Abstract
The cell cycle is governed by a family of cyclin-dependent kinases (Cdks). Cdk2 forms a functional complex with cyclin E and plays a pivotal role in the regulation of G1/S transition. Cdk2 activity is negatively regulated by interactions with inhibitors. p27Kip1, one of the most potent inhibitors of Cdk2, was recently identified as a powerful negative prognostic marker in non-small cell lung cancer as well as in colorectal and breast cancer. In the present study, the expression of p27 and Ki-67 antigen in nonneoplastic and cancerous lung tissues was determined by immunohistochemistry. After establishing that the antibody-measured p27 labeling index was a good reflection of the level of p27 expression measured by Western blotting, we show that p27 labeling index is decreased in cancerous lung tissues, compared with nonneoplastic lung tissues, and exhibits a significant inverse relation to the proliferation marker Ki-67 antigen, detected with monoclonal antibody MIB-1. Consistent with these data, all cancerous lung tissues showed enhanced degradation activity of p27 compared with nonneoplastic lung tissues and, in addition, increased levels of the phosphorylated form of Cdk2, as determined with Western blot analysis. The H1 histone kinase activity associated with Cdk2 was also increased in non-small cell lung cancers. Statistical analysis showed that proliferative activity as measured by MIB-1 labeling index was highly correlated with Cdk2 activity (r = 0.767, P < 0.0015). These results suggest that p27 and Cdk2 may play an important role in the proliferation of non-small cell cancer.
Lung cancer is one of the leading causes of cancer death throughout the world. Despite major advances in cancer treatment in the past two decades, the prognosis of patients with lung cancer has improved only minimally. In cases not eligible for surgery, which amount to almost two-thirds of all lung cancers, chemotherapy or radiotherapy are commonly used. 1 Despite these treatments, local recurrence or distant metastasis develops in most cases.
Recent progress in the study of the molecular biology of cancer has contributed to a better understanding of its molecular pathogenesis. Additionally, advances in cell cycle research have paved the way for the identification of proteins responsible for the regulation of cell proliferation. The cell cycle is governed by a family of cyclin-dependent kinases (Cdks). Among various Cdks, Cdk2 plays a pivotal role in the regulation of the cell cycle at the G1/S transition. For complete kinase activity, Cdks require physical association with regulatory subunits called cyclins. 2-4 Cdk2 forms a functional complex with cyclin E during the G1/S phase; the expression of Cdk2 and abundance of the cyclin E/Cdk2 complex are maximal at the G1/S transition. 5 Cdk2 activity is negatively regulated by interactions with inhibitors, among which p27Kip1 (p27) is the most potent. 6,7 p27 is present in large amounts in quiescent cells and declines when cells proliferate in response to mitogenic signals such as growth factors and cytokines. 8-10 Thus, the loss of p27 may contribute to oncogenesis and tumor progression. Unlike traditional tumor suppressor genes, the p27 gene rarely exhibits homozygous deletions or point mutations. 11-14 Further, it was recently demonstrated that the absence of p27 expression is a powerful negative prognostic marker in non-small cell lung cancers (NSCLCs), as well as in colorectal and breast cancers. 15-17 Thus, the potential function of p27 as a tumor suppressor provides new insight into the link between the cell cycle and oncogenesis in lung, colon, and breast tissues.
The proliferation index is a potent biological marker that estimates the growth of neoplasms quantitatively and can aid in determining the prognosis of patients with neoplasms. 18-20 A variety of methods have been used for the estimation of the proliferation index of human cancers. Ki-67 is the most reliable antibody for assessing the growth fraction by immunohistochemistry. 21 The Ki-67 antigen represents a DNA-binding nuclear protein of 345 and 395 kd, encoded by a gene on chromosome 10. 22-24 It is expressed throughout the cell cycle in proliferating cells, but not in cells in the G0 phase or early G1 phase. It can thus be used to distinguish between growing and nongrowing cells. 25 The value of Ki-67 antibody in the assessment of cell-proliferating activity has been widely documented for various human tumors, including lung cancer. 26
Use of the Ki-67 antibody has methodological drawbacks, in particular, the restriction of its use in frozen tissue assays. Nevertheless, MIB-1, a recently developed antibody against an epitope of the Ki-67 antigen, can be used on paraffin material after antigen retrieval. Its reactivity has been shown to correlate strongly with Ki-67 staining and has the advantage of reacting with epitopes in routinely fixed, wax-embedded specimens. 27 Thus, the MIB-1 labeling index (LI) is a useful prognostic factor and may enhance the accuracy of conventional morphological grading and pathological staging systems. 19,28 The relationship between Ki-67 antigen expression and the estimated activities of cell cycle regulators that govern G1/S transition has not been well documented. Thus, it may be of interest to evaluate the correlation between the immunohistochemical labeling of cell nuclei with both MIB-1 and the expression of cell cycle regulators that govern G1/S transition in surgically resected NSCLCs.
The aim of the present study was to determine: 1) whether any relationship existed between the proliferative potential represented by the MIB-1 LI and the immunoreactivity of p27 protein in surgically resected NSCLCs, and 2) the relationship between MIB-1 LI and Cdk2 activity as H1 histone kinase activity in tissue lysates.
Materials and Methods
Patient Characteristics and Tumor Specimens
Lung cancer tissues and nonneoplastic lung tissues were obtained from 63 patients with primary lung cancers (36 men and 27 women; median age, 65 years) who underwent resection of NSCLC at the Hamamatsu Medical Center (Hamamatsu, Japan) and East Matsudo City Hospital (Matsudo City, Chiba, Japan). They had received no other therapy before surgery. Twenty-nine patients were in stage I, 24 in stage II, and 15 in stage IIIA. 29 All cancers were independently classified, according to the standard criteria of the World Health Organization, by two pathologists; there were 37 adenocarcinomas (16 well differentiated, 16 moderately differentiated, and 5 poorly differentiated) and 26 squamous cell carcinomas (4 well differentiated, 9 moderately differentiated, and 13 poorly differentiated), as summarized in Table 1 ▶ . 30 Forty-six of the 63 patients remain alive, with a median follow-up of 10 months (range, 1 to 32). For histological examination, portions of cancer tissues, as well as surrounding normal lung tissues, were fixed in buffered formalin overnight, dehydrated in graded ethanol at 4°C, and embedded in paraffin. Fresh samples were taken from pure-neoplastic tissues (excluding bronchial epithelium) and from nonneoplastic tissues for Western blot analysis.
Table 1.
Patient Characteristics (n = 63)
| Characteristic | No. |
|---|---|
| M/F | 39/24 |
| Mean age (standard deviation, range) | 66 (±8, 44 to 83) |
| Histology | |
| Adenocarcinoma | 37 |
| Squamous cell carcinoma | 26 |
| Nodal status | |
| N0 | 42 |
| N1 | 10 |
| N2 | 10 |
| N3 | 1 |
| Tumor status | |
| T1 | 33 |
| T2 | 24 |
| T3 | 3 |
| T4 | 3 |
| Stage of disease | |
| I | 40 |
| II | 8 |
| IIIA | 11 |
| IIIB | 4 |
| Resection type | |
| Lobectomy | 46 |
| Pneumonectomy | 17 |
Immunohistochemistry
Immunostaining for p27 and MIB-1 was performed on formalin-fixed, paraffin-embedded materials. Paraffin sections were deparaffinized with xylene, rehydrated, and microwaved for 20 minutes in 10 mmol/L citrate buffer (pH 6.0). Intrinsic and background stainings were blocked with 3% hydrogen peroxide in methanol before incubations with 0.1 mol/L phosphate-buffered saline at pH 6.0 containing either normal swine serum (1:10 dilution) for p27 staining or normal rabbit serum (1:10 dilution) for MIB-1 staining. Sections were then incubated overnight with a 1:200 (0.5 μg/ml) dilution of polyclonal p27 anti-body C-19 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or a 1:100 dilution of monoclonal MIB-1 antibody (Immunotech S. A., Marceille, France), both in phosphate-buffered saline. 15-17,31,32 After washing, slides were reacted with biotin-labeled anti-rabbit immunoglobulin G for p27 or anti-mouse immunoglobulin G for MIB-1 and then incubated with avidin-biotin-peroxidase complex (Dako Japan Ltd., Kyoto, Japan). 3,3′-Diaminobenzidine tetrahydrochloride substrate (Dojindo Laboratories, Kumamoto, Japan) was then added in the presence of hydrogen peroxide. Sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls using normal isotype-specific polyclonal rabbit immunoglobulin instead of primary antibody showed no evidence of staining. The pattern of p27 staining seen with the polyclonal antibody C-19 was confirmed using three other different p27 sera (one from Transduction Laboratories (Lexington, KY) and two from Santa Cruz Biotechnology). Specificity of the p27 staining was also assessed by preabsorption of the antibody with the recombinant p27 protein used.
Counting Procedure
Labeling indices for p27 and MIB-1 antibodies were determined in the same manner. Adjacent sections were used, and counting was performed in similar areas. After inspection, areas with the highest number of labeled cells were counted. Nuclei were considered positive if any nuclear staining was present. The degree of staining was scored independently by two different pathologists. Twenty high-power fields were chosen and scored for the percentage of cells showing nuclear staining of 1000 cells.
Preparation of Tissue Lysate
In tissues from 10 adenocarcinomas and 10 squamous cell carcinoma cases, we analyzed the expression of p27, its related Cdk2, and Cdk2 activity in both nonneoplastic and cancerous lung tissues. We confirmed histologically that these 20 samples did not show significant inflammatory involvement. Tissues were minced on dry ice into small pieces, and then 500 mg of each was quickly homogenized at 15,000 rpm with a Brinkmann Polytron homogenizer (PT 3000, Westbury, NY) in 1.0 ml of ice-cold lysis buffer containing 50 mmol/L Tris-HCl, pH 7.4, 250 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L N-ethylmalemide, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholic acid, 2 mmol/L sodium vanadate, 50 mmol/L sodium fluoride, 50 μg/ml leupeptin, 25 μg/ml aprotinin, and 10 μg/ml pepstatin. Lysates were sonicated and spun down at 15,000 rpm for 10 minutes at 4°C. Protein quantification, immunoprecipitation, Western blotting, and immunoprecipitation kinase assays were performed.
Western Blot Analysis
Samples were boiled in 62.5 mmol/L Tris-HCl buffer, pH 6.8, containing 2% SDS, 5% 2-mercaptoethanol, 7% glycerol, and 0.01% bromphenol blue for 10 minutes, and 10 μl of each was electrophoresed by SDS-polyacrylamide gel electrophoresis (12.5%) and transferred to immobilon-P membranes (Millipore) (Nihon Millipore Ltd., Yonezawa, Japan). Transfer was at 60 mA for 3 hours at 4°C in transfer buffer (25 mmol/L Tris, 190 mmol/L glycine, and 10% methanol). Blots were then incubated with blocking buffer (50 mmol/L Tris, 200 mmol/L NaCI, 0.2% Triton X-100, and 3% bovine serum albumin) for 90 minutes at room temperature. Western blot analysis was performed with rabbit polyclonal anti-Cdk2 (M2) (Santa Cruz) and anti-p27 (C-19), followed by incubation with goat anti-rabbit coupled to horseradish peroxidase (Amersham, Buckinghamshire, England). Western blotting was developed using an enhanced chemiluminescence detection kit (Amersham). 33
Northern Blot Analysis
Samples were lysed in 3 ml of 25 mmol/L sodium citrate solution (pH 7.0) containing 4 mol/L guanidinium isothiocynate and 0.1 mol/L 2-mercaptoethanol and then added to 1.7 ml of CsCl solution (5.7 mol/L CsCl (pH 7.2) and 0.1 mol/L ethylenediaminetetraacetic acid) and centrifuged at 80,000 × g for 36 hours at 16°C. The pellets were dissolved in Tris-ethylenediaminetetraacetic acid buffer, and RNA was precipitated with 1/10 volume of 3 mol/L sodium acetate (pH 5.2) and 2 volumes of ethanol at 80°C for 20 minutes. The concentration of RNA was determined by absorbance at 260 nm. Total RNA (10 μg) was separated on 1% formaldehyde agarose gels and transferred onto nitrocellulose membranes for 48 hours. Prehybridization was performed at 42°C for 16 hours in sodium citrate solution (750 mmol/L NaCl, 75 mmol/L sodium citrate (pH 7.0)) containing 1% Ficoll, 1% bovine serum albumin, 1% polyvinylpyrolidine, 50% formamide, 50 mmol/L sodium phosphate (pH 6.5), and 100 μg/ml salmon sperm DNA. The nylon filters were sequentially hybridized with 32P-labeled mouse p27 cDNA and human β-actin cDNA by overnight incubation and washed using a standard protocol. 34,35
Cdk2 Kinase Assay
Whole-cell lysates (300 μg/300 μl) obtained as above were precleared by incubation with 30 μl of protein A-Sepharose (50%, v/v; Pharmacia, Uppsala, Sweden) for 30 minutes, followed by centrifugation at 15,000 × g for 5 minutes. The lysate was then incubated with 1.5 μl of anti-Cdk2 antibody (M2) at 4°C overnight. After this, 20 μl of protein A-Sepharose (50%, v/v) was added and incubated for 30 minutes at 4°C. Immunoprecipitation was achieved after a brief centrifugation at 3000 × g. The activity of Cdk2 kinase was determined by the phosphorylation of H1 histone using the immunoprecipitates as reported previously. 36 Briefly, immunoprecipitates were incubated at 30°C for 30 minutes in 20 mmol/L Tris buffer, pH 7.0, 10 mmol/L H1 histone, 50 μmol/L ATP and 5.0 μCi γ-32P-ATP. Phosphorylated H1 histone was analyzed by SDS-polyacrylamide gel electrophoresis (10 to 20%) and autoradiography. 36
Degradation Assay of p27
Recombinant mouse p27-glutathione-S-transferase fusion protein was prepared using cDNA containing the full-length coding region for mouse p27. 34 Tissue homogenates were prepared as described by Pagano et al 8 with minor modifications. In brief, each frozen human tissue sample was sectioned and quickly homogenized with a Polytron homogenizer in 1 ml of ice-cold lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 250 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L N-ethylmalemide, 0.1% SDS, 0.5% deoxycholic acid, 1% Triton X-100, 50 mmol/L sodium fluoride, 2 mmol/L sodium vanadate, a protease inhibitor mixture (10 μg/ml of p-amidinophenyl methanesulfonyl fluoride hydrochloride, 10 mg/ml of pepstatin A, 10 mg/ml of chymostatin, 10 mg/ml leupeptin, and 10 mg/ml antipain). The lysate was microfuged at 19,000 × g for 5 minutes at 4°C to obtain supernatants. Purified recombinant p27-glutathione-S-transferase fusion protein (0.5 μg) was incubated at 37°C for different times in 30 μl volumes containing 300 μg of protein human tissue homogenates, 50 mmol/L Tris-HCI (pH 8.0), 5 mmol/L MgCl, 1 mmol/L DTT, 2 mmol/L ATP, 10 mmol/L creatine phosphokinase, and 10 mmol/L creatine phosphate. Adenosine-5′-(γ-thio)-triphosphate, a nonhydrolyzable ATP analog, decreased p27 proteolysis; MG-115, a proteasome inhibitor, but not E-64, also decreased p27 proteolysis. The reaction products were analyzed by immunoblotting with anti-p27 antibody. Purified recombinant glutathione-S-transferase was not degraded in these tissue lysates.
Statistical Analysis
Differences between groups were analyzed by the Mann-Whitney test and relations between groups by Pearson’s correlation coefficient. A rejection level of P < 0.05 was considered significant. This analysis was carried out using the StatView J 4.5 software statistical package (Abacus Concepts, Inc., Berkeley, CA).
Densitometry
For quantitative determinations of Western blot, Northern blot, and Cdk2 histone kinase activity, autoradiographic bands in the linear range were scanned with a DeskScan 4c (Hewlett-Packard, Tokyo, Japan) and analyzed by NIH Image 1.44 computer software. Values for Northern blots of p27 were calculated after normalization with β-actin.
Results
Assessment of MIB-1 and p27 Indices and Staining Patterns
For the group of 63 lung cancer specimens and 51 nonneoplastic lung tissues that were reassessed with respect to their MIB-1 and p27 staining, an average of 763 cells (range, 280 to l,000 nuclei) were counted. The subjective assessment of the distribution of positively labeled cells was identical in all cases. Antibody against p27 stained nuclei in diffuse fashion in most of the cases, whereas MIB-1 stained nuclei in a variety of patterns, from discrete intranuclear blobs consistent with nucleoli to diffuse staining of the whole nucleus.
Positive immunoreactivity of p27 was detected in nonneoplastic tissues such as the alveolar epithelium, bronchial epithelium and glandular cells, fibroblasts in connective tissues, and lymphocytes in lymph nodes. All of these were positively stained in the nuclei with a low to absent background, but staining intensity varied among cell types. Bronchial epithelium and lymphocytes in lymph nodes showed stronger staining intensity than alveolar epithelium and fibroblasts in connective tissues, and bronchial glandular cells showed modest positivity. In lung cancers, p27 was expressed in the nucleus with a low to absent background (Figure 1) ▶ . The number of cells expressing p27 and staining intensity varied from case to case.
Figure 1.
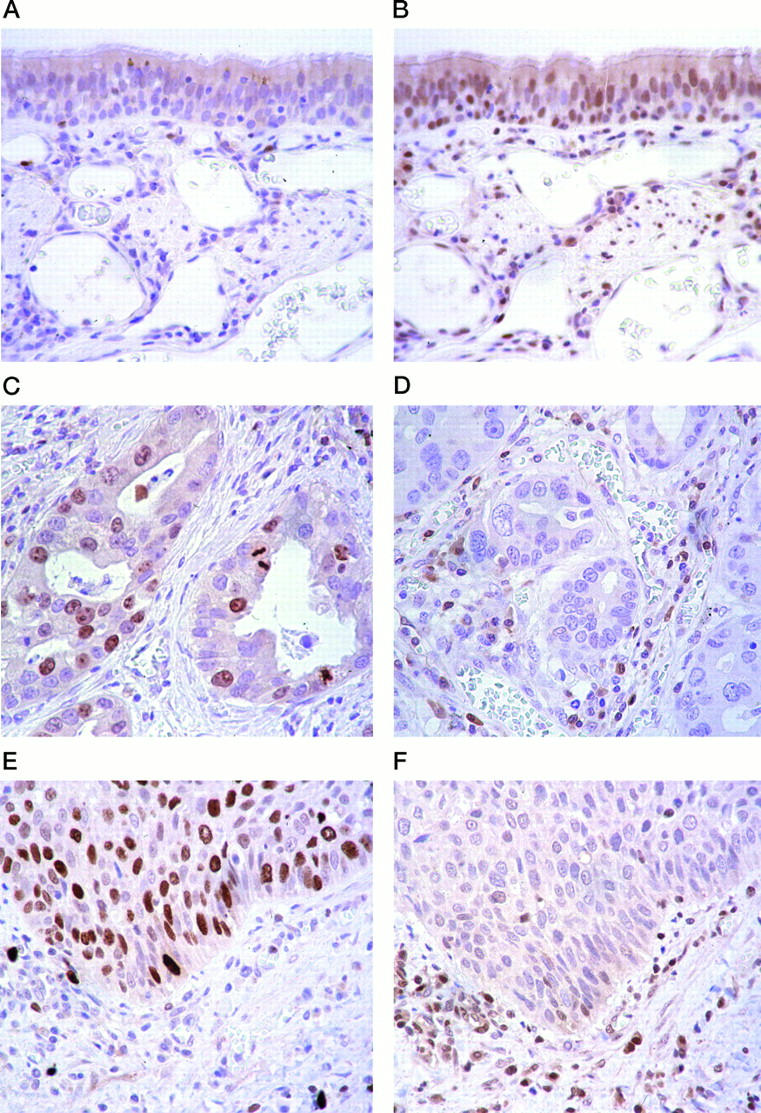
Comparison of Ki-67 antigen and p27 in nonneoplastic and cancerous lung tissues. Anti-Ki-67 antigen and anti-p27 immunostaining of sections of normal human lung tissue (A and B), adenocarcinoma (C and D), and squamous cell carcinoma (E and F) are compared. Ki-67 antigen expression was analyzed using monoclonal MIB-1 antibody. In normal lung tissue, immunostaining with MIB-1 showed few positive cells (A), whereas that with p27 showed positive nuclei in about 60 to 70% of cells (B). In adenocarcinoma tissue, immunostaining with MIB-1 revealed that 30% of cells had positive nuclei (C), whereas immunostaining of nuclei by anti-p27 showed few positive cells (D). In squamous cell carcinoma tissue, immunostaining with MIB-1 revealed positive nuclei in 60% of cells (E), whereas p27 immunostaining revealed few positive cells (F). Original magnification, ×400.
p27 LI
The mean percentage of p27 LI was 17.9% (SD, 13; range, 0 to 58%) for all cancers. For adenocarconomas it was 19.8% (SD, 13.8; range, 0 to 58%) and for squamous cell carcinomas 15.2% (SD, 10.6; range, 1 to 48%). In normal bronchial epithelium, the p27 LI was 43.1% (SD, 6.7; range, 38 to 62%). The differences between cancers and normal tissues were statistically significant (P < 0.001) (Figure 2A) ▶ . In contrast, differences in LI between the histological types of cancers with different degrees of differentiation were statistically not significant.
Figure 2.

Comparison of p27 LI and MIB-1 LI in nonneoplastic and cancerous lung tissues. Median p27 LI is significantly higher in normal lung tissues than in lung cancer tissues. Median MIB-1 LI is significantly lower in nonneoplastic lung tissues than in lung cancer tissues.
There were two atypical adenomatous hyperplasia (AAH) lesions in 2 of the 37 adenocarcinomas. These AAH lesions were observed adjacent to the primary cancer and were distinguished from the cancer on the basis of the degree of nuclear and structural atypia as well as by cell density, 37 although microscopic difference from well-differentiated adenocarcinoma was subtle. p27 LIs of these two lesions were 35% and 39%, respectively.
MIB-1 LI: Proliferative Activity
In most lesions, heterogeneous staining was observed. However, MIB-1 LI was significantly higher in lung cancers than in the control group consisting of normal bronchial epithelium. The mean of MIB-1 LI was 26.3% (SD, 14; range, 1 to 61%) for all cancers, 23% (SD, 15.2; range 1 to 61%) for adenocarcinomas, and 31% (SD, 12.4; range, 10 to 51%) for squamous cell carcinomas. The mean LI for the normal bronchial epithelium was 0.5% (SD, 0.8; range, 0 to 3.5%). The difference of MIB-1 LI between cancerous and normal lung tissues was statistically significant (P < 0.001) (Figure 2B) ▶ . MIB-1 LI was not significantly different between lung cancers with different histological features and different degrees of differentiation. MIB-1 LIs of the two AAH lesions were 2.6% and 11.3%, respectively.
Relationship between p27 LI and MIB-1 LI
Pearson’s relative coefficient estimation revealed a weak inverse correlation between p27 LI and MIB-1 LI, with a correlation coefficient of −0.459 (P < 0.001).
Relation to Clinical Findings
There was no significant difference in both MIB-1 LI and p27 LI that could be related to the degree of differentiation, nodal status, and clinical staging of the lung cancers.
Relationship between Immunohistochemistry and Western Blot Analysis
In tissues from 20 cases of lung cancer (10 adenocarcinoma and 10 squamous cell carcinoma), p27 levels were varied in each cancer. Despite this, p27 staining was a good reflection of the level of p27 expression on Western blotting (Table 2) ▶ .
Table 2.
Alterations in p27 Protein, mRNA, and Cdk2 Activity Detected in 20 Fresh Specimens of Non-Small Cell Lung Cancer
| Case | Sex | Age (years) | T/N/M | Stage | Diagnosis | Follow-up (Months) | % of p27 LI | % of MIB-1 LI | p27 protein | *p27 mRNA | Phosphorylated Cdk2 | Cdk2 activity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 62 | 2 /2/0 | 3 | Sq | 14 | 20.9 | 32.3 | 4,947 | 1.15 | 1,293 | 11,604 |
| 2 | M | 72 | 2 /0/0 | 1 | Sq | 10 | 26.0 | 12.0 | 3,451 | 1.14 | 0 | 18,322 |
| 3 | M | 50 | 2 /1/0 | 2 | Sq | 2 | 22.3 | 15.1 | 2,996 | 1.24 | 1,994 | 18,479 |
| 4 | M | 79 | 2 /0/0 | 1 | Sq | 9 | 18.8 | 23.3 | 2,597 | 0.74 | 2,773 | 30,737 |
| 5 | M | 71 | 2 /0/0 | 1 | Sq | 12 | 23.4 | 21.1 | 2,243 | 0.31 | 3,549 | 38,940 |
| 6 | M | 83 | 2 /0/0 | 1 | Sq | 2 | 25.1 | 30.0 | 1,574 | 1.08 | 3,329 | 38,184 |
| 7 | M | 65 | 4 /0/0 | 3 | Sq | 11 | 9.1 | 38.5 | 1,281 | 0.89 | 3,762 | 46,781 |
| 8 | F | 55 | 1 /0/0 | 1 | Sq | 12 | 7.5 | 30.4 | 0 | 0.61 | 3,440 | 42,804 |
| 9 | M | 66 | 2 /0/0 | 1 | Sq | 14 | 5.4 | 41.3 | 0 | 0.74 | 3,667 | 56,234 |
| 10 | M | 60 | 2 /0/0 | 1 | Sq | 4 | 1.0 | 48.2 | 0 | 0.80 | 4,156 | 57,007 |
| 11 | F | 71 | 1 /0/0 | 1 | Ad | 1 | 34.7 | 8.2 | 3,528 | 0.71 | 800 | 11,384 |
| 12 | M | 59 | 1 /0/0 | 1 | Ad | 2 | 21.2 | 12.6 | 3,149 | 0.61 | 0 | 16,492 |
| 13 | M | 79 | 1 /0/0 | 1 | Ad | 13 | 39.1 | 8.5 | 3,528 | 0.71 | 800 | 11,384 |
| 14 | F | 76 | 2 /0/0 | 1 | Ad | 10 | 30.3 | 21.4 | 1,460 | 0.75 | 2,225 | 25,117 |
| 15 | M | 69 | 1 /0/0 | 1 | Ad | 42 | 22.5 | 21.6 | 1,384 | 0.21 | 3,894 | 26,461 |
| 16 | M | 64 | 1 /1/0 | 2 | Ad | 4 | 15.5 | 29.3 | 812 | 1.03 | 3,494 | 37,481 |
| 17 | M | 74 | 2 /0/0 | 1 | Ad | 32 | 20.1 | 26.2 | 690 | 0.82 | 4,180 | 34,429 |
| 18 | F | 57 | 2 /2/0 | 3 | Ad | 5 | 12.8 | 25.3 | 487 | 0.48 | 4,983 | 41,800 |
| 19 | F | 72 | 2 /0/0 | 1 | Ad | 3 | 15.0 | 15.3 | 0 | 0.74 | 5,160 | 31,668 |
| 20 | M | 66 | 1 /0/0 | 1 | Ad | 4 | 11.0 | 20.9 | 0 | 0.83 | 6,363 | 42,861 |
T, primary tumor; N, nodal involvement; M, distant metastasis; Sq, squamous cell carcinoma; Ad, adenocarcinoma.
*The values of p27 mRNA levels were estimated as described in Material and Methods after normalization for the amount of β-actin mRNA.
Northern Blot Analysis
p27 mRNA was detected in all fresh cancerous samples. The levels of p27 protein expression were not proportional to expression of p27 mRNA (Table 2) ▶ .
Western Blot Analysis of Cdk2 and Cdk2 Activity
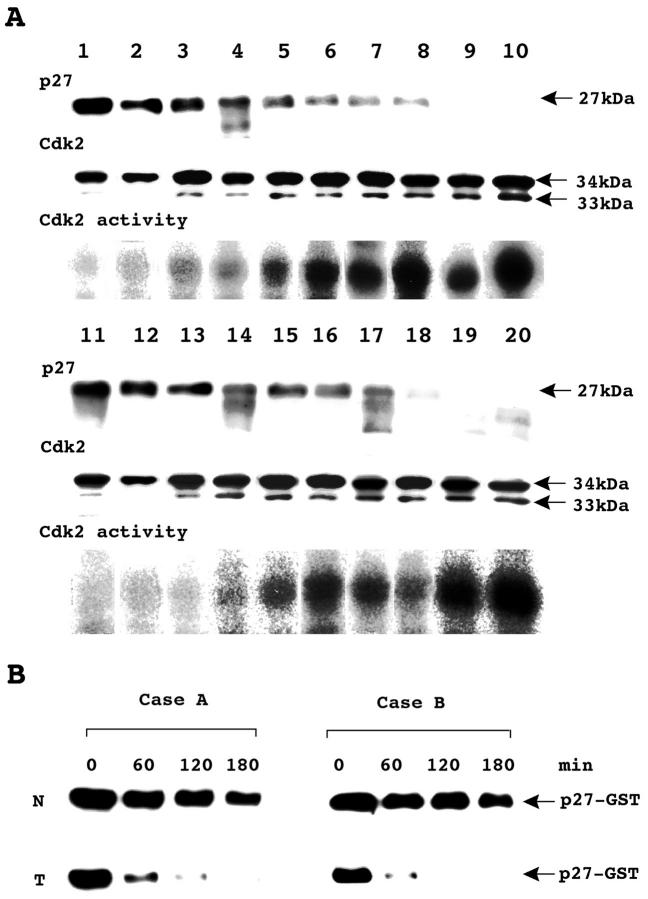
Eighteen of the 20 cancers exhibited increased levels of the rapidly migrating, 33-kd phosphorylated form of Cdk2. 34 Cdk2 activity was found at high levels in cancer tissues that showed decreased expression of p27 (Figure 3A) ▶ . Densitometric analysis showed that the amounts of 33-kd Cdk2 expressed in lung cancer tissues significantly correlated with Cdk2 activity (r = 0.702, P < 0.001). Statistical analysis also clearly showed that p27 LI has a strong and inverse correlation with Cdk2 activity. The correlation coefficient was −0.767 (P < 0.0001).
Figure 3.
Activation of Cdk2 and p27 elimination in cancerous lung tissues. A: Western blot analysis of p27, Cdk2, and Cdk2 activity in adenocarcinomas (lanes 1 to 10) and squamous cell carcinomas (lanes 11 to 20). p27 was found in both adenocarcinomas and squamous cell carcinomas, but expression levels were varied. A 33-kd band representing the phosphorylated form of Cdk2 was found in adenocarcinomas and squamous cell carcinomas. The kinase activity associated with Cdk2 in lung cancer tissues varied, both in individual adenocarconomas and in individual squamous cell carcinomas. B: Degradation of recombinant p27 in lysate of nonneoplastic and cancerous lung tissues. Purified recombinant p27-glutathione-S-transferase fusion protein was incubated for the indicated times with extracts from nonneoplastic tissue and cancerous lung tissue from a patient with adenocarcinoma (Case A) or squamous cell carcinoma (Case B). N, nonneoplastic lung tissue; T, cancerous lung tissue. Case A corresponds to lane 10 and case B to lane 20 in A.
The levels of 34-kd Cdk2 protein an inactive and nonphosphorylated form of Cdk2, were found to be in similar amounts.
Relationship between Cdk2 Activity and MIB-1 LI
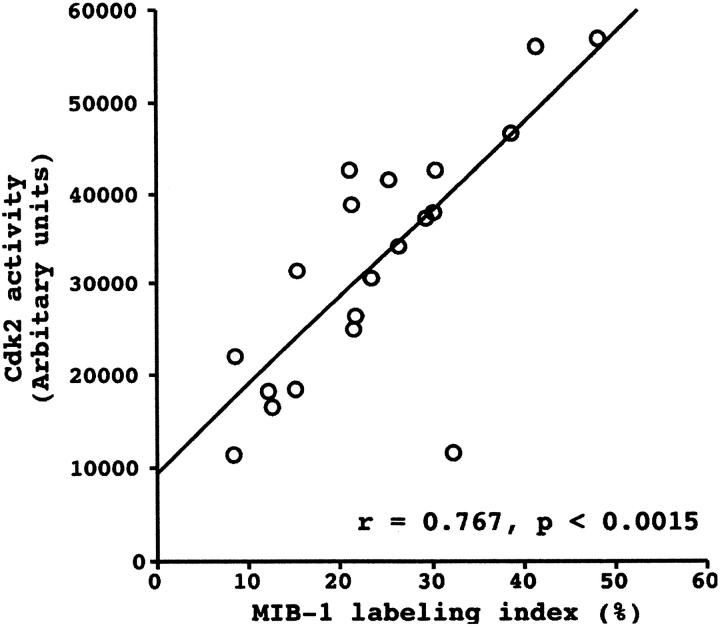
Pearson’s correlation coefficient demonstrated a strong correlation between Cdk2 activity and MIB-1 LI with a correlation coefficient of 0.767 (P < 0.0015) (Figure 4) ▶ .
Figure 4.
Relationship between Cdk2 activity and MIB-1 LI in lung cancer tissue. Scatter plot of Cdk2 activity versus MIB-1 LI with regression line showing a strong correlation of the two cell-cycle regulators using Pearson’s correlation coefficient.
p27 Degradation Activity
Eight cases of 10 lung cancer specimens showed higher degradation activity than corresponding nonneoplastic lung specimens. For these specimens, p27 was reduced by approximately 50% within 120 minutes. Figure 3B ▶ shows two samples with high degradation activity.
Discussion
Our first objective was to examine the relationship between the proliferative potential represented by MIB-1 LI and the immunoreactivity of p27 in surgically resected NSCLCs.
The reliability of the immunohistochemical assay for p27 LI in NSCLC was first described by Esposito et al. 16 A tight correlation between immunohistochemistry and Western blot analysis of p27 in NSCLC was mentioned, although the data were not shown in their paper. In the present study, our data made a clear and strong correlation between the immunohistochemical assay for p27 LI and their Western blot analysis (r = 0.656, P < 0.0001). These results support the reliability of the immunohistochemical assay for p27 LI in NSCLC.
Ki-67 is the most reliable antibody for assessing growth by immunohistochemistry. 21 High proliferative activity, as measured by Ki-67 antigen, has been shown to correlate with reduced p27 in lymphoid neoplasms, carcinomas of the oral cavity, and endocrine tumors, including pituitary, thyroid, and parathyroid gland hyperplasia. 31,32,38 In contrast, studies showing no correlation between tumor cell proliferation and p27 have been reported in colorectal and breast cancers. 39,40 A relation between Ki-67 and p27 protein was not previously analyzed in primary NSCLC. 15,16,31,32 Therefore, we performed the study to investigate the association of proliferative activity (as measured by the expression of Ki-67) with alterations of p27 in primary NSCLC. Our results revealed that the expression for p27 and Ki-67 antigen had a weak but significant inverse correlation in primary NSCLC (r = −0.459, P < 0.001). This may indicate that p27 protein contributes to, or reflects, increased cell proliferation in primary NSCLC; however, within the limited population studied here, there was variability in this relationship from case to case. Thus, other mechanisms that influence the changes in proliferation in the NSCLC cannot be excluded.
Of the various histological types of human lung carcinoma, the most prevalent in Japan is adenocarcinoma. Little is known about the lesions preceding this type of lung cancer. 41 Some investigators regard AAH lesions as precursors of adenocarcinomas, particularly bronchiolo-alveolar carcinomas. 42-45
In the present study, the value of p27 LI and MIB-1 LI in AAH lay between those in normal bronchial epithelium and adenocarcinomas, but only two such cases were available for analysis. A decrease in the number of cells expressing p27 may play a role during progression from normal lung tissue to AAH and then to adenocarcinomas, although larger numbers of AAH lesions will have to be analyzed.
All cancerous lung tissues showed p27 transcripts at various levels and enhanced degradation activity of p27 compared with nonneoplastic lung tissues. We used samples from lung tissues as a source of ubiquitinating enzymes and proteasomes. Our results support the view that p27 abundance is mostly regulated at a posttranscriptional level by ubiquitin-proteasome-mediated proteolysis, and also that the p27 degradation pathway in NSCLC is enhanced as reported in previous studies. 16,39
Our second objective was to analyze the relationship between MIB-1 LI and Cdk2 activity in lung cancer tissues. There have been a few reports measuring Cdk2 activity in fresh-frozen tumor tissues. 46 We analyzed the expression of Cdk2 by Western blotting and Cdk2 activity by H1 histone kinase assay in nonneoplastic lung tissues and lung cancer tissues. To our knowledge, this is the first report demonstrating Cdk2 activity in lung cancer tissues. Densitometric analysis of the Western blots showed that the amounts of 33-kd Cdk2 expressed in lung cancer tissues were significantly correlated with Cdk2 activity (r = 0.702, P < 0.001). p27 LI and Cdk2 activity also showed a tight inverse correlation (r = −0.784, P < 0.0001). These results indicate that the proliferative activity of lung cancer tissues was closely related to Cdk2 activation in these tissues. It would be of value to study whether p27 degradation is directly linked to Cdk2 activation in NSCLC. 5,47 In addition to the elimination of p27, Cdk2 activation requires the binding with cyclin E and the phosphorylation of threonine 160 by Cdk-activating kinase. 48 These Cdk regulators may also play a role in the proliferation of NSCLC.
p21Cip1/Waf1 is a member of the Cip/Kip family of cyclin-dependent kinase inhibitors, which includes the p21, p27, and p57 proteins. 6,7 A recent study has shown that p21 protein and mRNA in the majority of lung neoplasms are expressed at higher levels than in the corresponding normal tissues and that their expression is independent of p53 gene and protein alterations. 35 The role of p21 in the proliferation of NSCLC will require further investigation.
The present study clearly demonstrates that decreased p27 expression correlates with increased Cdk2 activity and increased proliferative activity measured by MiB-1 LI in a series of NSCLCs. We also show that the degrading activity of p27 in NSCLCs was higher than in nonneoplastic lung tissues. The results support the conclusion that p27 and Cdk2 have an essential role in the proliferation of non-small cell cancers. The molecular mechanism of p27 degradation in proliferating cells has not been well documented. Clarification of the mechanism by which degradation of p27 in NSCLC is increased may eventually yield a new form of therapy for this kind of neoplasia.
Acknowledgments
The authors thank K. Azuma, T. Umemiya, Y. Tsuchikawa, Y. Okuda, M. Maemori, and E. Miyagawa for their excellent technical assistance and Y. Noguchi, I. Tatsuno, and T. Oeda for helpful discussions and suggestions. We are also grateful to F. Okamoto and K. Ohiwa for their assistance in tissue acquisition. Special thanks to Prof. L. D. Kohn for his comments on the manuscript.
Footnotes
Address reprint requests to Dr. H. Kawana, First Department of Pathology, Chiba University School of Medicine, 2600856, 1-8-1 Inohana, Chu-ou-ku, Chiba, Japan. E-mail: khideta@pathoh.m.chiba-u.ac.jp.
References
- 1.Schottenfeld D: Epidemiology of lung cancer. Pass H Mitchell JB Johnson DH Turrisi AT eds. Lung Cancer: Principles and Practice. 1996, :pp 305-321 Lippincott-Raven Publishers, Philadelphia [Google Scholar]
- 2.Minshull J: Cyclin synthesis: who needs it? (review). Bioessays 1993, 15:149-155 [DOI] [PubMed] [Google Scholar]
- 3.Pines J: Cyclins and cyclin-dependent kinases: take your partners (review). Trends Biochem Sci 1993, 18:195-197 [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ: Mammalian G1 cyclins (review). Cell 1993, 73:1059-1065 [DOI] [PubMed] [Google Scholar]
- 5.Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM: Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 1992, 257:1689-1694 [DOI] [PubMed] [Google Scholar]
- 6.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A: p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev 1994, 8:9-22 [DOI] [PubMed] [Google Scholar]
- 7.Toyoshima H, Hunter T: p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78:67-74 [DOI] [PubMed] [Google Scholar]
- 8.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M: Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27 (see comments). Science 1995, 269:682-685 [DOI] [PubMed] [Google Scholar]
- 9.Hengst L, Reed SI: Translational control of p27Kip1 accumulation during the cell cycle. Science 1996, 271:1861-1864 [DOI] [PubMed] [Google Scholar]
- 10.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM: Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 1994, 372:570-573 [DOI] [PubMed] [Google Scholar]
- 11.Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S, Lee YY, Bartram CR, Koeffler HP: Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 1995, 55:2266-2269 [PubMed] [Google Scholar]
- 12.Shiohara M, el-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R, Chen DL, Vogelstein B, Koeffler HP: Absence of WAF1 mutations in a variety of human malignancies. Blood 1994, 84:3781-3784 [PubMed] [Google Scholar]
- 13.Pietenpol JA, Bohlander SK, Sato Y, Papadopoulos N, Liu B, Friedman C, Trask BJ, Roberts JM, Kinzler KW, Rowley JD, Vogelstein B: Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res 1995, 55:1206-1210 [PubMed] [Google Scholar]
- 14.Morosetti R, Kawamata N, Gombart AF, Miller CW, Hatta Y, Hirama T, Said JW, Tomonaga M, Koeffler HP: Alterations of the p27KIP1 gene in non-Hodgkin’s lymphomas and adult T-cell leukemia/lymphoma. Blood 1995, 86:1924-1930 [PubMed] [Google Scholar]
- 15.Catzavelos C, Bhatacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM: Decreased levels of the cell-cycle inhibitor p27(Kip1) protein: prognostic implications in primary breast cancer. Nat Med 1997, 3:227-230 [DOI] [PubMed] [Google Scholar]
- 16.Esposito V, Baldi A, Luca A, Groger A, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A: Prognostic role of the cyclin-dependent inhibitor p27 in non-small cell lung cancer. Cancer Res 1997, 57:3381-3385 [PubMed] [Google Scholar]
- 17.Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM: Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients (see comments). Nat Med 1997, 3:222-225 [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Suzuki M, Kono S, Shinomiya N, Rokutanda M, Takagi K, Ogata T, Tamai S: Proliferating cell nuclear antigen and Ki-67 in lung carcinoma: correlation with DNA flow cytometric analysis. Cancer 1994, 74:2468-2475 [DOI] [PubMed] [Google Scholar]
- 19.Bohm J, Koch S, Gais P, Jutting U, Prauer HW, Hofler H: Prognostic value of MIB-1 in neuroendocrine tumours of the lung. J Pathol 1996, 178:402-409 [DOI] [PubMed] [Google Scholar]
- 20.Pujol JL, Simony J, Jolimoy G, Jaffuel D, Demoly P, Quantin X, Marty AC, Boher JM, Charpentier R, Michel FB: Hypodiploidy: Ki-67 growth fraction and prognosis of surgically resected lung cancers. Br J Cancer 1996, 74:964-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DC, Gatter KC: Monoclonal antibody Ki-67: its use in histopathology (review). Histopathology 1990, 17:489-503 [DOI] [PubMed] [Google Scholar]
- 22.Gerdes J, Lemke H, Baish H, Wacher H-H, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by monoclonal antibody Ki-67. J Immunol 1984, 133:1710-1715 [PubMed] [Google Scholar]
- 23.Gerdes J, Schwab U, Lemke H, Stein H: Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983, 31:3-20 [DOI] [PubMed] [Google Scholar]
- 24.Gerdes J, Schulter C, Dochrow M, Wohlenberg C, Gerlach C: Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 1991, 138:867-873 [PMC free article] [PubMed] [Google Scholar]
- 25.Hall P, Woods A: Immunohistochemical markers of cellular proliferation: achievements, problems and prospects. Cell Tissue Kinet 1990, 23:505-522 [DOI] [PubMed] [Google Scholar]
- 26.Fontanini G, Pingitore R, Bigini D, Vignati S, Pepe S, Ruggiero A, Macchiarini P: Growth fraction in non-small cell lung cancer estimated by proliferating cell nuclear antigen and comparison with Ki-67 labeling and DNA flow cytometry data. Am J Pathol 1992, 141:1285-1290 [PMC free article] [PubMed] [Google Scholar]
- 27.Cattorei G, Becker M, Key G: Monoclonal antibodies against recombinant parts of Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 1992, 168:357-363 [DOI] [PubMed] [Google Scholar]
- 28.Tungekar MF, Gatter KC, Dunnhill MS, Mason DY: Ki-67 immunostaining and survival in operable lung cancer. Histopathology 1991, 19:545-550 [DOI] [PubMed] [Google Scholar]
- 29.Mountain CF: A new international system for staging lung cancer. Chest 1986, 89(suppl):225S. [DOI] [PubMed] [Google Scholar]
- 30.: World Health Organization: ed 2 Histological Typing of Lung Tumors. 1981, World Health Organization, Geneva
- 31.Sanchez-Beato M, Saez AI, Martinez-Montero JC, Mateo MS, Sanchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin-dependent kinase inhibitor p27(KIP1) in lymphoid tissue: p27(KIP1) expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd RV, Jin L, Qian X, Kulig E: Aberrant p27kip1 expression in endocrine and other tumors. Am J Pathol 1997, 150:401-407 [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto K, Hirai A, Ban T, Saito J, Tahara K, Terano T, Tamura Y, Saito Y, Kitagawa M: Thyrotropin induces G1 cyclin expression and accelerates G1 phase after insulin-like growth factor I stimulation in FRTL-5 cells. Endocrinology 1996, 137:2036-2042 [DOI] [PubMed] [Google Scholar]
- 34.Hirai A, Nakamura S, Noguchi Y, Yasuda T, Kitagawa M, Tatsuno I, Oeda T, Tahara K, Terano T, Narumiya S, Kohn L, Saito Y: Geranylgeranylated rho small GTPase(s) are essential for the degradation of p27kip1 and facilitate the progression from G1 to S phase in growth stimulated rat FRTL-5 cells. J Biochem 1997, 272:13-16 [PubMed] [Google Scholar]
- 35.Marchetti A, Doglioni C, Barbareschi M, Buttitta F, Pellegrini S, Bertacca G, Chella A, Merlo G, Angeletti CA, Dalla Palma P, Bevilacqua G: p21 RNA, and protein expression in non-small cell lung carcinomas: evidence of p53-independent expression and association with tumoral differentiation. Oncogene 1996, 12:1319-1324 [PubMed] [Google Scholar]
- 36.Kitagawa M, Okabe T, Ogino H, Matsumoto H, Suzuki-Takahashi I, Kokubo T, Higashi H, Saitoh S, Taya Y, Yasuda H, Ohba Y, Nishimura S, Tanaka N, Okuyama A: Butyrolactone I, a selective inhibitor of cdk2, and cdc2 kinase. Oncogene 1993, 8:2425-2432 [PubMed] [Google Scholar]
- 37.Kitamura H, Kameda Y, Nakamura N, Nakatani Y, Inayama Y, Iida M, Noda K, Ogawa N, Shibagaki T, Kanisawa M: Proliferative potential and p53 overexpression in precursor and early stage lesions of bronchiolo-alveolar lung carcinoma. Am J Pathol 1995, 146:876-887 [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan RCK, Bradley G, Slingerland J: Reduced levels of the cell-cycle inhibitor p27(Kip1) in epithelial dysplasia and carcinoma of the oral cavity. Am J Pathol 1998, 152:585-590 [PMC free article] [PubMed] [Google Scholar]
- 39.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M: Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997, 3:231-234 [DOI] [PubMed] [Google Scholar]
- 40.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzzi C, Lavin P, Draetta G, Pagano M, Loda M: The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res 1997, 57:1259-1263 [PubMed] [Google Scholar]
- 41.Saccomano G, Archer V, Auerbach O, Saunders R, Brennan L: Development of carcinoma of the lung as reflected in exfoliated cells. Cancer 1974, 33:256-270 [DOI] [PubMed] [Google Scholar]
- 42.Mori M, Chiba R, Takahashi T: Atypical adenomatous hyperplasia of the lung and its differentiation from adenocarcinoma. Cancer 1993, 72:2331-2340 [DOI] [PubMed] [Google Scholar]
- 43.Miller R: Bronchiolo-alveolar adenoma. Am J Surg Pathol 1990, 14:904-912 [DOI] [PubMed] [Google Scholar]
- 44.Nakanishi K: Alveolar epithelial hyperplasia and adenocarcinoma of the lung. Am J Surg Pathol 1990, 14:904-912 [PubMed] [Google Scholar]
- 45.Weng S, Tsuchiya E, Satou Y, Kitagawa T, Nakagawa K, Sagano H: Multiple atypical adenomatous hyperplasia of type II pneumocytes and bronchiolo-alveolar carcinoma. Histopathology 1990, 16:101-103 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto H, Monden T, Ikeda K, Izawa H, Fukuda K, Fukunaga M, Tomita N, Shimano T, Shiozaki H, Monden M: Coexpression of cdk2/cdc2 and retinoblastoma gene products in colorectal cancer. Br J Cancer 1995, 71:1231-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Robert JM: Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 1991, 66:1217-1228 [DOI] [PubMed] [Google Scholar]
- 48.Morgan DO: Principles of CDK regulation (review). Nature 1995, 374:131-134 [DOI] [PubMed] [Google Scholar]