Oxford, OX3 9DU, UK. E-mail: susan.fuggle@nds.ox.ac.uk.
Supported by a grant from the National Kidney Research Fund.
References
- 1.Najarian JS, Gillingham KJ, Sutherland DE, Reinsmoen NL, Payne WD, Matas AJ: The impact of the quality of initial graft function on cadaver kidney transplants. Transplantation 1994, 57:812-816 [DOI] [PubMed] [Google Scholar]
- 2.Peters TG, Shaver TR, Ames JE, Santiago-Delpin EA, Jones KW, Blanton JW: Cold ischemia and outcome in 17,937 cadaveric kidney transplants. Transplantation 1995, 59:191-196 [PubMed] [Google Scholar]
- 3.Troppmann C, Gillingham KJ, Benedetti E, Almond PS, Gruessner RW, Najarian JS, Matas AJ: Delayed graft function, acute rejection, and outcome after cadaver renal transplantation: the multivariate analysis. Transplantation 1995, 59:962-968 [DOI] [PubMed] [Google Scholar]
- 4.Troppmann C, Gillingham KJ, Gruessner RWG, Dunn DL, Payne WD, Najarian JS, Matas AJ: Delayed graft function in the absence of rejection has no long-term impact. Transplantation 1996, 61:1331-1337 [DOI] [PubMed] [Google Scholar]
- 5.Shoskes DA, Halloran PF: Delayed graft function: etiology, management and long-term significance. J Urol 1996, 155:1831-1840 [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Milford EL, Mackenzie HS, Brenner BM: Antigen-independent determinants of cadaveric kidney transplant failure. JAMA 1996, 276:1732-1736 [PubMed] [Google Scholar]
- 7.Nicholson ML, Wheatley TJ, Horsburgh T, Edwards CM, Veitch PS, Bell PRF: The relative influence of delayed graft function and acute rejection on renal transplant survival. Transplant Int 1996, 9:415-419 [DOI] [PubMed] [Google Scholar]
- 8.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL: Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997, 63:968-974 [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal JT, Danovitch GM, Wilkinson A, Ettenger RB: The high cost of delayed graft function in cadaveric renal transplantation. Transplantation 1991, 51:1115-1118 [PubMed] [Google Scholar]
- 10.Yokoyama I, Uchida K, Kobayashi T, Tominaga Y, Orihara A, Takagi H: Effect of prolonged delayed graft function on long-term graft outcome in cadaveric kidney transplantation. Clin Transplant 1994, 8:101-106 [PubMed] [Google Scholar]
- 11.Barry JM, Shively N, Hubert B, Hefty T, Norman DJ, Bennett WM: Significance of delayed graft function in cyclosporine-treated recipients of cadaver kidney transplants. Transplantation 1988, 45:346-348 [DOI] [PubMed] [Google Scholar]
- 12.Sanfilippo F, Vaughn WK, Spees EK, Lucas BK: The detrimental effects of delayed graft function in cadaver donor renal transplantation. Transplantation 1984, 38:643-648 [DOI] [PubMed] [Google Scholar]
- 13.Lehtonen SRK, Isoniemi HM, Salmela KT, Taskinen EI, von Willebrand EO, Ahonen JP: Long-term graft outcome is not necessarily affected by delayed onset of graft function and early acute rejection. Transplantation 1997, 64:103-107 [DOI] [PubMed] [Google Scholar]
- 14.Korthuis RJ, Granger DN: Reactive oxygen metabolites, neutrophils, and the pathogenesis of ischemic-tissue/reperfusion. Clin Cardiol 1993, 16:I19-I26 [DOI] [PubMed] [Google Scholar]
- 15.Granger DN, Kvietys PR, Perry MA: Leukocyte—endothelial cell adhesion induced by ischemia and reperfusion. Can J Physiol Pharmacol 1993, 71:67-75 [DOI] [PubMed] [Google Scholar]
- 16.Grace PA: Ischaemia-reperfusion injury. Br J Surg 1994, 81:637-647 [DOI] [PubMed] [Google Scholar]
- 17.Land W, Messmer K: The impact of ischaemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplant Rev 1996, 10:108-127 [Google Scholar]
- 18.Patel KD, Zimmerman GA, Prescott SM, McEver RP, McIntyre TM: Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol 1991, 112:749-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellak H, Franzini E, Hakim J, Pasquier C: Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood 1994, 83:2669-2677 [PubMed] [Google Scholar]
- 20.Arnould T, Michiels C, Remacle J: Hypoxic human umbilical vein endothelial cells induce activation of adherent polymorphonuclear leukocytes. Blood 1994, 83:3705-3716 [PubMed] [Google Scholar]
- 21.Rainger GE, Fisher A, Shearman C, Nash GB: Adhesion of flowing neutrophils to cultured endothelial cells after hypoxia and reoxygenation in vitro. Am J Physiol 1995, 269:H1398-H1406 [DOI] [PubMed] [Google Scholar]
- 22.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM: Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies: a mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest 1996, 97:493-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa H, Flores S, Kvietys PR, Wolf RE, Yoshikawa T, Granger DN, Aw TY: Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ Res 1997, 81:922-931 [DOI] [PubMed] [Google Scholar]
- 24.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL: The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney: inhibition by a soluble P-selectin ligand. J Clin Invest 1997, 99:2682-2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL: The role of the B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest 1997, 100:1199-1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoskes DA, Partrey NA, Halloran PF: Increased major histocompatibility complex antigen expression in unilateral ischemic acute tubular necrosis in the mouse. Transplantation 1990, 49:201-207 [DOI] [PubMed] [Google Scholar]
- 27.Goes N, Urmson J, Ramassar V, Halloran PF: Ischemic acute tubular necrosis induces an extensive local cytokine response. Transplantation 1995, 59:565-572 [PubMed] [Google Scholar]
- 28.Tullius SG, Tilney NL: Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation 1995, 59:313-318 [PubMed] [Google Scholar]
- 29.Rabl H, Khoschsorur G, Colombo T, Tatzber F, Esterbauer H: Human plasma lipid peroxide levels show a strong transient increase after successful revascularization operations. Free Radical Biol Med 1992, 13:281-288 [DOI] [PubMed] [Google Scholar]
- 30.Pincemail J, Defraigne JO, Franssen C, Bonnet P, Deby-Dupont G, Pirenne J, Deby C, Lamy M, Limet M, Meurisse M: Evidence for free radical formation during human kidney transplantation. Free Radical Biol Med 1993, 15:343-348 [DOI] [PubMed] [Google Scholar]
- 31.Davenport A, Hopton M, Bolton C: Measurement of malondialdehyde as a marker of oxygen free radical production during renal allograft transplantation and the effect on early graft function. Clin Transplant 1995, 9:171-175 [PubMed] [Google Scholar]
- 32.Kincaid-Smith P, Morris PJ, Saker BM, Ting A, Marshall VC: Immediate renal-graft biopsy and subsequent rejection. Lancet 1968, 2:748-749 [DOI] [PubMed] [Google Scholar]
- 33.Williams GM, Hume DM, Hudson RP, Morris PJ, Kano K, Milgrom F: “Hyperacute” renal-homograft rejection in man. N Engl J Med 1968, 279:611-618 [DOI] [PubMed] [Google Scholar]
- 34.Perloff LJ, Goodloe SJ, Jenis EH, Light JA, Spees EK: Value of one-hour renal-allograft biopsy. Lancet 1973, 2:1294-1295 [DOI] [PubMed] [Google Scholar]
- 35.McDicken IW, Hawking KM, Lameyer LD, Blok APR, Westbroek DL: Prognostic value for immediate function of one-hour renal allograft biopsy. Br Med J 1975, 4:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela R, Hamway SA, Deodhar SD, Braun WE, Banowsky LH, Magnusson MO, Osborne DG: Histologic, ultrastructural, and immunomicroscopic findings in 96 one hour human renal allograft biopsy specimens. Hum Pathol 1980, 11:187-195 [DOI] [PubMed] [Google Scholar]
- 37.Gaber LW, Gaber AO, Tolley EA, Hathaway DK: Prediction by postrevascularization biopsies of cadaveric kidney allografts of rejection, graft loss, and preservation nephropathy. Transplantation 1992, 53:1219-1225 [DOI] [PubMed] [Google Scholar]
- 38.Jones RM, Murie JA, Allen RD, Ting A, Morris PJ: Triple therapy in cadaver renal transplantation. Br J Surg 1988, 75:4-8 [DOI] [PubMed] [Google Scholar]
- 39.Fuggle SV, McWhinnie DL, Chapman JR, Taylor HM, Morris PJ: Sequential analysis of HLA-class II antigen expression in human renal allografts: induction of tubular class II antigens and correlation with clinical parameters. Transplantation 1986, 42:144-150 [DOI] [PubMed] [Google Scholar]
- 40.Dalchau R, Kirkley J, Fabre JW: Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol 1980, 10:737-744 [DOI] [PubMed] [Google Scholar]
- 41.Beverley PC, Callard RE: Distinctive functional characteristics of human “T” lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol 1981, 11:329-334 [DOI] [PubMed] [Google Scholar]
- 42.Hogg N, MacDonald S, Slusarenko M, Beverley PC: Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology 1984, 53:753-767 [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly PM, Bliss E, Morton JA, Burns J, McGee JO: Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol 1988, 41:510-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEver RP, Martin MN: A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem 1984, 259:9799-9804 [PubMed] [Google Scholar]
- 45.Bernstein ID, Andrews RG, Cohen SF, McMaster BE: Normal and malignant human myelocytic and monocytic cells identified by monoclonal antibodies. J Immunol 1982, 128:876-881 [PubMed] [Google Scholar]
- 46.McKenzie JL, Fabre JW: Studies with a monoclonal antibody on the distribution of Thy-1 in the lymphoid and extracellular connective tissues of the dog. Transplantation 1981, 31:275-282 [DOI] [PubMed] [Google Scholar]
- 47.McMichael AJ, Parham P, Rust N, Brodsky F: A monoclonal antibody that recognizes an antigenic determinant shared by HLA-A2 and B17. Hum Immunol 1980, 1:121-129 [DOI] [PubMed] [Google Scholar]
- 48.Brodsky FM, Parham P, Barnstable CJ, Crumpton MJ, Bodmer WF: Monoclonal antibodies for analysis of the HLA system. Immunol Rev 1979, 47:3-62 [DOI] [PubMed] [Google Scholar]
- 49.Fuggle SV, Sanderson JB, Gray DW, Richardson A, Morris PJ: Variation in expression of endothelial adhesion molecules in pretransplant and transplanted kidneys: correlation with intragraft events. Transplantation 1993, 55:117-123 [DOI] [PubMed] [Google Scholar]
- 50.Abbassi O, Kishimoto TK, McIntyre LV, Anderson DC, Smith CW: E-selectin supports neutrophil rolling in vitro under conditions of flow. J Clin Invest 1993, 92:2719-2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrence MB, Springer TA: Neutrophils roll on E-selectin. J Immunol 1993, 151:6338-6346 [PubMed] [Google Scholar]
- 52.Nagareda T, Kinoshita Y, Tanaka A, Takeda M, Sakano T, Yawata K, Sugimoto T, Nishizawa Y, Terada N: Clinicopathology of kidneys from brain-dead patients treated with vasopressin and epinephrine. Kidney Int 1993, 43:1363-1370 [DOI] [PubMed] [Google Scholar]
- 53.Takada M, Nadeau KC, Chandraker A, MacKenzie HS, Shaw GD, Hancock WW, Sayegh MH, Tilney NL: Brain death selectively stimulates expression of renal inflammatory mediators and cytokine expression: means of inhibition. Surg Forum 1997, 48:470-472 [Google Scholar]
- 54.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ: Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem 1989, 264:7768-7771 [PubMed] [Google Scholar]
- 55.Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP: Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 1990, 343:757-760 [DOI] [PubMed] [Google Scholar]
- 56.Sugama Y, Tiruppathi C, Offakidevi K, Andersen TT, Fenton JWD, Malik AB: Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1: a mechanism for stabilizing neutrophil adhesion. J Cell Biol 1992, 119:935-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blockmans D, Deckmyn H, Vermylen J: Platelet activation. Blood Rev 1995, 9:143-156 [DOI] [PubMed] [Google Scholar]
- 58.Closse C, Seigneur M, Renard M, Pruvost A, Dumain P, Belloc F, Boisseau MR: Influence of hypoxia and hypoxia-reoxygenation on endothelial P-selectin expression. Haemostasis 1996, 26:177-181 [DOI] [PubMed] [Google Scholar]
- 59.Philippeaux MM, Vesin C, Tacchini-Cottier F, Piguet PF: Activated human platelets express β2 integrin. Eur J Haematol 1996, 56:130-137 [DOI] [PubMed] [Google Scholar]
- 60.Buttrum SM, Hatton R, Nash GB: Selectin-mediated rolling of neutrophils on immobilized platelets. Blood 1993, 82:1165-1174 [PubMed] [Google Scholar]
- 61.Yeo EL, Sheppard J-AI, Feuerstein IA: Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model). Blood 1994, 83:2498-2507 [PubMed] [Google Scholar]
- 62.Sheikh S, Nash GB: Continuous activation and deactivation of integrin CD11b/CD18 during de novo expression enables rolling neutrophils to immobilize on platelets. Blood 1996, 87:5040-5050 [PubMed] [Google Scholar]
- 63.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA: Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the β2-integrin CD11b/CD18. Blood 1996, 88:146-157 [PubMed] [Google Scholar]
- 64.Kuijper PHM, Gallardo Torres HI, Lammers J-WJ, Sixma JJ, Koenderman L, Zwaginga JJ: Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood 1997, 89:166-175 [PubMed] [Google Scholar]
- 65.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu Y-M, Sajer SA, Furie B: Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 1992, 359:848-851 [DOI] [PubMed] [Google Scholar]
- 66.Nagata K, Tsuji T, Todoroki N, Katagiri Y, Tanoue K, Yamazaki H, Hanai N, Irimura T: Activated platelets induce superoxide anion release by monocytes and neutrophils through P-selectin (CD62). J Immunol 1993, 151:3267-3273 [PubMed] [Google Scholar]
- 67.Colli S, Eligini S, Lalli M, Tremoli E: Platelet-neutrophil interaction and superoxide anion generation: involvement of purine nucleotides. Free Radical Biol Med 1996, 20:271-278 [DOI] [PubMed] [Google Scholar]
- 68.Cywes R, Packham MA, Tietze L, Sanabria JR, Harvey PRC, Phillips MJ, Strasberg SM: Role of platelets in hepatic allograft preservation injury in the rat. Hepatology 1993, 18:635-647 [PubMed] [Google Scholar]
- 69.Okada Y, Marchevsky AM, Zuo X-J, Pass JA, Kass RM, Matloff JM, Jordan SC: Accumulation of platelets in rat syngeneic lung transplants: a potential factor responsible for preservation-reperfusion injury. Transplantation 1997, 64:801-806 [DOI] [PubMed] [Google Scholar]
- 70.Haug CE, Colvin RB, Delmonico FL, Auchincloss HJ, Tolkoff-Rubin N, Preffer FI, Rothlein R, Norris S, Scharschmidt L, Cosimi AB: A Phase I trial of immunosuppression with anti-ICAM-1 (CD54) mAb in renal allograft recipients. Transplantation 1993, 55:766-773 [DOI] [PubMed] [Google Scholar]
- 71.Hourmant M, Bedrossian J, Durand D, Lebranchu Y, Renoult E, Caudrelier P, Buffet R, Soulillou J-P: A randomized multicenter trial comparing leukocyte function-associated antigen-1 monoclonal antibody with rabbit antithymocyte globulin as induction treatment in first kidney transplantation. Transplantation 1996, 62:1565-1570 [DOI] [PubMed] [Google Scholar]
- 72.Land W, Schneeberger H, Schleibner S, Illner WD, Abendroth D, Rutili G, Arfors KE, Messmer K: The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation 1994, 57:211-217 [DOI] [PubMed] [Google Scholar]