Abstract
Retinoids are effective growth modulators of human ovarian carcinoma cell lines. Their effects are mediated by nuclear retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which are transcriptional factors and members of the steroid/thyroid receptor superfamily. To our knowledge, until now, the cellular distribution of RAR proteins in human ovarian tumor specimens is unknown. This study provides new data on the differential cellular localization of RARα protein in 16 serous adenocarcinomas originating from the ovaries, fallopian tubes, and the peritoneum. Using an affinity-purified antiserum specific for RARα and a monoclonal antibody recognizing the full-length estrogen receptor molecule (clone 6F11), we performed immunohistochemistry on frozen tissue sections and examined the relationship between RARα and estrogen receptor protein expression by comparing the percentage of immunostained tumor cells for either receptor. Our findings indicate a strong linear relationship between the percentages of RARα- and estrogen receptor-labeled tumor cells as determined by linear regression analysis (P < 0.005, r = 0.825). A modest inverse relationship was found between the percentage of RARα-positive tumor cells and histological grade, attesting to a differentiation-dependent trend (P < 0.04). No significant relationship was found between RARα-labeled cells and clinical stage (P = 0.139), site of tumor origin (ovaries versus fallopian tubes versus peritoneum) (P = 0.170), and primary versus metastatic lesion (P = 0.561). Thus, serous adenocarcinomas are capable of expressing RARα and estrogen receptor despite high histological grade and advanced stage of neoplastic disease. Compared with the heterogeneous localization of RARα in cancer cells, there was widespread RARα immunoreactivity in tumor-infiltrating lymphocytes, vascular endothelial cells, and stromal fibroblasts, underscoring the value of immunohistochemistry in the accurate determination of RAR/(RXR) content in tumor specimens.
Ovarian epithelial cancer (adenocarcinoma) is responsible for the largest number of deaths from malignancies of the female genital tract and is the fifth leading cause of cancer death in women. 1 Most ovarian adenocarcinomas are of the serous histological type. 1 Clinically, about two-thirds of serous neoplasms of the ovary present de novo as advanced-stage tumors, reflecting their propensity for intra-abdominal/peritoneal spread. 1,2 These tumors arise from transformed cells of the celomic surface epithelium of müllerian origin that accounts for their ontogenetic and phenotypic kinship, histological overlap, and sometimes coexistence with carcinomas of the endometrium and endocervix. 3-5 Occasionally, identical serous neoplasms may arise from the so-called secondary müllerian system 6 involving the pelvic and lower abdominal mesothelium. These extraovarian serous adenocarcinomas, or papillary tumors of the peritoneum, are very closely related to their ovarian counterparts and are different, both in terms of phenotype and clinical behavior, from the mesotheliomas of the peritoneum. 5,7,8 Ovarian carcinomas, like carcinomas of the breast and endometrium, are steroid hormone-dependent epithelial neoplasms. One of the unifying features of the female genital cancer is the presence of steroid receptors in tumor cells, including estrogen, progesterone, and androgen receptors. 9
Retinoids are metabolites of retinol (vitamin A) and are considered to be important signaling molecules in the modulation of growth and differentiation of normal and neoplastic cells. 10-13 They have been shown to prevent mammary carcinogenesis in rodents, 13 inhibit the growth of human cancer cells in vitro, 10-12 and be effective chemopreventive and chemotherapeutic agents in a variety of human epithelial and hematopoietic neoplasms. 14,15 Retinoic acid (RA) has been shown to be an effective growth modulator of human ovarian carcinoma cell lines, imparting an inhibitory effect on ovarian tumor cell growth using RA either alone or in combination with other differentiation-inducing agents. 16-19 The effects of RA on ovarian cancer cells are thought to be mediated by nuclear RA receptors (RARs) and retinoid X receptors (RXRs). 20-22 These nuclear receptors are members of the steroid/thyroid receptor superfamily and can modulate gene transcription through a variety of mechanisms. 23-25 RARs include RARα, RARβ, and RARγ, each of which exhibit high affinity for both all-trans-RA and 9-cis-RA, whereas RXRs include RXRα, RXRβ, and RXRγ and are activated by 9-cis-RA. 26,27 Each RAR subtype comprises various isoforms as a result of different promoters and alternative splicing: the RARα gene contains two promoters transcribing two distinct isoforms, identified as RARα1 and RARα2 in humans. 28
Concomitant with ligand binding, the receptor-ligand complexes bind to their respective response elements (RARE and RXRE), located in the regulatory regions of a number of retinoid target genes. 11,21,29 Receptor binding to specific response elements and the resulting gene activation or inhibition occurs through the formation of heterodimers between RARs and RXRs; however, gene activation can also occur through RAR or RXR homodimers. 12 It has been suggested that although RXR ligands mediate transactivation through RXR homodimers, they are largely inactive in mediating transactivation through RAR/RXR heterodimers. 30-32 This raises the possibility that RARs, and not RXRs, are active in the various retinoid-mediated processes. 33
Despite several previous studies looking at the RAR/RXR signaling pathways in RA-sensitive and RA-resistant human ovarian cancer cell lines, until now, the cellular distribution of RAR proteins in human ovarian tumor specimens is unknown. In the present study, we have evaluated by immunohistochemistry the cellular localization of RARα protein and have examined whether there is a relationship between RARα and estrogen receptor (ER) labeling of tumor cells in surgical specimens of serous adenocarcinoma originating in the ovaries and the secondary müllerian system.
Materials and Methods
Patient Data
Patient data are summarized in Table 1 ▶ . All 16 patients had undergone exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, and debulking surgery. Patient ages ranged from 36 to 80 (median age, 64.5). Fifteen of 16 patients harbored intermediate (grade II) to high grade (grade III) serous papillary adenocarcinomas, and in one patient the tumor contained mixed serous papillary and endometrioid adenocarcinomas (Table 1 ▶ , case 4). In 11 of 16 patients there was a clearly defined ovarian primary lesion, in 3 patients the tumors were of peritoneal origin (Table 1 ▶ , cases 5, 15, and 16), and in one instance the origin of the serous adenocarcinoma was traced to the fallopian tube (Table 1 ▶ , case 11). The majority of patients had advanced Fédération Internationale des Gynaecologistes et Obstetristes (FIGO) stage III/IV disease at presentation (13 of 16 stage III, 1 of 16 stage IV). Two patients with ovarian and tubal serous papillary adenocarcinomas, respectively, were considered to be stage IC (Table 1 ▶ , cases 3 and 11). In 6 of 16 lesions, the specimens evaluated in this study were derived from metastatic deposits to the contralateral fallopian tube (Table 1 ▶ , case 9), pelvis (Table 1 ▶ , case 12), omentum (Table 1 ▶ , cases 5 and 7), and transverse mesentery (Table 1 ▶ , case 2).
Table 1.
Patient Characteristics
| No. | Age | Source of specimen | Histopathology/Grade | Extent of disease at surgery/Stage | FIGO stage |
|---|---|---|---|---|---|
| 1 | 75 | Left ovary | Serous papillary CA | Primary: left ovary | IIIB |
| Metastases to left fallopian tube, right mesovarium, uterine serosa, omentum, left pelvic peritoneum, sigmoid colon and mesentery, and mesentery and serosa of small intestine and hemidiaphragm | |||||
| 2 | 50 | Transverse mesentery | Serous papillary CA/II (metastatic) | Primary: N/D | IIIC |
| Metastases to transverse mesentery, sigmoid colon, vagina, spleen, REIA lymph node, falciform ligament, right upper/diaphragmatic peritoneum | |||||
| 3 | 47 | Right ovary | Serous papillary CA/II | Primary: right ovary | IC |
| 4 | 36 | Right ovary | Mixed serous papillary CA, endometrioid CA/II | Primary: right ovary | IIIA/B |
| Metastases to fallopian tubes bilaterally, uterine serosa, omentum, sigmoid colon, appendix, and right hemidiaphragm | |||||
| 5 | 56 | Omentum | Serous papillary CA/III (metastatic) | Primary: c/w peritoneal | IIIC |
| Metastases to gastrohepatic ligament, transverse mesocolon, and perisplenic region | |||||
| 6 | 66 | Left ovary | Serous papillary CA/III | Primary: left ovary | IIIA/B |
| Metastases to omentum, left and right fallopian tube and ovary, myometrium, cecum, sigmoid colon, soft tissues around REIA, and cul-de-sac | |||||
| 7 | 79 | Omentum | Serous papillary CA/III (metastatic) | Primary: left ovary | IIIB |
| Metastases to ipsilateral fallopian tube, omentum, periumbilical region, and small intestine | |||||
| 8 | 70 | Right ovary | Serous papillary CA/II | Primary: right ovary | IIIB |
| Metastases to right and contralateral (left) fallopian tubes and omentum; serosal metastatic deposits, uterine cervix and corpus uteri; synchronous poorly differentiated intramucosal serous papillary CA of the endometrium | |||||
| 9 | 47 | Left fallopian tube | Serous papillary CA/III (metastatic) | Primary: right ovary | IIIC |
| Metastases to contralateral (left) fallopian tube, left renal, and right para-aortic lymph nodes | |||||
| 10 | 63 | Left ovary | Serous papillary CA/III | Primary: left ovary | IIIB |
| Metastases to right ovary, parametria, uterine wall, omentum, perirenal soft tissues, falciform and round ligaments, right diaphragmatic peritoneum, hemidiaphragm, appendix, rectosigmoid | |||||
| 11 | 80 | Right adnexa | Serous papillary CA/III | Primary: right fallopian tube (11.5 cm); focal tumor extension through serosal surface | IC |
| 12 | 79 | Right pelvic mass | Serous papillary CA/III | Primary: right ovary | IIIC |
| Metastases to the right pelvis/cul-de-sac, myometrium, omentum, transverse colon, appendix, gastrocolic, and REIA lymph nodes (two of two) | |||||
| 13 | 53 | Left ovary | Serous papillary CA/III | Primary: left ovary | IIIB |
| Metastases to left fallopian tube, peritoneum/urinary bladder, sigmoid colon, mesentery; proliferative endometrium with focal endometrial hyperplasia; focal atypical tubal and clear cell metaplasia | |||||
| 14 | 85 | Left ovary | Serous papillary CA/II to III | Primary: left ovary | IIIA/B |
| Metastases to bladder peritoneum and sigmoid colon; history of breast CA with current new breast CA | |||||
| 15 | 49 | Omentum | Serous papillary CA/III (metastatic) | Primary: peritoneal | IIIB |
| Metastases to omentum; transverse colon; descending colon; sigmoid colon; cul-de-sac; and surfaces of the uterus, both ovaries, and right fallopian tube | |||||
| 16 | 72 | Peritoneal nodule | Serous papillary CA/III | Primary: peritoneal | IV |
| Metastases to omentum; surface of left ovary; lymphatic invasion of right ovary; serosal surface of anterior uterine wall; left infundibulopelvic ligament; serosal surface of right colon, sigmoid colon, and rectum; liver; lesser gastric curvature; and gastrohepatic ligament |
CA, carcinoma; ND, not determined; C/W, consistent with; REIA, right external iliac artery.
Tumor Specimens
Ovarian tumor specimens were obtained from the Department of Pathology, Fox Chase Cancer Center, under institutional Internal Review Board approval. All specimens were collected prospectively during a 2-year period (1995 to 1997). The tumor tissue samples procured for this investigational study were microdissected from, and were representative of, the surgical pathology specimens. The presence of viable (nonnecrotic) tumor tissue was determined grossly by the prosector. Surgically resected tumor samples were promptly embedded in ornithine carbamoyltransferase and were kept frozen in −70°C until further processing. Patient characteristics, source of tumor specimens, histopathological diagnosis, tumor grade, and FIGO stage at the time of surgery are summarized in Table 1 ▶ (also, see above).
Antibodies
A rabbit polyclonal antibody to RARα and a mouse monoclonal antibody to ER were used. RARα1 (C-20; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), is an affinity-purified polyclonal antibody raised against a peptide corresponding to amino acids 443 to 462 mapping at the COOH terminus of the RARα1 of human origin, which is identical in sequence to the corresponding region of RARα2. C-20 does not cross-react with RARβ or RARγ isoforms. The immunogen of the immunoglobulin (Ig) G1 mouse monoclonal antibody (clone 6F11) to human ER (Novocastra Laboratories Ltd., Newcastle on Tyne, UK) is prokaryotic recombinant protein corresponding to the full-length ER molecule.
Determination of Specificity of the RARα Antibody by Western Blot
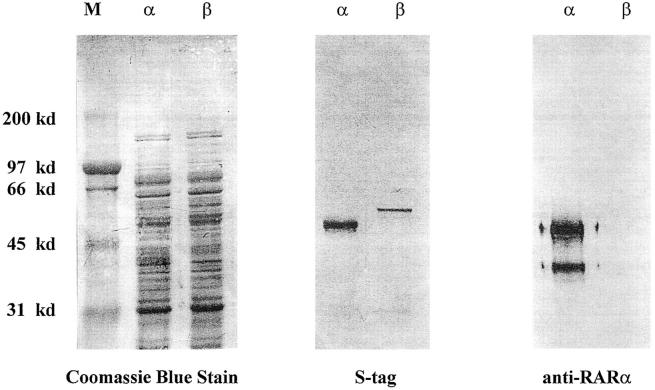
Recombinant RARα and recombinant RARβ proteins were prepared as S-Tag fusion proteins as previously described. 34 Briefly, Escherichia coli K12 strain BL21(DE3) cells were transformed with the expression of plasmid pET-29a (Novagen, Madison, WI) containing full-length cDNA of mouse RARα or mouse RARβ. Because the cDNAs are cloned in frame with an S-Tag marker, the proteins are expressed as fusion proteins that can be monitored by probing for the presence of the S-Tag. Bacterial extracts containing either recombinant RARα or recombinant RARβ were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride paper by electroblotting. The blots were probed with either S-Tag antibody or RARα antibody (C-20), and protein bands were detected by alkaline phosphatase as described previously. 35 The anti-RARα antibody only recognizes the RARα protein and not the RARβ protein (Figure 1) ▶ . This confirms the specificity of the RARα antibody used in this study.
Figure 1.
Demonstration of the specificity of the RARα antibody. Bacterial protein extract containing either RARα or RARβ S-Tag fusion protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The gels were either stained with Coomassie blue (left) or transferred to polyvinylidene difluoride paper by electroblotting (middle and right). The blots were then probed with either antibody to the S-Tag (middle) or antibody to RARα (right). Protein bands were detected by alkaline phosphatase. Left: Coomassie blue stain of all of the proteins present in the bacterial extracts used in these experiments. Middle: Results when the blot is probed with the anti-S-Tag antibody. It is evident that both RAR proteins are present in the extracts. Right: Results of probing the blot with the anti-RARα antibody. Clearly, the anti-RARα antibody only recognizes the RARα protein and not the RARβ protein. This confirms the specificity of the RARα antibody. The additional band is probably a degraded product that has lost its S-tag.
Immunoperoxidase Procedure
Cryostat sections (5 μm thick) were air-dried for 1 hour and then fixed in cold acetone at −20°C for 1 hour. Immunohistochemical staining was then performed according to the avidin biotin complex peroxidase method as previously described, 36 using commercially available kits, rabbit IgG and mouse IgG ABC Elite Vectastain (Vector Laboratories, Burlingame, CA) for polyclonal and monoclonal antibodies respectively. Briefly, sections were incubated with goat (rabbit IgG kit) or horse (mouse IgG kit) sera for 1 hour to reduce nonspecific binding. Sections were incubated with either anti-RARα (dilution 1:50) or anti-ER (dilution 1: 50) antibodies according to the manufacturers’ recommendations for 1 hour at room temperature. Antigen-antibody complexes were detected with anti-rabbit (or anti-mouse)-biotinylated avidin-horseradish peroxidase complex. The sections were then developed with 3,3′-diaminobenzidine as the peroxidase substrate. All experiments with the RARα antibody were performed in duplicate. Two complete immunostained sets of slides were generated: one without counterstaining to ensure unambiguous nuclear localization or lack thereof, and the other with light counterstaining with Mayer’s hematoxylin (Sigma Chemical Co., St. Louis, MO) to facilitate histological evaluation. The RA-sensitive human ovarian carcinoma cells (CAOV) grown in coverslips served as the positive control for RARα (Figure 2) ▶ . The CAOV cell line has been shown previously to constitutively express RARα mRNA. 19 Negative controls included normal rabbit IgG (1.25 μg/ml) and nonspecific mouse ascites fluid (Becton Dickinson, Mountain View, CA), which were used instead of specific rabbit anti-RARα1 or mouse monoclonal antibody 6F11 to ER, respectively.
Figure 2.
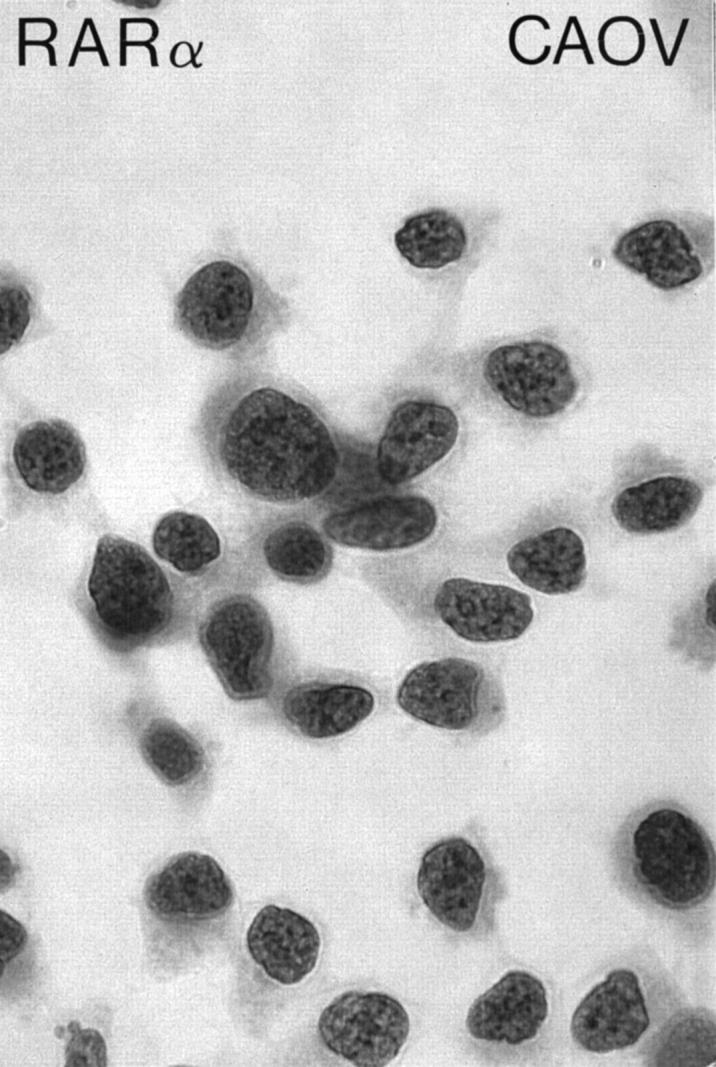
RARα immunostaining of CAOV cells grown as monolayers. CAOV is an RA-sensitive human ovarian carcinoma cell line and has been used in this study as a positive control. Avidin-biotin complex peroxidase method without hematoxylin counterstaining; original magnification, ×1000.
Analysis of Staining
Morphological assessment of immunostained tissue preparations and manual cell counting of immunolabeled tumor cells were performed. Initially, representative areas of the histological tumor specimen were selected under low-power magnification. Between 633 and 1271 epithelial tumor cell nuclei were evaluated per case, in 20 representative high-power fields (40×). Only tumor cells with unequivocal nuclear localization, irrespective of intensity of staining, were counted as positive. Staining of the nuclei of tumor-infiltrating lymphocytes (TILs) and the nuclei of nonneoplastic mesenchymal cells, such as fibroblasts and vascular endothelial cells, was excluded from the cell counts. Immunostained preparations were evaluated by two observers (CDK, IS) independently. The number of positive tumor cells (the numerator) in relation to the total number of tumor cells (the denominator) was recorded as the labeling count for each individual case. The percentage of immunolabeled tumor cells for a given tumor specimen was expressed as a mean labeling percentage based on the number of high-power fields examined. Mean labeling percentages for histological grades II and III were calculated from the total number of representative specimens examined. Interobserver agreement was within 15% (κ = 0.82).
Statistical Methods
RARα- and ER-labeled cell fractions were expressed in percentages. Overall variations in the percentages of RARα-labeled cells were evaluated by analysis of covariance using a main effects model for tumor origin, histological tumor grade, clinical (FIGO) stage, and metastasis, with ER percentages as the covariate. Relationships between the percentages of RARα-labeled cells and ER-labeled cells were evaluated by linear regression analysis, the Pearson correlation coefficient, and data plotting; the individual relationships between the percentages of RARα-labeled cells with metastatic source, tumor origin, histological grade, and FIGO stage were evaluated by one-way analysis of variance. A P value less than 0.05 was considered to be significant. The general linear model with LSMEANS posthoc testing and the plot procedures of the SAS package (SAS Institute, Cary, NC) were used for these statistical analyses.
Results
Cellular Localization of RARα and ER Proteins
RARα immunohistochemical staining was detected in all specimens, although the degree and cell-type distribution of immunolabeled cells varied widely among tissue specimens. Overall, RARα-positive tumor cells were present in more well differentiated portions of tumor growing as papillary fronts (Figures 3 ▶ , A, B, and C, and Figure 4 ▶ , A and C) and much less prominent in poorly differentiated areas of tumor typified by dense cellularity and a predominantly solid pattern of growth (Figures 3E and 4 ▶ ▶ , E and F). The percentage of RARα-labeled tumor cell nuclei was somewhat increased in the moderately differentiated, intermediate-grade (grade II) tumors containing variably prominent tubulopapillary structures, as compared with the less differentiated, high-grade (grade III) serous adenocarcinomas: the mean RARα labeling percentages for grades II and III were 67% (range 58 to 84%) and 30% (range 2 to 66%), respectively. A certain degree of RARα intratumoral staining heterogeneity was present in high-grade tumors, and when present it was usually associated with better differentiated papillary foci (Figure 3D) ▶ . However, RARα staining was also detected in poorly differentiated areas of tumor, either in scattered, individual cells, or in clusters of tumor cells (Figure 3E) ▶ .
Figure 3.
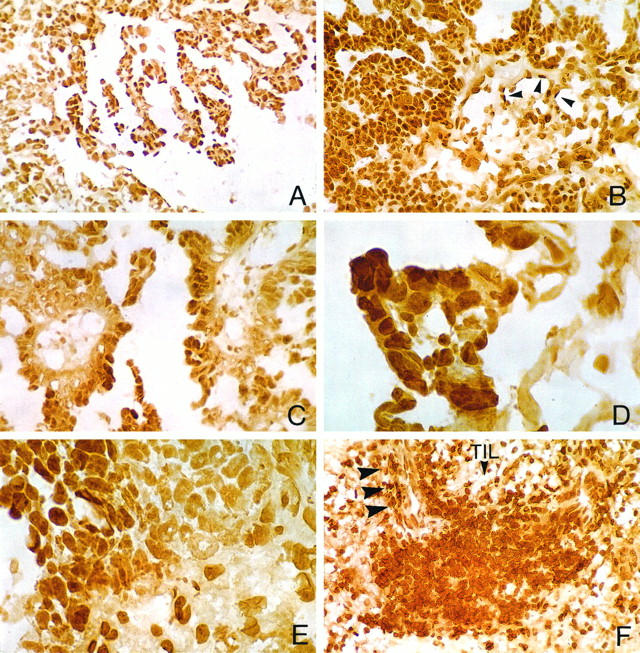
Panel featuring differential cellular localization of RARα in serous adenocarcinomas. A to C: Widespread RARα immunohistochemical staining of neoplastic cells in well to moderately differentiated areas of grade II serous tumors with papillary morphology. B depicts, in addition, RARα staining of endothelial cells in vessels of the fibrovascular core (arrowheads). D and E illustrate, respectively, intratumoral staining heterogeneity associated with better differentiated, albeit abortive, RARα-positive papillary foci (D) or clusters of RARα-positive poorly differentiated tumor cells (E). F: Prominent nodular aggregate of TILs with robust RARα staining. Arrowheads point to a frequently observed perivascular predilection of RARα-positive TILs. Avidin-biotin complex peroxidase method without hematoxylin counterstaining; original magnifications: A and B, ×400; C to F, ×1000.
Figure 4.
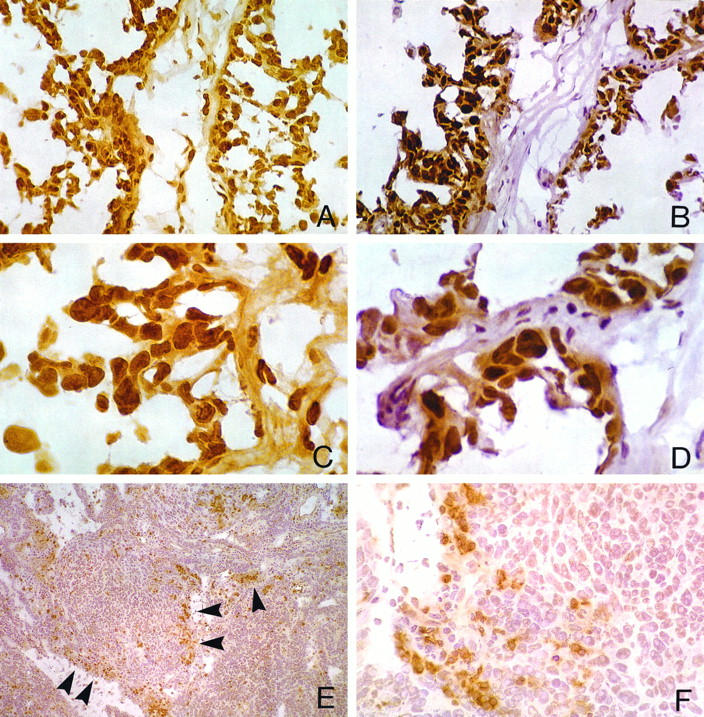
A through D demonstrate a comparison between RARα (A and C) and ER (B and D) nuclear staining in homologous fields from adjacent cryostat sections of a grade II serous adenocarcinoma (C and D are higher magnifications of A and B, respectively). Tumor cell immunoreactivity for both receptors is widespread in papillary areas. Note distinctive nuclear localization of both RARα and ER antibodies (C and D). E and F demonstrate absence or paucity of RARα immunoreactivity in tumor cells of a poorly differentiated (grade III) serous adenocarcinoma of peritoneal origin (F is a higher magnification of E). By contrast, there is robust RARα staining in TILs infiltrating haphazardly the tumor stroma (E, arrowheads, and F). Avidin-biotin complex immunoperoxidase method with (B, D, E, and F) and without (A and C) hematoxylin counterstaining. Original magnifications: A and B: ×400; C and D: ×1000; E: ×100; F: ×400.
Robust RARα staining was detected in benign, mononuclear cells infiltrating haphazardly the tumor stroma, known as TILs (Figures 3F and 4 ▶ ▶ , E and F). RARα-positive TILs occurred either as prominent nodular aggregates (Figure 3F) ▶ or as scanty stromal mononuclear cell infiltrates (Figure 4 ▶ , E and F). In one of our cases representing a high-grade, FIGO stage IV serous adenocarcinoma of peritoneal origin (Table 1 ▶ , case 16), RARα staining was present in TILs but was largely absent in tumor cells (Figure 4 ▶ , E and F). Also, RARα staining was present in nonneoplastic mesenchymal cells, consistent with fibroblasts of the desmoplastic tumor stroma, in endothelial cells of tumor blood vessels (Figure 3B ▶ , arrowheads), and in resident ovarian stromal cells (not shown).
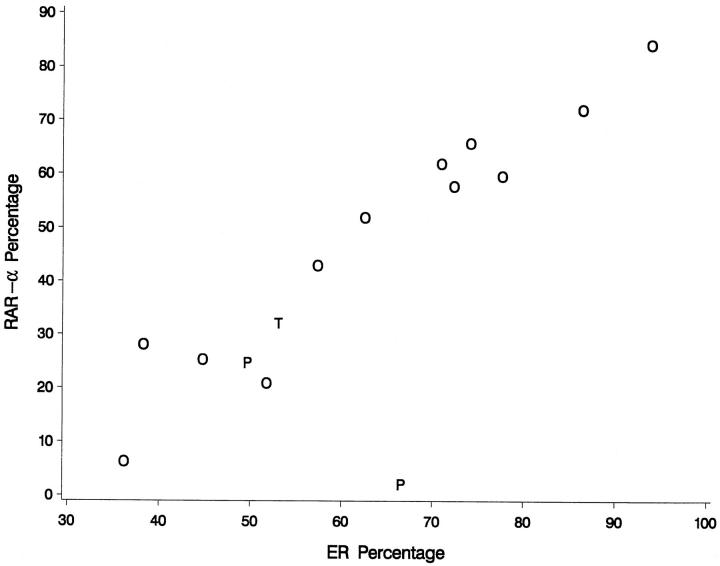
A distinctive ER nuclear staining was detected variously in all specimens. Immunoreactivity among tumor cells was particularly widespread in papillary areas (Figure 4 ▶ , B and D) but was also present in poorly differentiated areas of tumor (not shown). The number of ER-labeled cells in any given tissue specimen was consistently higher as compared with RARα (the mean ER labeling percentages for grades II and III were 80% (range 71 to 94%) and 53% (range 36 to 74%), respectively), but followed a differentiation-dependent trend similar to that of RARα. A strong linear relationship was found between percentages of RARα- and ER-immunolabeled tumor cells (Table 2 ▶ , Figure 5 ▶ ) (also, see below).
Table 2.
Regression and Variant Analyses of the Relationship of the Percentage of RARα-Labeled Tumor Cells with Several Continuous and Categorical Variables
| Variable* | P value |
|---|---|
| Continuous | |
| ER percentage† | 0.0002 |
| Categorical | |
| Histological grade | 0.038 |
| FIGO stage | 0.139 |
| Tumor origin‡ | 0.170 |
| Metastasis§ | 0.561 |
*Also see Figure 5 ▶ .
†Percentage of ER-positive tumor cells (numerator) relative to total number of tumor cells (denominator).
‡Ovarian, tubal, or peritoneal.
§Tumor specimen derived from an intra-abdominal metastasis (as opposed to the primary site).
Figure 5.
Data plotting depicting a strong linear relationship is found between percentages of RARα- and ER-immunolabeled tumor cells (r = 0.825). A significant relationship between tumor origin (O, ovary; T, fallopian tube; P, peritoneum) and the percentage of RARα-positive tumor cells is found only when the covariate effects of ER labeling are taken into account. This relationship appears to derive from the single data point of peritoneal tumor origin (P) that plots below the rest of the data (lower right portion of the graph). However, more data from serous tumors of peritoneal origin are needed before any conclusions may be drawn.
A deviation of this pattern was observed only in two specimens in which the percentage of ER-positive tumor cells was disproportionately higher as compared with RARα-positive tumor cells (Table 1 ▶ , cases 7 and 16) (Figure 5) ▶ : The first, case 7, was derived from an omental metastasis of a grade III/FIGO stage IIIB serous adenocarcinoma of ovarian origin. The second specimen, case 16, was from a grade III/FIGO stage IV serous adenocarcinoma of peritoneal origin (Figure 4 ▶ , D and F).
TILs and stromal fibroblasts of the tumor were ER negative; however, ovarian stromal cells were ER positive (not shown).
Statistical Analysis
The percentages of ER-labeled cells and the tumor origin were found to be jointly related to the percentages of RARα-labeled cells using analysis of covariance (P < 0.005), and on posthoc testing, the percentages of RARα-labeled cells when corrected for the covariate effects of ER labeling in the analysis of covariance model were found to be significantly higher in serous adenocarcinomas of ovarian origin than in homologous tumors of peritoneal origin (P < 0.01). Interestingly, when the relationship of RARα and tumor origin was examined by analysis of variance (without the information supplied by the ER labeling), no statistical significance was found (Table 2) ▶ . Results of statistical analysis of the individual relationships between RARα immunohistochemical labeling percentages of tumor cells and ER percentages, metastatic source of the specimen, tumor grade, and FIGO stage are shown in Table 2 ▶ . A strong linear relationship was found between the percentages of RARα- and ER-immunostained tumor cells (r = 0.825) by linear regression analysis and data plotting (Table 2 ▶ , Figure 5 ▶ ). A modest relationship (P < 0.04) was found between the percentages of RARα-positive tumor cells and histological grade with slightly higher labeling counts noted in grade II as compared with grade III serous tumors. No statistically significant relationship was found, respectively, between RARα immunoreactivity and FIGO stage or specimen sampling from metastatic versus primary tumor sites.
Discussion
Cellular Distribution of RARα Protein in Serous Adenocarcinomas
This study provides new data with regard to the cellular distribution of RARα protein in 16 frozen, surgically resected serous adenocarcinoma specimens originating in the ovaries, fallopian tubes and the pelvic peritoneum, ie, the so-called secondary müllerian system. Also, it examines the relationship between RARα and ER protein expression by comparing the percentage of immunostained tumor cells for either receptor in surgical specimens. The relatively small number of cases evaluated notwithstanding, this study indicates a strong linear relationship between the percentages of RARα- and ER-immunostained tumor cells as determined by linear regression analysis (Table 2 ▶ , Figure 5 ▶ ). RARα and ER immunoreactivities are present in both intermediate- (grade II) and high-grade (grade III) lesions, corresponding, for the most part, to advanced FIGO stage serous ovarian adenocarcinomas. A modest inverse relationship is found between the percentage of RARα-positive tumor cells and histological grade, attesting to a differentiation-dependent trend (Table 2) ▶ . Because histological grade in ovarian adenocarcinomas is largely a function of differentiation, there is a higher percentage of RARα-positive tumor cells in grade II tumors with papillary areas as compared with the grade III tumors. However, grade III tumors may also contain papillary foci, as well as a relatively small number of poorly differentiated RARα-positive cells. Conversely, no significant relationship is found between RARα-labeled cells and such categorical variables as FIGO stage; site of origin of tumor, ie, from ovary, fallopian tubes, or pelvic peritoneum; and source of specimen from an intra-abdominal metastasis (as opposed to the primary site) (Table 2) ▶ . Thus, RARα tumor cell labeling is present in specimens from primary ovarian, as well as metastatic tumor implants in the omentum, peritoneum, and parametria, indicating that serous carcinomas are capable of expressing RARα, and also ER, despite high histological grade and advanced clinical stage.
Previous in vitro studies have shown that RARα plays a major role in the growth inhibition of mammary cancer cells 37,38 and ovarian cancer cells 39,40 by retinoids in a dose-dependent manner. Still, the presence of RARα in intermediate- to high-grade, advanced-stage serous tumors demonstrated in this study is similar to that described previously in breast carcinomas. 41 van der Leede and collaborators 41 have proposed an apparent uncoupling of RARα expression and proliferation inhibition, offering a threefold explanation for this phenomenon: 1) perturbed transcriptional regulation as part of tumor progression; 2) loss of mechanisms of RARα down-regulation; and/or 3) insufficient retinoid levels to achieve down-regulation, hence culminating in overexpression of RARα. 41 It remains to be determined whether there are alterations in the levels of RARα isoforms in cancer cells because of alterations in the factors regulating RARα gene transcription at the promoter level. In this regard, there is evidence of estrogen-induced expression of RARα1 mRNA, but lack of RARα2 transcripts, in ER-positive breast carcinoma cell lines. 38 Interestingly, serum retinol has been found to be significantly lower in ovarian cancer patients, 42 although the actual content of retinoids in ovarian adenocarcinoma specimens is unknown. Collectively, malignant tumors in vivo may exhibit dysregulation of cellular differentiation signaling pathways, which may also involve RARs/RXRs.
To date, there has been only a small number of immunohistochemical studies aimed at the localization of RARs in normal and neoplastic tissues. This may be attributed to several confounding factors. The low concentration of individual RAR epitopes may hinder their detection by immunohistochemistry, 43,44 even in neoplastic cells, such as leukemia cells. 45 At this time, there is only a limited number of commercially available subtype-specific antibodies. Their immunohistochemical performance in chemically fixed tissues is not well defined and may be punctuated by unexpected (or even spurious) localizations. Also, the presence of cellular retinoid binding proteins may hinder the localization of RARs, a problem that has been addressed previously in the context of autoradiography. 46 Thus far, immunohistochemical studies on surgical tumor specimens are limited to the evaluation of RARα in breast carcinomas. 41,47 The latter two studies have used archival, formalin-fixed, paraffin-embedded tissue sections and different antibodies. In one of them, unexpected RARα cytoplasmic staining of tumor cells has been detected in addition to nuclear staining. 41 We have also observed a similar cytoplasmic localization for RARα in a large number of formalin-fixed, paraffin-embedded ovarian tumor specimens, which led us to withdraw these data from the present study. Clearly, an abnormal processing of RARα in tumor cells deserves consideration in this respect; 41 however, in our view, this appears less likely, because cytoplasmic staining has also been detected, by us, in benign epithelial and mesenchymal cells in paraffin, but not in frozen, acetone-fixed sections of ovarian, adnexal, and other unrelated tissues (CDK, Y. Yu, IS, and KJS, unpublished observations).
To minimize potential artifacts introduced either by the use of cross-linking fixatives or by conventional tissue processing and paraffin embedding, we elected to perform immunohistochemical staining only on frozen sections fixed briefly in cold acetone in −20°C. This is a reliable method that renders unambiguous nuclear localizations, at least as evidenced with the RARα antibody used in this study. That the anti-RARα antibody recognizes RARα in bacteria is also supported by the expected nuclear immunolocalizations in human cells in situ, thus collectively providing strong evidence for specificity. Additional issues regarding accurate determination of steroid receptors in general and RARs in particular in surgical specimens include tumor cell heterogeneity and receptor expression by nonneoplastic cell types within the tumor, such as TILs, vascular endothelial cells, and stromal fibroblasts. Thus, immunohistochemistry is the single most appropriate and accurate method of determining the cellular source of steroid receptor protein in ovarian tumor specimens. 48
RARα Localization in TILs
The widespread presence and intensity of RARα staining in TILs is noteworthy. It is detected in >80% of mononuclear inflammatory cells randomly dispersed either in the periphery or within sheets of cancer cells. Although all 16 specimens contain RARα-positive mononuclear cells in varying proportions, 6 of 16 tumors exhibit prominent TILs. A similar RARα staining pattern of the TILs has been described, in passing, in a series of breast carcinomas. 41 Although the immunological significance of TILs in epithelial tumors in general and in ovarian carcinomas in particular is unclear, it has been suggested that they may represent tumor cytolytic oligoclonal T-cell responses. 49
Retinoids are multicellular immunomodulators both in vivo and in vitro, including but not limited to various tumor cell types, human and murine thymocytes, fibroblasts, Langerhans’ cells, natural killer cells, and T lymphocytes. 50 Both retinol and RA induce vigorous proliferative responses on human peripheral blood mononuclear cells after stimulation with anti-CD3 antibodies, which is specifically mediated through the clonotypic T-cell receptor-CD3 complex and correlates with the up-regulation of surface T-lymphocyte adhesion/activation markers, as well as an augmentation of interleukin-2 and interferon-γ transcripts. 50 It has been shown that RA promotes proliferation and induces RARα gene expression in murine T lymphocytes and that RA and RARα might function in T cells as ligand-inducible transcriptional enhancer factors. 51 It remains to be determined whether the expression of RARα in ovarian carcinoma TILs is related to tumor antigen-specific or oligoclonal T-cell response(s).
Relationship of RARα and ER Immunoreactivity Profiles
Despite the exclusion of RARA, the gene for RARα, as a candidate gene for brca-1 (the susceptibility gene for hereditary breast-ovarian cancer), 52 there is nonetheless evidence to suggest that ovarian and breast cancer cells may exploit similar signaling pathways for growth and differentiation, insofar as they are both steroid hormone-dependent neoplasms. Compared with mammary 53 and endometrial cancers 54 in which the ER status is prognostically significant, a linear relationship between ER level and survival has not been established in ovarian carcinomas. 55-57 By biochemical radioligand assays, ovarian carcinomas have been shown to contain ER in the 60% tumor range. 9 Others have reported that this percentage declines to 38% when immunohistochemistry is used as the method of detection. 48 Using solely cryostat sections and monoclonal antibody 6F11, we have found ER mean labeling percentages of 80% for grade II and 53% for grade III serous tumors. Interestingly, Slotman and coworkers 58 have found no correlation between tumor ploidy and histological grade, stage of disease, and ER content in ovarian adenocarcinomas.
There are several lines of evidence suggesting a positive relationship between RARα and ER gene 59 and protein 57 expression in breast carcinomas and human mammary carcinoma cell lines. RARα appears to be required for inhibition of anchorage-independent growth either by natural (all-trans-RA) or by conformationally restricted retinoids with agonist activity in the MCF-7 ER-positive breast carcinoma cell line. 60 Moreover, although a statistically significant correlation has been established between RARα and ER mRNA in primary breast tumors, no such correlation has been found to exist between ER levels and expression of either RARβ, RARγ, RXRα, RXRβ, or RXRγ mRNA levels. 61,62 It is believed that the relationship between RARα and ER gene expression is partly caused by estradiol enhancement of RARα gene expression, 59,63 which is mediated through an imperfect half-palindromic estrogen response element and Sp1 motifs. 63 In contrast to RARα, the mechanism responsible for the retinoid sensitivity of breast cancer cells does not involve transcriptional modulation of the RXRs by RA. 64 Most ER-negative breast carcinomas express lower levels of RARα and are largely resistant to the growth-inhibition effects of RA compounds in vitro. 65 However, estradiol-independent enhancement of RARα gene expression has been demonstrated in certain ER-negative breast cancer cell lines, such as SKBR-31 and MDA-MB-435. 65 RA-mediated growth inhibition in these lines is accomplished via a 72-bp fragment of RARα promoter that contains unique cis elements. 65 To date, there are conflicting reports with regard to a relationship between RARα and ER in breast tumors at the protein level. Recently, Han and co-workers 47 have reported that RARα expression is significantly increased in ER-positive breast tumors as determined by immunohistochemistry and image cytometry. This is in contrast to a previous immunohistochemical study claiming that no such relationship exists between RARα and ER status. 41
It has been previously shown that 17-β-estradiol may regulate growth in human ovarian carcinoma lines. 66 17-β-Estradiol can stimulate the growth of populations of ER-positive ovarian carcinoma cells that may in turn be associated with changes in the cellular levels of steroid hormone receptors. 66 Our findings in serous ovarian carcinomas are consistent with those by Han and collaborators 47 in breast carcinomas, insofar as they support a relationship trend between RARα and ER expression in certain steroid hormone-dependent epithelial neoplasms. However, as alluded to above, a definitive determination in this regard awaits the evaluation of a larger sample of tumors.
Concluding Remarks and Future Directions
In the past, encouraging results have been obtained with RA-based treatment of patients with locally advanced squamous cell carcinoma of the uterine cervix 67 and cisplatin-resistant metastatic endometrial adenocarcinoma. 68 Because RARα plays a major role in retinoid-mediated growth inhibition of breast and ovarian cancer cells in vitro, it is also possible that patients with breast 41 and ovarian carcinomas could be responsive to retinoids independently of their responsiveness to antiestrogen regimens. Based on their specific RAR/RXR profile and method of delivery in vivo, ovarian carcinomas may be amenable to a variety of therapeutic interventions using conformationally restricted retinoid ligands. 40 This underscores the importance of future studies aiming to elucidate the full RAR/RXR profile in serous adenocarcinomas of the ovary and secondary müllerian system.
Acknowledgments
We are grateful to Tina Rader, Pathologist Assistant, Department of Pathology, Fox Chase Cancer Center, for coordinating the procurement of tumor specimens. Dr. Yunxia Yu and Sister Anne Donigan, Ph.D., participated in the initial phases of this study.
Footnotes
Address reprint requests to Dr. Christos D. Katsetos, Section of Neurology/Research Laboratories, St. Christopher’s Hospital for Children, Erie Avenue at Front Street, Philadelphia, PA 19134.
Supported by National Cancer Institute grant RO1 CA64945 (to KJS) and by Research Supplement for Individuals with Disabilities grant 3 RO1-CA64945-02S1 from the National Cancer Institute’s Comprehensive Minority Biomedical Program (to CDK).
This work is dedicated to Dr. Demetrios Katsetos on the occasion of his 70th birthday.
References
- 1.Piver MS, Baker TP, Piedmonte M, Sandecki AM: Epidemiology and etiology of ovarian cancer. Semin Oncol 1991, 18:177-185 [PubMed] [Google Scholar]
- 2.Rose PG, Piver MS, Tsukada Y, Lau TS: Metastatic patterns in histologic variants of ovarian cancer: an autopsy study. Cancer 1989, 64:1508-1513 [DOI] [PubMed] [Google Scholar]
- 3.Eifel P, Hendickson M, Ross J, Ballon S, Martinez A, Kempson R: Simultaneous presentation of carcinoma involving the ovary and the uterine corpus. Cancer 1982, 50:163-170 [DOI] [PubMed] [Google Scholar]
- 4.Kaminski PF, Norris HJ: Coexistence of ovarian neoplasms and endocervical adenocarcinoma. Obstet Gynecol 1984, 64:553-556 [PubMed] [Google Scholar]
- 5.Rosai J: Female reproductive system (ovary). ed 8 Ackerman’s Textbook of Surgical Pathology, 1996, :pp 1461-1539 Mosby, St. Louis [Google Scholar]
- 6.Blaustein A: Peritoneal mesothelium and ovarian surface cells: shared characteristics. Int J Gynecol Pathol 1984, 3:361-375 [PubMed] [Google Scholar]
- 7.Foyle A, Al-Jabi M, McGaughey WTE: Papillary peritoneal tumors in women. Am J Surg Pathol 1981, 5:241-249 [DOI] [PubMed] [Google Scholar]
- 8.Wick MR, Mills SE, Dehner LP, Bollinger DJ, Fechner RE: Serous papillary carcinomas arising from peritoneum and the ovaries: clinicopathologic and immunohistochemical comparison. Int J Gynecol Pathol 1989, 8:179-188 [DOI] [PubMed] [Google Scholar]
- 9.Rao BR, Slotman BJ: Endocrine factors in common epithelial ovarian cancer. Endocr Rev 1991, 12:14-26 [DOI] [PubMed] [Google Scholar]
- 10.De Luca LM: Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J 1991, 5:2924-2933 [PubMed] [Google Scholar]
- 11.Gudas LJ: Retinoids, retinoid-responsive genes, cell differentiation, and cancer. Cell Growth Differ 1992, 3:655-662 [PubMed] [Google Scholar]
- 12.Mangelsdorf DJ, Umesono K, Evans RM: The retinoid receptors. ed 2 Sporn MB Roberts AB Goodman DS eds. The Retinoids: Biology, Chemistry and Medicine, 1994, :pp 314-349 Raven Press, New York [Google Scholar]
- 13.Moon RC, Metha RG, Rao KVN: Retinoids and cancer in experimental animals. ed 2 Sporn MB Roberts AB Goodman DS eds. The Retinoids: Biology, Chemistry and Medicine, 1994, :pp 573-596 Raven Press, New York [Google Scholar]
- 14.Smith MA, Parkinson DR, Cheson BD, Friedman MA: Retinoids in cancer therapy. J Clin Oncol 1992, 10:839-864 [DOI] [PubMed] [Google Scholar]
- 15.Hong WK, Itri LM: Retinoids and human cancer. ed 2 Sporn MB Roberts AB Goodman DS eds. The Retinoids: Biology, Chemistry and Medicine, 1994, :pp 597-630 Raven Press, New York [Google Scholar]
- 16.Langdon SP, Hawkes MM, Hay FG, Lawrie SS, Schol DJ, Hilgers J, Leonard RCF, Smyth JF: Effect of sodium butyrate and other differentiation inducers on poorly differentiated human ovarian adenocarcinoma cell lines. Cancer Res 1988, 48:6161-6165 [PubMed] [Google Scholar]
- 17.Marth C, Helmberg M, Mayer I, Fuith LC, Daxenbichler G, Dapunt O: Effects of biological response modifiers on ovarian carcinoma cell lines. Anticancer Res 1989, 9:461-467 [PubMed] [Google Scholar]
- 18.Grunt TW, Somay C, Oeller H, Dittrich E, Dittrich C: Comparative analysis of the effects of dimethyl sulfoxide and retinoic acid on the antigenic pattern of human ovarian adenocarcinoma cells. J Cell Sci 1992, 103:501-509 [DOI] [PubMed] [Google Scholar]
- 19.Harant H, Korschineck I, Krupitza G, Fazeny B, Dittrich C, Grunt TW: Retinoic acid receptors in retinoid responsive cancer cell lines detected by polymerase chain reaction following reverse transcription. Br J Cancer 1993, 68:530-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caliaro MJ, Marmouget C, Guichard S, Mazars P, Valette A, Moisand A, Bugat R, Josan S: Response of four human ovarian carcinoma cell lines to all-trans-retinoic acid: relationship with induction of differentiation and retinoic acid receptor expression. Int J Cancer 1994, 56:743-748 [DOI] [PubMed] [Google Scholar]
- 21.van der Saag PT: Nuclear retinoid receptors: mediators of retinoid effects. Eur J Clin Nutr 1996, 50(suppl 3):S24-S28 [PubMed] [Google Scholar]
- 22.Soprano DR, Chen LX, Wu S, Donigan AM, Borgaei RC, Soprano KJ: Overexpression of both RAR and RXR restores AP-1 repression in ovarian adenocarcinoma cells resistant to retinoic acid-dependent growth inhibition. Oncogene 1997, 12:577-584 [PubMed] [Google Scholar]
- 23.O’Malley BW: The steroid superfamily, more excitement predicted in the future. Mol Endocrinol 1990, 4:363-369 [DOI] [PubMed] [Google Scholar]
- 24.Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS: The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 1991, 67:59-77 [DOI] [PubMed] [Google Scholar]
- 25.Bhat MK, Ashizawa K, Cheng S-Y: Phosphorylation enhances the target gene sequence-dependent dimerization of thyroid hormone receptor with retinoid X receptor. Proc Natl Acad Sci USA 1994, 91:7927-7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, Grippo JF: 9-cis-Retinoic acid stereoisomer binds and activates the nuclear receptor RXR-α. Nature 1992, 355:359-361 [DOI] [PubMed] [Google Scholar]
- 27.Heyman RA, Mangelsdorf DJ, Dyck JA, Sten RB, Eichele G, Evans RM, Thaller C: 9-cis-Retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 1992, 68:397-406 [DOI] [PubMed] [Google Scholar]
- 28.Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier J-M, Kastner P, Dierich A, Chambon P: Multiple isoforms of the mouse retinoic acid receptor α are generated by alternative splicing and differential induction by retinoic acid. EMBO J 1991, 10:59-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohnes D, Dierich A, Ghyselinck N, Kastner P, Lampron C, Le Meur M, Lufkin T, Mendelsohn C, Naksharti H, Chambon P: Retinoid receptors and binding proteins. J Cell Sci 1991, 16(suppl):69-76 [DOI] [PubMed] [Google Scholar]
- 30.Lehmann JM, Jong L, Fanjul A, Cameron JF, Lu XP, Haefner P, Dawson MI, Pgahl M: Retinoids selective for retinoid X receptor response pathways. Science 1992, 258:1944-1946 [DOI] [PubMed] [Google Scholar]
- 31.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld MG, Heyman RA, Glass CK: Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature 1994, 371:528-531 [DOI] [PubMed] [Google Scholar]
- 32.Mangelsdorf DJ, Evans RM: The RXR heterodimers and orphan receptors. Cell 1995, 83:841-850 [DOI] [PubMed] [Google Scholar]
- 33.Horn V, Minucci S, Ogryzko VV, Adamson ED, Howard BH, Levin AA, Ozato K: RAR and RXR selective ligands cooperatively induce apoptosis and neuronal differentiation in P19 embryonal carcinoma cells. FASEB J 1996, 10:1071-1077 [DOI] [PubMed] [Google Scholar]
- 34.Wolfgang CL, Zhang Z-p, Gabriel JL, Pieringer RA, Soprano KJ, Soprano DR: Identification of sulfhydryl-modified cysteine residues in the ligand binding pocket of retinoic acid receptor β. J Biol Chem 1997, 272:746-753 [DOI] [PubMed] [Google Scholar]
- 35.Scafonas A, Wolfgang CL, Gabriel JL, Soprano KJ, Soprano DR: Differential role of homologous positively charged amino acid residues for ligand binding in retinoic acid receptor α compared with retinoic acid receptor β. J Biol Chem 1997, 272:11244-11249 [DOI] [PubMed] [Google Scholar]
- 36.Cooper HS, Malecha MJ, Bass C, Fagel PL, Steplewski Z: Expression of blood group antigens H-2, Ley, and sialylated-Lea in human colorectal carcinoma: an immunohistochemical study using double-labeling techniques. Am J Pathol 1992, 38:103-110 [PMC free article] [PubMed] [Google Scholar]
- 37.Sheikh MS, Shao Z-M, Li X-S, Dawson M, Jetten AM, Wu S, Conley BA, Garcia M, Rochefort H, Fontana JA: Retinoid-resistant estrogen receptor-negative human breast carcinoma cells transfected with retinoic acid receptor-α acquire sensitivity to growth inhibition by retinoids. J Biol Chem 1994, 269:21440-21447 [PubMed] [Google Scholar]
- 38.van der Leede BM, Folkers GE, van den Brink, van der Saag PT, van der Burg B: Retinoic acid receptor α1 isoform is induced by estradiol and confers retinoic acid sensitivity in human breast cancer cells. Mol Cell Endocrinol 1995, 109:77-86 [DOI] [PubMed] [Google Scholar]
- 39.Wu S, Zhang Z, Ahang D, Soprano DR, Soprano KJ: Reduction of both RAR and RXR levels is required to maximally alter sensitivity of CA-OV3 ovarian tumor cells to growth suppression by all-trans-retinoic acid. Exp Cell Res 1997, 237:118-126 [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Zhang D, Donigan A, Dawson MI, Soprano DR, Soprano KJ: Effects of conformationally restricted synthetic retinoids on ovarian tumor cell growth. J Cell Biochem 1998, 68:378-388 [DOI] [PubMed] [Google Scholar]
- 41.van der Leede BM, Geertzema J, Vroom TM, Decimo D, Lutz Y, van der Saag PT, van der Burg B: Immunohistochemical analysis of retinoic acid receptor-α in human breast tumors: retinoic acid receptor-α expression correlates with proliferative activity. Am J Pathol 1996, 148:1905-1914 [PMC free article] [PubMed] [Google Scholar]
- 42.Das NP, Ma CW, Salmon YM: The relationship of serum vitamin A, cholesterol, and triglycerides to the incidence of ovarian cancer. Biochem Med Metab Biol 1987, 37:213-219 [DOI] [PubMed] [Google Scholar]
- 43.Cavey MT, Martin B, Carlavan I, Shroot B: In vitro binding of retinoids to the nuclear retinoic acid receptor α. Anal Biochem 1990, 186:19-23 [DOI] [PubMed] [Google Scholar]
- 44.Zhuang Y-H, Ylikomi T, Lindorfs M, Piippo SW, Tuohimaa P: Immunolocalization of retinoic acid receptors in rat, mouse and human ovary and uterus. J Steroid Biochem 1994, 48:61-68 [DOI] [PubMed] [Google Scholar]
- 45.Nevi C, Grippo JF, Sherman MI, George MD, Jetten AM: Identification and characterization of nuclear retinoic acid-binding activity in human myeloblastic leukemia HL-60 cells. Proc Natl Acad Acad Sci USA 1989, 86:5854-5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chytil F, Ong DE: Cellular retinoid-binding proteins. Sporn MB Roberts AB Goodman DS eds. The Retinoids, 1984, vol 2.:p 89 Academic Press, Orlando, FL [Google Scholar]
- 47.Han Q-X, Allegretto EA, Shao Z-M, Kute TE, Ordonez J, Aisner SC, Rishi AK, Fontana JA: Elevated expression of retinoic acid receptor-α (RARα) in estrogen-receptor-positive breast carcinomas as detected by immunohistochemistry. Diagn Mol Pathol 1997, 6:42-48 [DOI] [PubMed] [Google Scholar]
- 48.Kommoss F, Pfisterer J, Thome M, Schafer W, Sauerbri W, Pfleiderer A: Steroid receptors in ovarian carcinoma: immunohistochemical determination may lead to new aspects. Gynecol Oncol 1992, 47:317-322 [DOI] [PubMed] [Google Scholar]
- 49.Freedman RS, Platsoucas CD: Immunotherapy for peritoneal ovarian carcinoma metastasis using in vitro expanded tumor-infiltrating lymphocytes. Cancer Treat Res 1996, 82:115-146 [DOI] [PubMed] [Google Scholar]
- 50.Allende LM, Corell A, Madrono A, Gongora R, Rodriguez-Gallego C, Lopez-Goyanes A, Rosai M, Arnaiz-Villena A: Retinol (vitamin A) is a cofactor in CD3-induced human T-lymphocyte activation. Immunology 1997, 90:388-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman A, Halevy O, Schrift M, Arazi Y, Sklan D: Retinoic acid promotes proliferation and induces expression of retinoic acid receptor-α gene in murine T lymphocytes. Cell Immunol 1993, 152:240-248 [DOI] [PubMed] [Google Scholar]
- 52.Simard J, Feunteun J, Lenoir G, Tonin P, Normand T, Luu Thé V, Vivier A, Lasko D, Morgan K, Rouleau GA, Lynch H, Labrie F, Navod SA: Genetic mapping of the breast-ovarian cancer syndrome to a small interval on chromosome 17q12–21: exclusion of candidate genes EDH17B2 and RARA. Hum Mol Genet 1993, 2:1193-1199 [DOI] [PubMed] [Google Scholar]
- 53.McGuire WL: Steroid hormone receptors in breast cancer treatment. Recent Prog Horm Res 1980, 36:135-149 [DOI] [PubMed] [Google Scholar]
- 54.Martin JD, Hacknel R, McCartney AJ, Woodlings TL: The effect of estrogen receptor status on survival in patients with endometrial cancer. Am J Obstet Gynecol 1983, 147:322-324 [DOI] [PubMed] [Google Scholar]
- 55.Schwartz PE, MacLusky N, Merino MJ, LiVolsi VA, Kohorn EI, Eisenfeld A: Are cytosol estrogen and progestin receptors of prognostic significance in the management of epithelial ovarian cancers? Obstet Gynecol 1986, 68:751-758 [PubMed] [Google Scholar]
- 56.Slotman BJ, Rao BR: Ovarian cancer (review): etiology, diagnosis, prognosis, surgery, radiotherapy, and endocrine therapy. Anticancer Res 1988, 8:417-434 [PubMed] [Google Scholar]
- 57.Kieback DG, McCamant SK, Press MF, Atkinson EN, Gallager HS, Edwards CL, Hajek RA, Jones LA: Improved prediction of survival in advanced adenocarcinoma of the ovary by immunocytochemical analysis and the composition adjusted receptor level of the estrogen receptor. Cancer Res 1993, 53:5188-5192 [PubMed] [Google Scholar]
- 58.Slotman BJ, Baak JP, Rao BR: Correlation between nuclear DNA content and steroid receptor status in ovarian cancer. Eur J Obstet Gynecol Reprod Biol 1991, 38:221-227 [DOI] [PubMed] [Google Scholar]
- 59.Roman SD, Ormandy CJ, Manning DL, Blamey RW, Nicholson RI, Sutherland RL, Clarke CL: Estradiol induction of retinoic acid receptors in human breast cancer cells. Cancer Res 1993, 53:5940-5945 [PubMed] [Google Scholar]
- 60.Dawson MI, Chao WR, Pine P, Jong L, Hobbs PD, Rudd CK, Quick TC, Niles RM, Zhang XK, Lombardo A, Ely KR, Shroot B, Fontana JA: Correlation of retinoid binding affinity to retinoic acid receptor α with retinoid inhibition of growth of estrogen receptor-positive MCF-7 mammary carcinoma cells. Cancer Res 1995, 55:4446-4451 [PubMed] [Google Scholar]
- 61.Sheikh MS, Shao Z-M, Cjen J-C, Hussein A, Jetten AM, Fontana JA: Estrogen receptor-negative breast cancer cells transfected with estrogen receptor exhibit increased RARα gene expression and sensitivity to growth inhibition by retinoic acid. J Cell Biochem 1993, 53:394-404 [DOI] [PubMed] [Google Scholar]
- 62.van der Burg B, van der Leede BM, Kwakkenbos-Isbrucker L, Salverda S, de Laat SW, van der Saag PT: Retinoic acid resistance of estradiol-independent breast cancer cells coincides with diminished retinoic acid receptor function. Mol Cell Endocrinol 1993, 91:149-157 [DOI] [PubMed] [Google Scholar]
- 63.Rishi AK, Shao Z-M, Baumann RG, Li X-S, Seikh MS, Kimura S, Bashirelahi N, Fontana JA: Estradiol regulation of human retinoic acid receptor α gene in human breast carcinoma cells is mediated by an imperfect half-palindromic estrogen response element and Sp1 motifs. Cancer Res 1995, 55:4999-5006 [PubMed] [Google Scholar]
- 64.Zhao Z, Zhang ZP, Soprano DR, Soprano KJ: Effect of 9-cis-retinoic acid on growth and RXR expression in human breast cancer cells. Exp Cell Res 1995, 219:555-561 [DOI] [PubMed] [Google Scholar]
- 65.Rishi AK, Gerald TM, Shao ZM, Li XS, Baumann RG, Dawson MI, Fontana JA: Regulation of the human retinoic acid receptor α gene in the estrogen receptor-negative human breast carcinoma cell lines SKBR-3 and MDA-MB-435. Cancer Res 1996, 56:5246-5252 [PubMed] [Google Scholar]
- 66.Langdon SP, Hirst GL, Miller EP, Hawkins RA, Tesdale AL, Smyth JF, Miller WR: The regulation of growth and protein expression by estrogen in vitro: a study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol 1994, 50:131-135 [DOI] [PubMed] [Google Scholar]
- 67.Lippman SM, Kavanagh JJ, Paredes-Espinoza M, Delgadillo-Madrueno F, Paredes-Cancillas P, Hong WK, Holdener E, Krakoff I: 13-cis-Retinoic acid plus interferon-α-2a: highly active systemic therapy for squamous cell carcinoma of the cervix. J Natl Cancer Inst 1992, 84:241-245 [DOI] [PubMed] [Google Scholar]
- 68.Kudelka AP, Freedman RS, Edwards CL, Lippman SM, Tornos CF, Krakoff IH, Kavanagh JJ: Metastatic adenocarcinoma of the endometrium treated with 13-cis-retinoic acid plus interferon α. Anticancer Drugs 1993, 4:335-337 [DOI] [PubMed] [Google Scholar]