Abstract
Sexually transmitted diseases, genital ulcer disease, and progesterone therapy increase susceptibility to lentivirus transmission. Infection of cells by human immunodeficiency virus (HIV) is dependent on expression of specific chemokine receptors known to function as HIV co-receptors. Quantitative kinetic reverse transcription-polymerase chain reaction was developed to determine the in vivo expression levels of CCR5, CXCR4, CCR3, CCR2b, and the cytomegalovirus-encoded US28 in peripheral blood mononuclear cells and cervical biopsies from 12 women with and without sexually transmitted diseases, genital ulcer disease, and progesterone-predominant conditions. Our data indicate that CCR5 is the major HIV co-receptor expressed in the female genital tract, and CXCR4 is the predominantly expressed HIV co-receptor in peripheral blood. CCR5 mRNA expression in the ectocervix was 10-fold greater than CXCR4, 20-fold greater than CCR2b, and 100-fold greater than CCR3. In peripheral blood, CXCR4 expression was 1.5-fold greater than CCR5, 10-fold greater than CCR2b, and 15-fold greater than CCR3. US28 was not expressed in cervical tissue despite expression in peripheral blood mononuclear cells from five individuals. CCR5 was significantly increased (p < 0.02) in biopsies from women with sexually transmitted diseases and others who were progesterone predominant. In vitro studies demonstrate that progesterone increases CCR5, CXCR4, and CCR3 expression and decreases CCR2b expression in lymphocytes and monocytes/macrophages. Characterization of chemokine receptors at the tissue level provides important information in identifying host determinants of HIV-1 transmission.
The susceptibility to sexual transmission of human immunodeficiency virus (HIV)-1 is extremely variable as infection by HIV-1 may occur after a single or a few exposures, 1 or not at all, even after multiple high-risk exposures. 2 In addition, many studies have noted a gender imbalance for heterosexual HIV transmission that places women at greater risk for acquisition from infected male partners. 3,4 Aside from gender, other factors may increase susceptibility to HIV-1 infection. The most convincing factors associated with enhancing HIV-1 transmission are co-infection with sexually transmitted diseases (STDs) or genital ulcer disease (GUD). The synergy between STDs, GUD, and HIV-1 has been described from an epidemiological and behavioral perspective. 5,6 The cellular mechanism by which STDs and GUD facilitate HIV-1 transmission, however, has not been well characterized. Similarly, the role of endogenous or exogenous hormonal influences in increasing or decreasing susceptibility to HIV infection has not been determined. STDs, GUD, and hormonal influences may increase the number of target cells present, increase immune activation, or increase expression of cell type-specific chemokine receptors, co-receptors that are necessary for HIV-1 infection.
Specific variants of HIV-1, non-syncytium-inducing, macrophage-tropic isolates, have been postulated to be the major sexually transmitted variants. 7 The second requirement for infection involves the expression of specific co-receptors on host cell types that express CD4. 8-13 The chemokine receptor CCR5 is the predominant receptor for macrophage-tropic isolates. It has previously been shown that the regulation of CCR5 expression is influenced by type 1 cytokine (eg, interleukin (IL)-2) activity and inflammatory responses in general. In addition, cells infiltrating inflammatory sites maintain the capacity to express other types of chemokine receptors (eg, CXCR4, CCR3, or CCR2b) that may serve as HIV co-receptors.
Thus, susceptibility to sexual transmission of HIV-1 undoubtedly involves features associated with both the virus and the local state of immune activation. Because of the invasive procedures involved, studying the female genital tract mucosa has been difficult in humans. Animal studies involving macaques have yielded abundant information on the early events of simian immunodeficiency virus (SIV) transmission, including the elucidation of Langerhans’ cells as the major infectable cell type immediately after inoculation. 14 Studies in macaques have also shown that progesterone increases susceptibility to intravaginal SIV challenge by undetermined mechanisms. 15 Because infection with SIV may be mediated by receptors other than CCR5 and CXCR4, 16 the purpose of this study was to examine factors influencing the expression and regulation of HIV-1 co-receptors in human tissue.
Here, using immunological and extremely sensitive molecular methods, we report on the expression and localization of macrophage-tropic 8-10 (CCR5 and CCR3), dual-tropic 11,12 (CCR2b and US28), and T-cell-tropic 13 (CXCR4) HIV co-receptors in the female genital tract. Our findings show distinct patterns of chemokine receptor expression in the cervix compared with peripheral blood. The pattern of chemokine receptor expression in the cervix is influenced by infiltrates of cells expressing various chemokine receptors and microbial as well as hormonal factors that affect the local state of immune activation.
Materials and Methods
Patients
Patients were enrolled in this study from the Northwestern Memorial Hospital Outpatient Clinic and the Prentice Women’s Hospital Ambulatory Care Clinic. All women were in the preovulatory phase of their menstrual cycles at the time of biopsy, except one woman, who was postmenopausal. Informed consent was obtained from all patients. Cervical biopsies were obtained from the superior portion of the ectocervix using biopsy forceps. Peripheral blood (16 ml) was drawn in acid citrate dextrose tubes. Patients 1 to 4 and 6 were undergoing routine examinations, patients 5 and 8 to 12 were undergoing diagnostic procedures, and patient 7 was undergoing a hysterectomy. The clinical history of all patients in this study is shown in Table 1 ▶ .
Table 1.
Clinical History of Patients in the Study
| Patient | Age | HIV status | CD4 count | Reproductive status | Hormonal exposure | Inflammatory conditions |
|---|---|---|---|---|---|---|
| 1 | 39 | Negative | NA | Nonpregnant | None | None |
| 2 | 23 | Negative | NA | Nonpregnant | None | None |
| 3 | 26 | Negative | NA | Nonpregnant | None | None |
| 4 | 48 | Negative | NA | Nonpregnant | None | None |
| 5 | 27 | Negative | NA | Nonpregnant | OCP | GUD (Behcet’s) |
| 6 | 52 | Negative | NA | Postmenopausal | None | None |
| 7 | 45 | Negative | NA | Nonpregnant | Prog. | None |
| 8 | 29 | Positive | 238 | Nonpregnant | None | LGSIL, HPV |
| 9 | 28 | Positive | 533 | Nonpregnant | None | LGSIL, HPV |
| 10 | 26 | Positive | 15 | Nonpregnant | None | HPV, HSV |
| 11 | 26 | Positive | 230 | Nonpregnant | None | HPV |
| 12 | 35 | Positive | 181 | Nonpregnant | None | HGSIL, HPV |
Abbreviations: NA, not applicable; OCP, oral contraceptives; Prog, progesterone; HGSIL, high-grade squamous intraepithelial lesion; LGSIL, low-grade squamous intraepithelial lesion; HSV, herpes simplex virus.
Blood Processing and Cell Culture
Peripheral blood mononuclear cells (PBMCs) from homozygous wild-type (wt) CCR5 volunteers were isolated on a Ficoll-Hypaque gradient. PBMCs (2 × 106 cells) were immediately placed in TriReagent (Molecular Research Center, Cincinnati, OH) for RNA extraction as per the manufacturer’s protocol or cultured in RPMI 1640 supplemented with 2 mmol/L l-glutamine 10% fetal bovine serum, 10 mmol/L HEPES, and penicillin/streptomycin. PBMCs were incubated with 50 ng/ml progesterone (Sigma Chemical Co., St. Louis, MO) for up to 6 days.
Tissue and Cell Preparation
Tissue samples from uterine ectocervix were trisected and either homogenized for RNA extraction, snap frozen in ornithine carbamoyltransferase embedding compound or fixed in Streck Tissue Fixative 17-19 (Streck Laboratories, Omaha, NE). RNA was extracted from biopsy specimens by homogenizing fresh biopsies of 5 mm3 in 500 μl TriReagent using diethyl pyrocarbonate-treated, autoclaved, disposable homogenizers. After homogenization, RNA was purified as per the manufacturer’s protocol. RNA pellets were resuspended in 1× transcription buffer (Promega, Madison, WI) with 2 units RQ1 RNase-free DNase (Promega, Madison, WI) and incubated for 30 minutes at 37°C to remove contaminating DNA. The mixture was extracted once with phenol:chloroform:isoamyl alcohol and once with chloroform:isoamyl alcohol. The aqueous layer was removed, and the RNA was precipitated in 3 volumes ethanol and 1/40 volume 3 mol/L sodium acetate overnight at −20°C.
Immunohistochemistry/Image Analysis
Tissue sections were cut to 5 μm, adhered to silanized slides, and deparaffinized through xylenes and graded alcohols. After peroxidase quenching and blocking with mouse serum in phosphate-buffered saline, pH 7.4, with 5% nonfat dry skim milk, immunohistochemistry was performed using the Vectastain ABC-HP kit (Vector Laboratories, Burlingame, CA) as per the manufacturer’s recommendations. Diaminobenzidine was used as substrate with hematoxylin counterstain. Frozen tissue sections for quantitative image analysis were allowed to air dry for 5 minutes, followed by postfixation in cold acetone for 20 minutes or 2% formaldehyde for 15 minutes. Sections were washed in phosphate-buffered saline, and an optimized dilution of primary antibody was applied. Cytokine and chemokine expression was quantified using assisted computerized image analysis as previously described. 20 IL-2-producing cells were identified by a juxtanuclear focal staining pattern surrounded by extracellular immune reactivity caused by adherence of cytokines to matrix proteins. Commercially available antibodies to CD4, CD45RO, CD68, S-100, IL-2, IL-4, and IL-10 (PharMingen, San Diego, CA) were used at concentrations optimized on control tissues.
Immunofluorescence/Flow Cytometry
After the appropriate incubation with progesterone, cells were pretreated with 0.5 mmol/L EDTA three times for 10 minutes each to remove adherent cells. Cells were washed three times with phosphate-buffered saline, pH 7.4/0.5% bovine serum albumin. Cells were then treated with 1 μg human immunoglobulin IgG/1 × 105 cells for 15 minutes at room temperature. Cells were stained with anti-CD4-fluorescein isothiocyanate or anti-CD14-fluorescein isothiocyanate (Becton Dickinson Immunochemistry Systems, San Jose, CA) and anti-CCR5-phycoerythrin or anti-CXCR4-phycoerythrin (PharMingen, San Diego, CA) for 30 minutes at room temperature, washed in phosphate-buffered saline, pH 7.4/0.5% bovine serum albumin, and fixed in 2% formaldehyde. Analysis was performed on a FACSCalibur flow cytometer using Cell Quest software.
CCR5 Genotyping
Total DNA was prepared by adding 400 μl of cell lysis buffer/200 μg/ml proteinase K to 1 × 106 cells from peripheral blood. The mixture was incubated at 58°C for 2 hours followed by extraction in 25:24:1 phenol:chloroform:isoamyl alcohol. The aqueous layer was recovered, and DNA was precipitated by the addition of 3 volumes of ethanol and 1/40 volume sodium acetate. DNA polymerase chain reaction (PCR) was performed by adding 45 μl reaction mix (1× PCR buffer (PEG> Applied Biosystems, Foster City, CA), 4.0 mmol/L MgCl2, 200 μmol/L dATP, 200 μmol/L dCTP, 200 μmol/L dGTP, 200 μmol/L dTTP, 200 nmol/L upstream CCR5 primer (TGTTTGCGTCTCTCCCAGGA), and 200 nmol/L CCR5 downstream primer (TGAAGATAAGCCTCACAGCCCT)) to approximately 500 ng DNA. The amplified product was resolved on a 2% metephor gel (FMC Bioproducts, Rockland, ME).
Chemokine Receptor mRNA Quantification
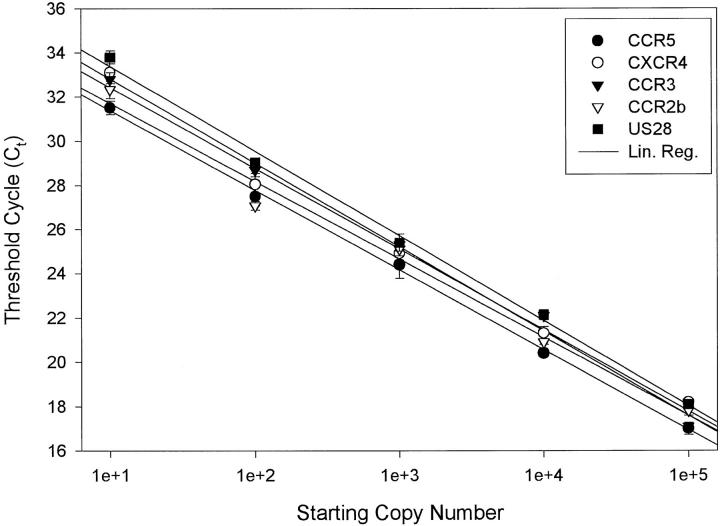
Quantitative kinetic reverse transcription-PCR was performed by adding 45 μl of reaction mix (1× RT Taqman EZ buffer (PE Applied Biosystems, Foster City, CA), 4.0 mmol/L Mn(O)Ac2, 300 μmol/L dATP, 300 μmol/L dCTP, 300 μmol/L dGTP, 300 μmol/L dTTP, 200 nmol/L upstream primer, 200 nmol/L downstream primer, 200 nmol/L internally conserved fluorogenic probes, and 10 units rTth polymerase) directly to 100 ng of total RNA in 5 μl RNase, DNase free water (Ambion, Austin, TX). Input RNA was normalized using glyceraldehyde-3-phosphate dehydrogenase mRNA quantification (PE Applied Biosystems). Reverse transcription and thermal amplification were performed using the following linked profile: reverse transcription, 30 minutes at 60°C; cDNA denaturation, 5 minutes at 95°C; 40 cycles of denaturation (95°C for 15 seconds); and annealing/extension (60°C for 1 minute) in a 7700 sequence detection system (PE Applied Biosystems). Duplicate standard curves with copy number controls ranging from 10 to 105 copies were run with each optical 96-well plate (PE Applied Biosystems). In addition, no template controls were included with each plate. We assessed the efficiency of amplification and the linearity of the assay by plotting the threshold cycle number, the cycle number at which the fluorescence signal exceeds background, versus the log target copy number (Figure 1) ▶ . To exclude potential signal due to plasmid DNA in the copy number standards, or to genomic DNA in the patient samples, we performed duplicate experiments with Taq polymerase rather than rTth polymerase. These experiments revealed a lack of amplification signal due to contaminating chemokine receptor DNA (data not shown). Amplification of heterologous transcripts or squamous cell RNA known to be negative for chemokine receptors revealed a lack of amplification signal. Amplification of RNA from a homozygous Δ32 CCR5 individual also revealed a lack of wt CCR5 amplification signal.
Figure 1.
Chemokine receptor mRNA quantification is linear over a range of at least 105 copies. Threshold cycle number (Ct) refers to the cycle number at which the reporter signal exceeds background. Results were based on triplicate determinations. The correlation coefficient for all curves was 0.99.
Primers and Probes
The primers and their respective probes used were as follows: wt CCR5, 5′-TGTTTGCGTCTCTCCCAGGA-3′ and 5′-TGAAGATAAGCCTCACAGCCCT-3′ (probe, 5′-FAM-CAGTCAGTATCAATTCTGGAAGAATTTCCAGACAT-TAMRA-3′); CXCR4, 5′-TATGACTCCATGAAGGAACCCTGT-3′ and 5′-AGCCTGTACTTGTCCGTCATGC-3′ (probe, 5′-FAM-TCC TGCCCACCATCTACTCCATCATC-TAMRA-3′); CCR3, 5′-AAAGCTGATACCAGAGCACTGATGG-3′ and 5′-GTTGGTCATAATTCGGAGCCTCC-3′ (probe, 5′-FAM-TTCACTGTGGGCCTCTTGGGCAAT-TAMRA-3′); CCR2b, 5′-CCTGTAAAGCAGGTGCCCAA-3′ and 5′-AGAGTCAAAGTCTCTACCCACAGTTTTT-3′ (probe, 5′-FAM-CCAATGCATATCCAACATGTGCTCAG-TAMRA-3′); US28, 5′-GACTCCCTGTGTCCTCACCG-3′ and 5′-CCAAGAAGTTGCCGATGGAA-3′ (probe, 5′-FAM-ACGTTGTTTCTGTACGGCGTTGTCTTTC-TAMRA-3′); IL-2, 5′-CCACAATATGCTATTCACATGTTCAGT-3′ and 5′-CAATTAACGCCTTCTGTATGAAACAG-3′ (probe, 5′-FAM-TTTCTGAGTTACTTTTGTATCCCCACCC-TAMRA-3′); IL-4, 5′-CTGTTCCCTGTGAGCTGCCT-3′ and 5′-GTATAGTTATCCGCACTGACCACG-3′ (probe, 5′-FAM-AGCTGGTTTTTCTGCTCTCCGAAGCC-TAMRA-3′); and IL-10, 5′-CCCAAGTATAGCTGAACCTTCCAA-3′ and 5′-TGTGGATGCCTGCTGTGTG-3′ (probe, 5′- FAM-CACGTAGGGTTGCAGGTTTCCTAGTGAG-TAMRA-3′).
Statistical Analysis
Comparisons between samples were performed using the Student’s t-test. Comparisons yielding a P < 0.05 were considered significant.
Results
Quantification of the Chemokine Receptor Repertoire in Cervical Biopsies and Peripheral Blood
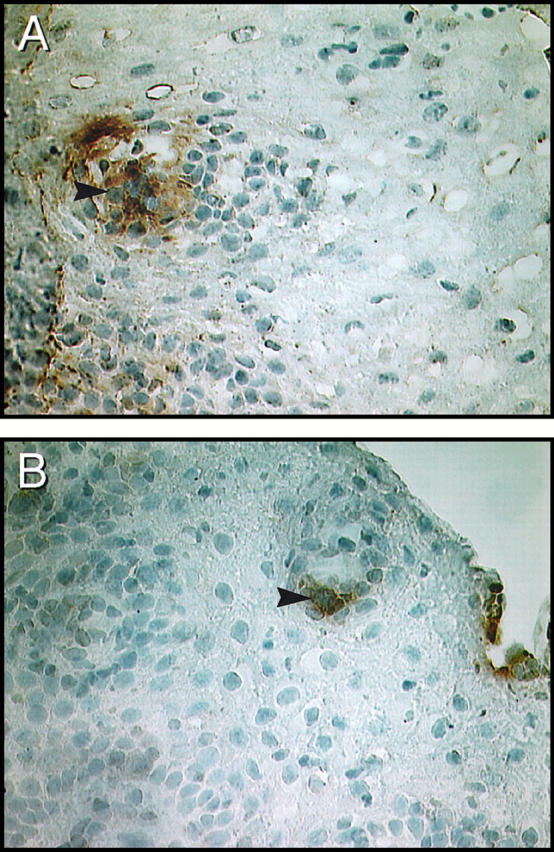
To quantify the expression of chemokine receptor mRNA in peripheral blood and biopsies from the female genital tract, we extracted and purified RNA and DNA from 12 women. Seven HIV-seronegative and five HIV-seropositive women with or without STDs, GUD, and progesterone predominance were evaluated using these techniques (Table 1) ▶ . To control for variable levels of wt and Δ32 CCR5 allelic expression in heterozygotes relative to homozygous wt individuals (manuscript in preparation), we selected women with a homozygous wt CCR5 genotype. Using 10,000 copies of glyceraldehyde-3-phosphate dehydrogenase mRNA (∼100 cells) in each replicate, at least duplicate determinations of chemokine receptor mRNA levels were performed (Table 2) ▶ . The level of CCR5 mRNA in the cervix was significantly greater than CXCR4, CCR3, CCR2b, and US28 (P < 0.001) regardless of the clinical state of the patient (Table 2 ▶ , Figure 2 ▶ ). Levels of CCR5 mRNA in biopsies with increased inflammation (Table 2 ▶ , patients 5 to 12) were significantly increased compared with levels in biopsies without increased inflammation (Table 2 ▶ , patients 1 to 4) (P < 0.02). Levels of CCR5 were also increased in biopsies from progesterone-predominant women (Table 2 ▶ , patients 6 and 7) relative to premenopausal, exogenous progesterone-naïve women (Table 2 ▶ , patients 1 to 4). Immunohistochemistry using monoclonal antibodies was performed to localize and quantify CXCR4 and CCR5 protein-expressing cells and to confirm the relative expression levels of CXCR4 and CCR5 determined by quantitative reverse transcription-PCR (Figure 3) ▶ . We found an 8-fold greater CCR5 protein expression level compared with CXCR4 (P < 0.02). The 8-fold difference in protein expression approximates the 10-fold difference in mRNA expression. Double-label immunofluorescence staining revealed that all cells expressing CCR5 or CXCR4 co-expressed CD4 in the biopsies studied (Figure 4) ▶ .
Table 2.
Quantification of Chemokine Receptor mRNA in Blood and Cervical Biopsies
| Patient | CCR5 mRNA* | CXCR4 mRNA* | CCR3 mRNA* | CCR2b mRNA* | US28* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CX | PBLs | CX | PBLs | CX | PBLs | CX | PBLs | CX | PBLs | |
| 1 | 91 ± 1 | 189 ± 4 | <10 | 744 ± 71 | Negative | 52 ± 12 | <10 | <10 | Negative | Negative |
| 2 | 94 ± 2 | 1104 ± 204 | 42 ± 5 | 368 ± 19 | Negative | 15 ± 6 | <10 | 113 ± 13 | Negative | <10 |
| 3 | 292 ± 43 | 133 ± 28 | 278 ± 17 | 674 ± 33 | <10 | 67 ± 8 | 36 ± 1 | 336 ± 82 | Negative | Negative |
| 4 | 209 ± 15 | 77 ± 13 | 60 ± 9 | 914 ± 55 | <10 | <10 | 48 ± 19 | 52 ± 7 | Negative | 1832 ± 177 |
| 5 | 123 ± 17 | 112 ± 10 | <10 | 964 ± 44 | Negative | 25 ± 2 | <10 | 76 ± 33 | Negative | Negative |
| 6 | 441 ± 43 | 860 ± 38 | 55 ± 8 | 1860 ± 124 | Negative | 23 ± 11 | <10 | 95 ± 20 | Negative | 2997 ± 291 |
| 7 | 678 ± 211 | 144 ± 36 | 27 ± 8 | 1287 ± 41 | Negative | 116 ± 28 | 18 ± 7 | 184 ± 33 | Negative | Negative |
| 8 | 704 ± 82 | 3717 ± 30 | 86 ± 27 | 865 ± 92 | Negative | 80 ± 22 | <10 | 64 ± 16 | Negative | 822 ± 119 |
| 9 | 922 ± 71 | 1409 ± 54 | <10 | 421 ± 12 | <10 | 348 ± 41 | 100 ± 37 | 529 ± 111 | Negative | Negative |
| 10 | 642 ± 27 | 706 ± 48 | <10 | 655 ± 149 | 26± 11 | 72 ± 17 | 51 ± 21 | 266 ± 14 | Negative | Negative |
| 11 | 1216 ± 167 | 85 ± 6 | 14 ± 6 | 1232 ± 211 | Negative | <10 | <10 | 12 ± 3 | Negative | Negative |
| 12 | 1176 ± 114 | 106 ± 21 | 153 ± 31 | 5531 ± 249 | <10 | <10 | 17 ± 3 | <10 | Negative | <10 |
CX, cervix; PBLs, peripheral blood lymphocytes.
*Number of copies per 10,000 glyceraldehyde-3-phosphate dehydrogenase RNA copies.
Figure 2.
Comparison of chemokine receptor expression in PBMCs and uterine cervix. CCR5 mRNA expression in the cervix was 10-fold greater than CXCR4, 20-fold greater than CCR2b, and 100-fold greater than CCR3. In PBMCs, CXCR4 expression was 1.5-fold greater than CCR5, 10-fold greater than CCR2b, and 15-fold greater than CCR3. US28 was not expressed in any of the cervical biopsies despite expression in PBMCs from five individuals.
Figure 3.
Digital microscopic images of chemokine receptor expression in an immunohistochemically stained cervical biopsy from HIV-1-seropositive patient 12 (Tables 1 and 2) ▶ ▶ . Chemokine receptor-expressing cells appear brown (arrows) in sections counterstained with hematoxylin (blue). A: CCR5-expressing cells are clustered beneath the surface epithelium. B: CXCR4-expressing cells from the same biopsy have a similar localization as the CCR5-expressing cells. Increased numbers of CCR5-expressing cells were found compared with CXCR4-expressing cells. Magnification, ×350.
Figure 4.
Localization of cells co-expressing CD4 and CCR5 (yellow) using double-label immunofluorescence and laser confocal image analysis (patient 6). Cells expressing only CD4 appeared red (arrowheads), and cells expressing only CCR5 were not identified. The epithelium-submucosa junction is denoted by arrows.
The levels of CXCR4, CCR3, CCR2b, and US28 were significantly elevated in blood (P < 0.001) relative to the cervix when the whole study population was assessed, whereas the level of CCR5 in peripheral blood was not significantly elevated relative to the cervix (Table 2 ▶ , Figure 2 ▶ ). The difference between the level of CXCR4 and the level of CCR5 in peripheral blood was not statistically significant.
Quantification of Immune Cells in the Cervical Mucosa
Increases in chemokine receptor expression in a particular tissue can be attributed to an increase in the number of cells expressing a particular chemokine receptor, up-regulation of chemokine receptors in cells present in a particular tissue, or both. To determine whether increases in the number of cells known to express specific chemokine receptors contributed to the repertoire of chemokine receptor expression, we characterized the immune cells present in the ectocervix of women with normal, proinflammatory, and progesterone-predominant conditions.
Using immunohistochemistry and assisted computerized image analysis, 20 we quantified the number of cells known to express CCR5 (CD4+ and CD45RO+ T cells, CD68+ macrophages, and S-100+ Langerhans’ cells), CXCR4 (CD4+ and CD45RA+ T cells and CD68+ macrophages), CCR3 (CD4+ T cells, CD68+ macrophages, eosinophils, and basophils), CCR2b (CD68+ macrophages, natural killer cells), or US28 (CD4+ T cells and monocytes/macrophages) (Table 3) ▶ . Langerhans’ cells, macrophages, and lymphocytes were evenly distributed in low quantities throughout the submucosa in four healthy controls such as patient 1 (Table 3 ▶ , Figure 5 ▶ ) and were rarely found in the epithelium. Dramatic yet heterogeneous increases in cellular infiltrates were seen in biopsies from women with GUD, progesterone predominance, or genital tract infections. The woman with noninfectious GUD (patient 5, Table 3 ▶ , Figure 5 ▶ ) had a moderate increase in macrophages and CD45RO+ T cells with a CD4/CD8 ratio of 4.9. In patients 6 and 7, an increase in CD45RO+ T cells and macrophages was observed in the progesterone-predominant biopsies. Women with human papillomavirus (HPV) or herpes simplex virus and HIV, such as patient 8 (Table 3 ▶ , Figure 5 ▶ ), had profound increases in CD45RO+ T cells and macrophages. Despite the presence of inflammatory conditions, neither the intensity of CD4 cell surface staining nor the number of Langerhans’ cells changed in the women studied.
Table 3.
Quantification of Immune Cells in Cervical Biopsies Using Image Analysis
| Patient | Immune cells (cells/HPF) | ||
|---|---|---|---|
| CD45RO | CD68 | S-100 | |
| 1 | 1 (0–1) | 2 (0–5) | 1 (0–4) |
| 2 | 2 (0–3) | 1 (0–3) | 2 (0–4) |
| 3 | 4 (2–8) | 3 (1–4) | 1 (0–2) |
| 4 | 1 (0–2) | 5 (0–9) | 3 (2–4) |
| 5 | 8 (0–25) | 15 (4–31) | 2 (1–6) |
| 6 | 13 (7–26) | 7 (2–19) | 5 (1–8) |
| 7 | 22 (11–34) | 13 (8–18) | 3 (1–5) |
| 8 | 57 (43–86) | 2 (0–4) | 1 (0–3) |
| 9 | 32 (18–59) | 15 (11–17) | 2 (0–3) |
| 10 | 65 (28–97) | 24 (14–33) | 2 (0–5) |
| 11 | 38 (22–49) | 17 (14–21) | 3 (0–6) |
| 12 | 18 (14–31) | 28 (19–43) | 4 (2–7) |
HPF, high-power field.
Figure 5.
Immunophenotypic characterization of cell types known to express CCR5 in representative cervical biopsies from patients listed in Table 1 ▶ . Activated/memory T lymphocytes (CD45RO), macrophages (CD68), and Langerhans’ cells (S-100) were visualized and quantified using image analysis. Positive cells appear brown (arrowheads) in sections counterstained with hematoxylin (blue). A lymphoid follicle was identified (arrow) in one of the biopsies. Magnification, ×200.
Regulation of Chemokine Receptor mRNA Expression in Vivo
To determine whether increased expression of cytokines involved in the mucosal immune response contributed to the pattern of chemokine receptor expression in our biopsies, we quantified the expression levels of IL-2, IL-4, and IL-10 using quantitative, kinetic reverse transcription-PCR and immunohistochemistry/assisted computerized image analysis. The type 1 cytokine IL-2 mRNA was up-regulated, whereas the type 2 cytokines IL-4 and IL-10 were below the limits of detection (10 mRNA copies) in biopsies from patients with STDs (Table 4 ▶ , Figure 6 ▶ ). To confirm the quantification and to localize the source of IL-2 up-regulation, we stained tissue sections with monoclonal antibodies against IL-2, IL-4, and IL-10. As might be expected, biopsies (Table 3 ▶ , patients 1 to 4) devoid of increased inflammatory cell infiltrates were negative for IL-2, as well as IL-4 and IL-10. Biopsies from women such as patients 8 and 9 (Figure 6) ▶ with the most inflammation and the highest level of CCR5 mRNA expression (Table 3 ▶ , patients 8 to 12) exhibited numerous cells in the epithelium and submucosa expressing IL-2 but lacked cells expressing IL-4 and IL-10. Biopsies from patients 6 and 7 revealed high levels of CCR5 expression in the absence of up-regulated IL-2.
Table 4.
Quantification of Cytokine mRNA in Cervical Biopsies
| Patient | IL-2 | IL-4 | IL-10 |
|---|---|---|---|
| 1 | Negative | Negative | Negative |
| 2 | Negative | Negative | Negative |
| 3 | Negative | Negative | Negative |
| 4 | Negative | Negative | Negative |
| 5 | 102 ± 44 | Negative | Negative |
| 6 | Negative | 36 ± 9 | <10 |
| 7 | 876 ± 211 | Negative | Negative |
| 8 | 2249 ± 332 | Negative | Negative |
| 9 | 3211 ± 112 | Negative | Negative |
| 10 | 1692 ± 287 | Negative | Negative |
| 11 | 1871 ± 349 | Negative | Negative |
| 12 | 567 ± 37 | Negative | Negative |
Values equal number of cytokine mRNA copies per 10,000 glyceraldehyde-3-phosphate dehydrogenase mRNA copies.
Figure 6.
Assisted computerized image analysis photomicrograph of cervical biopsies from patients 8 (A) and 9 (B) stained by immunohistochemistry for IL-2. IL-2-producing cells were identified by a juxtanuclear focal staining pattern (arrows) surrounded by extracellular immune reactivity caused by adherence of cytokines to matrix proteins. Tissue sections were counterstained with hematoxylin (blue). Magnification, ×400.
Effect of Progesterone on Chemokine Receptor Expression
To determine the direct effects of progesterone on chemokine receptor expression at the cellular level, we incubated PBMCs with 50 ng/ml progesterone for 6 days. At time 0, 3 days, and 6 days of culture, we extracted total RNA for CCR5, CXCR4, CCR3, and CCR2b mRNA quantification (Figure 7A) ▶ . In addition, we performed flow cytometry on PBMCs from the same time points to localize increases of CCR5 and CXCR4 to specific cell types. Molecular and immunophenotypic analysis revealed 5- to 10-fold increases in CCR5, CXCR4, and CCR3 expression that peaked after 3 days of culture. Levels of CCR2b mRNA in PBMCs decreased after progesterone treatment. The increase in CCR5 after progesterone treatment was detected exclusively in CD14+ monocytes (Figure 7B) ▶ , whereas increased CXCR4 expression was detected equally in lymphocytes (CD4+ or CD8+) and CD14+ monocytes. The increase in CCR5/CXCR4 expression peaked at 3 days and remained elevated or slightly diminished by day 6 (data not shown).
Figure 7.
A: Quantification of progesterone-induced chemokine receptor mRNA up-regulation after 3 days of PBMC culture in the presence of 50 ng/ml progesterone. CCR5, CXCR4, and CCR3 mRNA were significantly increased after progesterone treatment, whereas CCR2b mRNA decreased after treatment. B: Representative histograms from immunophenotyping/flow cytometry experiments. Untreated (top) and progesterone-treated (bottom) cells were double labeled with CD14-fluorescein isothiocyanate and CCR5-PE and gated based on CD14 expression and side scatter. Progesterone up-regulated CCR5 protein expression fivefold in CD14+ monocytes. The increase in CCR5/CXCR4 expression peaked at 3 days and remained elevated or slightly diminished by day 6.
Discussion
Heterosexual transmission of HIV-1 is the leading mode of transmission worldwide. 21 Understanding the dynamics of HIV-1 sexual transmission from male to female is critical to designing effective prevention strategies. Male-to-female transmission of HIV-1 involves exposure of cell-free or cell-associated virus in semen to cervicovaginal mucosa. 22 The sexually transmitted macrophage-tropic, non-syncytium-inducing HIV-1 variants 23 primarily use the chemokine receptor CCR5 as co-receptor, 8,9 whereas T-cell-tropic, syncytium-inducing variants use CXCR4 as co-receptor. 13 Our data indicate that CCR5 is the major HIV-1 co-receptor expressed in the female genital tract and CXCR4 is the predominantly expressed HIV co-receptor in peripheral blood. In cervical biopsies, CCR5 mRNA expression was 10-fold greater than CXCR4, 20-fold greater than CCR2b, and 100-fold greater than CCR3. In PBMCs, CXCR4 expression was 1.5-fold greater than CCR5, 10-fold greater than CCR2b, and 15-fold greater than CCR3. US28 was not expressed in any of the biopsies despite expression in PBMCs from five individuals. In particular, CCR5 expression was significantly elevated in cervical tissue from women with HIV and HPV or progesterone-predominant conditions when compared with biopsies from healthy normal women. These data have important implications for HIV transmission, considering studies showing a correlation between the level of CCR5 expression and infectability of cells by macrophage-tropic isolates of HIV-1. 24
Significant increases in CD4+ and CD45RO+ T lymphocytes were found in all biopsies with increased CCR5 expression. With one exception, the increase of CCR5 expression was also associated with increased numbers of CD68+ macrophages. Our data support previous animal studies on lymphocyte recirculation that demonstrated memory T cells (CD45RO+ and CCR5+) migrating to peripheral tissues, whereas naïve T cells (CD45RA+ and CXCR4+) use a recirculation pathway that bypasses tissues. 25 The significant difference in CCR5 and CXCR4 expression in these cervical biopsies must be due to either increases in CD4+ and CD45RO+ T cells that differentially express more CCR5 than CXCR4, 26 or factors that preferentially up-regulate CCR5 expression. Monocytes and Langerhans’ cells express both CCR5 and CXCR4, so increases in these cell types would be expected to increase both CCR5 and CXCR4, although blocks in CXCR4 cell surface expression have been shown in Langerhans’ cells. 27 Langerhans’ cells, however, were not increased in any of the biopsies, raising the issue of their role in the increased HIV-1 susceptibility in women with STDs.
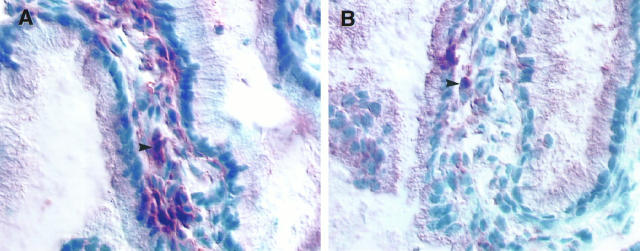
The first line of defense against HIV transmission in the female genital tract is the vaginal fluid and the cervical mucus. 22 A recent study has identified increased levels of HIV-1 env-specific immunoglobulin A in cervicovaginal fluid from HIV-exposed, uninfected partners of HIV-infected men. 28 Cervical mucus itself has been proposed to provide a physical barrier preventing HIV-1 from contacting the mucosal surface. 22 The second layer of defense is the vaginal and cervical epithelium. Breaches in this physical barrier have been suggested to increase the risk of HIV transmission. 15 This is not surprising in view of our data from the patient with noninfectious GUD. The base of the ulcer was lined by CD4+ and CD45RO+ T lymphocytes and macrophages, cell types that co-express CD4 and CCR5. The impact of oral contraceptives on genital-tract CCR5 expression in this patient, however, was not examined.
Studies have demonstrated an increased rate of HIV infection in women >45 years of age. 29 Progesterone, the predominant hormone in menopause, has been shown to enhance SIV transmission presumably by allowing more virions to move through the thinned epithelium. 15 In our study, a significantly increased level of CCR5 expression was found in cervical biopsies from progesterone-predominant women. In addition to epithelium only five to seven cells thick, immunohistochemical analysis revealed increased CD4+ and CD45RO+ T cells and macrophages localized to the epithelial-submucosa junction in cervical samples with immune activation (see Figure 3 ▶ ). In vitro stimulation of peripheral blood mononuclear cells with concentrations of progesterone known to increase female genital tract transmission of SIV in macaques revealed a 5- to 10-fold up-regulation of CCR5 in CD14+ monocytes/macrophages and 5-fold increase of CXCR4 expression in CD4+ lymphocytes and CD14+ monocytes/macrophages. The lack of CD4+ and CD45RA+ T cells, which predominantly express CXCR4, in tissue may explain why CXCR4 mRNA was higher in PBMCs when compared with cervical tissue (Table 2) ▶ . These data also suggest that CCR5 and CXCR4 have promoter regulatory elements responsive to the glucocorticoid/progesterone receptor pathway. 30 Progesterone-containing oral contraceptives have been shown, depending on the study, to either increase or decrease the risk of sexual transmission. 15,31 Further study is necessary to determine whether estrogen enhances or diminishes the effects of progesterone on chemokine receptor expression.
The last defense against HIV transmission in the female genital tract is the mucosal immune system. The immune system in the female genital tract consists of an inductive arm and an effector arm. 31 Pathogens encounter the inductive arm, during which time phagocytosis occurs and antigen is processed. During an immune response in the female genital tract, antigen is presented by mucosal macrophages and Langerhans’ cells. 32 These antigen-presenting cells migrate via afferent lymphatics to draining lymph nodes, where they stimulate B and T lymphocytes. Activated lymphocytes reenter peripheral blood and migrate to the female genital tract, functioning in the effector arm of the immune response. 31 We demonstrate that CCR5 mRNA is significantly increased when the effector arm of the cellular immune response is engaged in response to infectious and noninfectious inflammatory conditions. Local cytokine production is a critical correlate of an effective immune response. Preliminary data from our laboratory 34 have shown that type 1 cytokines, such as those would predominate in cellular immune responses (IL-2, interferon-γ, and IL-12), up-regulate CCR5 and to a lessor extent CXCR4. Type 2 cytokines (IL-4, IL-5, and IL-10), active in humoral immune responses, up-regulate CXCR4 but not CCR5. 33 In the present study, we determined that IL-2, a cytokine known to up-regulate CCR5, is increased in biopsies from women with STDs, whereas expression of IL-10, a cytokine known to decrease CCR5 mRNA expression in vitro, is low in all biopsies. 33 Although the contribution of genital HIV infection to this cytokine profile is unknown, HPV infection has been associated with an increase in IL-2 production. 34 These data support at the tissue level previous studies relating strong type 1 cytokine production and weak type 2 cytokine production, as defined by the IL-2/IL-10 ratio, with the presence of non-syncytium-inducing HIV-1 isolates. 35
The one-log range of CCR5 expression in the genital tract of homozygous wt CCR5 women may explain the wide variation in HIV infection rates and the increase of HIV sexual transmission in women with STDs. Here, we demonstrate that chemokine receptor expression was compartmentalized in the genital tract relative to peripheral blood; therefore, therapy directed at decreasing sexual transmission through modulating or blocking chemokine receptors should be locally targeted.
Acknowledgments
The authors acknowledge the expert technical assistance of Paul Jung and David Carter and thank Vanessa Jones for photographic assistance and Scott Brodie and Michael Socol for critical review of this manuscript. We also acknowledge the gift of the chemokine and chemokine receptor antibodies from Dr. Monica Tsang (R&D Systems, Inc., Minneapolis, MN).
Footnotes
Address reprint requests to Dr. Bruce K. Patterson, Northwestern University Medical School, 333 E. Superior Street, Suite 410, Chicago, IL 60611. E-mail: bpatters@nmh.org.
Supported by grants from the National Cancer Institute (grant 2490), the Swedish Medical Research Council (grant 10850), the Women’s Interagency HIV Study (grant 5 UO1 AI 34993-03), and the Northwestern Comprehensive AIDS Center.
References
- 1.Stakewski S, Schieck E, Rehmet S, Helm EB, Stille W: HIV transmission from a male after only two sexual contacts. Lancet 1987, 2:628-630 [DOI] [PubMed] [Google Scholar]
- 2.Liu R, Paxton WA, Choe S, Ceridini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR: Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply exposed individuals to HIV-1 infection. Cell 1996, 86:367-377 [DOI] [PubMed] [Google Scholar]
- 3.Padian NS, Shiboski SC, Jewell NP: Female to male transmission of human immunodeficiency virus. JAMA 1991, 266:1664-1667 [PubMed] [Google Scholar]
- 4.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR, Ngugi EN: Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis 1991, 163:233-238 [DOI] [PubMed] [Google Scholar]
- 5.Laga M, Diallo OM, Buve: Inter-relationship of sexually transmitted disease and HIV: where are we now? AIDS 1994, 8:S119-S124 [Google Scholar]
- 6.Kreiss JK, Coombs R, Plummer FA: Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J Infect Dis 1986, 160:380-384 [DOI] [PubMed] [Google Scholar]
- 7.Schuitemaker H, Kootstra NA, de Goede RE, de Wolf F, Miedema F, Termette M: Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable at all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol 1986, 65:356-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burhart M, Dimarzio P, Marmon S, Sutton RE, Hill CM, Peiper SC, Schall TJ, Littman DR, Landau NR: Identification of a major coreceptor for primary isolates of HIV-1. Nature 1996, 381:661-667 [DOI] [PubMed] [Google Scholar]
- 9.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA: CC-CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996, 272:1955-1958 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Broder CC, Kennedy PE, Berger EA: HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G-protein-coupled receptor. Science 1996, 272:872-877 [DOI] [PubMed] [Google Scholar]
- 11.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassem N, Ponath PD, Mackay CR: Chemokine receptor usage by human eosinophils. J Clin Invest 1997, 99:178-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doranz BJ, Rucker J, Yanjie Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW: A dual-tropic primary HIV-1 isolate that uses fusin, and the β-chemokine receptors CKR-5: CKR-3, and CKR-2b as fusion cofactors. Cell 1996, 85:1149-1158 [DOI] [PubMed] [Google Scholar]
- 13.Plekoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M: Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science 1997, 276:1874-1878 [DOI] [PubMed] [Google Scholar]
- 14.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD: Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 1996, 183:215-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ: Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 1996, 2:1084-1089 [DOI] [PubMed] [Google Scholar]
- 16.Deng HK, Unutmaz D, KewalRamani VN, Littman DR: Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 1997, 388:296-300 [DOI] [PubMed] [Google Scholar]
- 17.Frumkin L, Patterson BK, Leverenz J, Agy M, Wolinsky S, Morton W, Corey L: Infection of Macaca nemistrina brain with human immunodeficiency virus type 1. J Gen Virol 1995, 76:2467-2476 [DOI] [PubMed] [Google Scholar]
- 18.Korber B, Kunstman K, Patterson BK, Furtado M, McEvilly M, Levy R, Wolinsky S: HIV-1 sequence differences between blood and simultaneously obtained brain biopsy samples: conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol 1994, 68:7467-7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koffron AJ, Hummel M, Patterson BK, Yan S, Kaufman DB, Frye JP, Stuart FP, Abecassis MI: Cellular localization of latent murine cytomegalovirus. J Virol 1998, 72:95-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litton MJ, Dohlsten M, Hansson J, Rosendahl A, Ohlsson L, Kalland T, Andersson J, Andersson U: Tumor therapy with an antibody-targeted superantigen generates a dichotomy between local and systemic immune responses. Am J Pathol 1997, 150:1607-1618 [PMC free article] [PubMed] [Google Scholar]
- 21.: World Health Organization: The current global situation of the HIV/AIDS pandemic. Wkly Epidemiol Rec 1995, 70:7-87873345 [Google Scholar]
- 22.Stratton P, Alexander NJ: Heterosexual spread of HIV infection. Cotton D Watts DH eds. The Medical Management of AIDS in Women. 1997, :pp 15-43 Wiley-Liss New York [Google Scholar]
- 23.Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, Ho DD: Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol 1996, 70:3098-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, Choe H, Sodroski J, Newman W, Koup RA: CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med 1997, 185:1681-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay CR, Marston WL, Dudler L: Naïve and memory T-cells show distinct pathways of lymphocyte recirculation. J Exp Med 1990, 171:801-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR: The HIV coreceptors CXCR4, and CCR5 are differentially expressed and regulated on human T-lymphocytes. Proc Natl Acad Sci USA 1997, 94:1925-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaitseva M, Blauvelt A, Lee S, Lapham CK, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H: Expression and function of CCR5 and CXCR4 on human Langerhans’ cells and macrophages: implications for HIV primary infection. Nat Med 1997, 3:1369-1375 [DOI] [PubMed] [Google Scholar]
- 28.Mazzoli S, Trabattoni D, Caputo SL, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplicil F, Longhi R, Fusi ML, Tofani N, Biasin M, Villa ML, Mazzotta F, Clerici M: HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med 1997, 3:1250-1257 [DOI] [PubMed] [Google Scholar]
- 29.: European Study Group on Heterosexual Transmission of HIV: Comparison of female-to-male and male-to-female transmission of HIV in 563 stable couples. Br Med J 1992, 304:809-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beano M: Gene regulation by steroid hormones. Cell 1989, 56:335-344 [DOI] [PubMed] [Google Scholar]
- 31.Miller CJ, McGhee JR, Gardner MB: Biology of disease mucosal immunity, HIV transmission, and AIDS. Lab Invest 1992, 68:129-140 [PubMed] [Google Scholar]
- 32.Parr MB, Kepple L, Parr EL: Antigen recognition in the female reproductive tract. II. Endocytosis of horseradish peroxidase by Langerhans’ cells in murine vaginal epithelium. Biol Reprod 1991, 45:261-265 [DOI] [PubMed] [Google Scholar]
- 33.Patterson BK, Czerniewski MA, Su F, Andersson J, Jiyamapa D, Burki Z, Landay A: Regulation of CCR5 and CXCR4 expression by type 1 and type 2 cytokines: type 1 cytokine upregulation of CCR5 mRNA expression can be abolished by IL-10. 5th Conference on Retroviruses and Opportunistic Infections. Abstract 178
- 34.Tsukui T, Hildesheim A, Schiffman MH, Lucci J, III, Contois D, Lawler P, Rush BB, Lorincz AT, Corrigan A, Burk RD, Qu W, Marshall MA, Mann D, Carrington M, Clerici M, Shearer GM, Carbone DP, Scott DR, Houghten RA, Berzofsky JA: Interleukin 2 production in vitro by peripheral lymphocytes in response to human papilloma-derived peptides: correlation with cervical pathology. Cancer Res 1996, 56:3967-3974 [PubMed] [Google Scholar]
- 35.Clerici M, Balotta C, Salvaggio A, Riva C, Trabattoni D, Papagno L, Berusconi A, Rusconi S, Luisa Villa M, Moroni M, Galli M: Human immunodeficiency virus (HIV) phenotype and interleukin-2/interleukin-10 ratio are associated markers of protection and progression in HIV infection. Blood 1996, 88:574-579 [PubMed] [Google Scholar]