Abstract
Stanniocalcin (STC) is a glycoprotein hormone first found in fish, in which it regulates calcium homeostasis and protects against hypercalcemia. Human and mouse stc cDNA were recently cloned. We found a dramatically upregulated expression of STC during induced neural differentiation in a human neural crest-derived cell line, Paju. Immunohistochemical staining of sections from human and adult mouse brain revealed abundant presence of STC in the neurons with no activity in the glial cells. STC expression was not seen in immature brain neurons of fetal or newborn mice. Given that STC has been found to regulate calcium/phosphate metabolism in some mammalian epithelia, we suggest that STC may act as a regulator of calcium homeostasis in terminally differentiated brain neurons.
Stanniocalcin (STC) is a calcium-regulating glycoprotein hormone that was originally discovered in bony fish. STC is synthesized in specialized endocrine glands, the corpuscles of Stannius, in association with the fish kidney. 1 Elevated serum calcium level is a major trigger for secretion of STC. 2,3 The function of STC in fish is to counteract hypercalcemia by slowing the uptake of Ca2+ by the gills, 4,5 by increasing the renal reabsorption of inorganic phosphate, 6 and by inhibiting intestinal calcium transport. 7
The cDNAs for human 8 and mouse 9 stc were recently cloned. Human STC was found to contain 273 amino acids in a 73% sequence similarity with fish STC. Northern blot analysis revealed the presence of stc mRNA in different human tissues, with the strongest signals in ovary, prostate, and thyroid. Mouse stc mRNA has in addition been found in spleen and in 13.5-day embryonic tissue. 8,9 Immunostaining with antiserum raised against recombinant human STC demonstrated the presence of STC in distinct cells of the nephron tubule. 10 The kidney may also be a physiological target of mammalian STC activity, given that administration of human recombinant STC was found to inhibit renal phosphate excretion in rats. 11 Moreover, addition of STC to the serosal surface of rat and pig duodenal epithelium increased calcium fluxes and resulted in a net reduction in calcium absorption and an increased phosphate uptake. 12
We have been investigating gene expression during terminal differentiation of neurons in a model consisting of a human cell line called Paju. This cell line was established from a malignant, neural crest-derived tumor. Paju cells respond to different stimuli, including phorbol 12-myristate 13-acetate (PMA), by vigorous neural sprouting, cessation of proliferation, and de novo expression of different markers of terminally differentiated neurons. 13
To screen for gene expression in relation to neural differentiation, we performed a differential display (DD) reverse transcription-polymerase chain reaction (RT-PCR) on mRNA extracted from Paju cells before and after induced differentiation. Among genes with strongly upregulated expression during induced neural differentiation, we identified stc. Based on this observation, sections from human and mouse brain were stained by immunhistochemistry with antiserum to human STC. Here we report that the terminally differentiated neurons of the central nervous system express high levels of STC. STC was not found in the glial cells or in embryonic neurons. Given that influx of Ca2+ is considered a major pathogenic mechanism leading to neuron damage after hypoxia 14,15 together with the documented antihypercalcemic effects of STC, we suggest that terminally differentiated neurons upregulate STC expression to increase their cellular integrity.
Materials and Methods
DD-RT-PCR
DD 16 was carried out as previously described in a report from this laboratory. 17 For amplifying in the DD-RT-PCR, the 5′ and 3′ primers were 5′-CATTCAGCAC-3′ and 5′-GVTTTTTTTTTTTT-3′, respectively. The PCR products were separated on sequencing gels, and bands appearing differentially displayed were excised out and cloned into pCRII via TA cloning (Invitrogen; San Diego, CA), and sequencing of all cDNA clones was done on Applied Biosystems (Foster City, CA) 373 or 377 DNA sequencers according to the manufacturer’s protocol.
Cell Culture and Reagents
The Paju tumor cell line was established by one of us (LCA) from the pleural metastases of a neural-crest-derived tumor in a young patient. Uninduced Paju cells grow surface adherent when cultivated in RPMI-1640 supplemented with 10% fetal calf serum, penicillin G (10 U/ml), streptomycin sulfate (50 mg/ml), and 1 mmol/L glutamine. For subculturing, the cells were detached by treatment with Versene/ethylenediaminetetra-acetic acid (Life Technologies, Inc., Grand Island, NY). PMA was obtained from Sigma Chemical Co. (St. Louis, MO), dissolved in ethanol, and used at an optimal concentration of 10 nmol/L. Human recombinant STC and rabbit antiserum against human STC were prepared as described. 18
Western Blotting
Cells were collected after PMA treatment at the indicated time points and lysed in an ice-cold lysis buffer containing 20 mmol/L Tris/HCl pH 8.0, 0.2 mmol/L ethylenediaminetetra-acetic acid, 3% Nonidet P-40, 2 mmol/L orthovanadate, 50 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 100 mmol/L NaCl and 10 mg/ml each of aprotinin and leupeptin. After incubation on ice for 10 minutes, the samples were centrifuged at 14,000 × g for 15 minutes, and the supernatants were collected. An aliquot was removed for total protein estimation using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA). An aliquot corresponding to 30 μg of total protein of each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and transferred electrophoretically to nitrocellulose filters. Nonspecific binding of antibody was blocked with 3% bovine serum albumin in 20 mmol/L Tris/HCl pH 7.5, 150 mmol/L NaCl, and Triton X-100 for 2 hours. Immunoblotting was carried out with the 1:2000 diluted rabbit STC antibody followed by peroxidase-conjugated secondary anti-immunoglobulin antibodies, and the blots were developed with the enhanced chemiluminescence method (ECL, Amersham, Little Chalfont, UK).
Immunohistochemistry and Immunofluorescence Staining
Brain tissue was fixed in 4% buffered formaldehyde for 12 to 48 hours, routinely processed, and embedded in paraffin. Sections 4 μm thick were mounted on slides coated with 3-aminopropyl-triethoxy-silane (Sigma Chemical Co.) and dried for 12 hours at 37°C. The sections were deparaffinized in xylene and rehydrated through graded concentrations of ethanol to distilled water, processed in a microwave oven, 19 and treated with a methanol-perhydrol solution (0.5% hydrogen peroxide in absolute methanol) for 30 minutes at room temperature to block endogenous peroxidase activity. Immunohistochemical stainings were performed by using a commercial Elite ABC Kit (Vectastain, Vector Laboratories, Burlingame, CA). Blocking serum was applied for 15 minutes followed by a 60-minute incubation with the diluted primary antibody. The dilutions were made in phosphate-buffered saline (PBS; pH 7.2), and all incubations were done in a moist chamber at room temperature. Between the different staining steps, the slides were rinsed in three changes of PBS. The peroxidase staining was visualized with a 3-amino-9-ethylcarbazole (Sigma Chemical Co.) solution (0.2 mg/ml in 0.05 mol/L acetate buffer containing 0.03% perhydrol, pH 5.0) at room temperature for 15 minutes. Finally, the sections were lightly counterstained in Mayer’s hematoxylin and mounted in an aqueous mounting medium (Aquamount, BDH, UK). Slides stained with STC antibodies preabsorbed with recombinant STC protein and slides stained with normal rabbit serum served as negative controls.
For immunofluorescence staining, Paju cells were grown on glass coverslips with or without addition of PMA. The coverslips were gently washed with PBS, fixed for 10 minutes in 3.5% freshly made paraformaldehyde, and permeabilized by treatment for 10 minutes with PBS containing 0.05% Nonidet P-40. Optimally diluted antibodies were added. Two types of control stainings were performed: coverslips were treated 1) with normal rabbit serum at the same dilution and 2) with the antiserum to STC preabsorbed with the recombinant protein. After incubation for 40 minutes, the coverslips were washed twice with PBS containing 5% fetal calf serum and 1:50 diluted fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin was added for 30 to 40 minutes. After two washes with PBS-5% fetal calf serum, the coverslips were mounted on object slides with 50% glycerol in PBS, pH 8.
Northern Blotting
mRNA was extracted by oligodeoxythymidylic acid chromatography. Eight μg of mRNA per sample was separated on 0.8% agarose-formaldehyde gels and transferred to Hybond-N filters (Amersham). Equal loading was confirmed by hybridization with a glyceraldehyde-3-phosphate dehydrogenase probe. cDNA probes were 32P-labeled by random priming. The blots were hybridized overnight at 42°C, washed in 2× saline-sodium citrate and 0.5% sodium dodecyl sulfate at 60°C for 30 minutes, and subjected to autoradiography.
Results
DD-RT-PCR Revealed Upregulated Expression of stc after PMA Induced Neural Differentiation
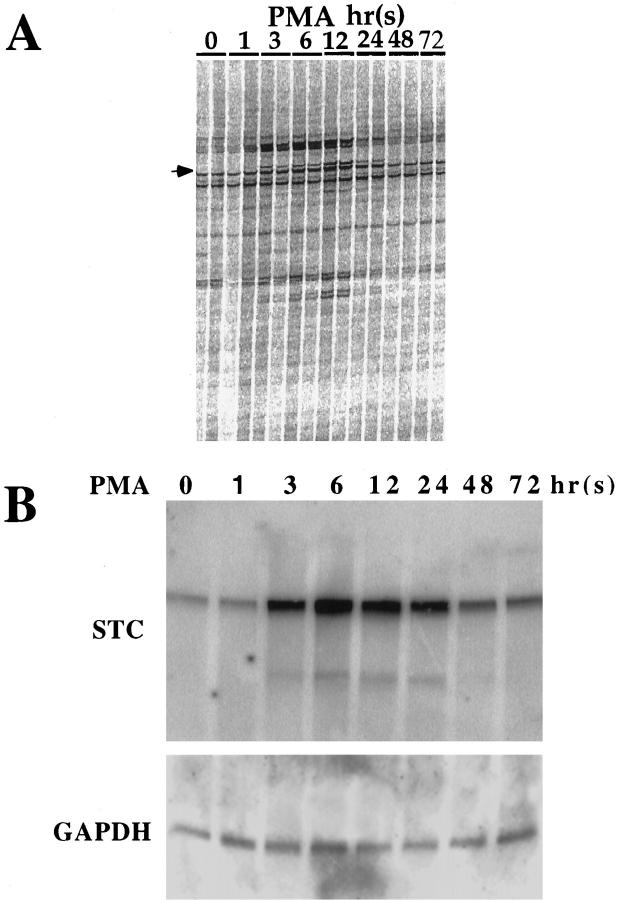
Cultivation of Paju cells in the presence of 10 nmol/L PMA induced neural differentiation, visualized as neural sprouting that was evident after only 6 hours (data not shown). We have previously reported that induced neural differentiation is accompanied by increased expression of neuron-specific enolase and Bcl-2. 13 mRNA was isolated from Paju cells at different times of PMA treatment and from cells kept in untreated parallel cultures and analyzed by DD-RT-PCR. Several products were seen differentially expressed in PMA-treated cells. Sequencing one of these differentially appearing bands revealed an identity to stc (Figure 1A) ▶ .
Figure 1.
Induction of stc expression in Paju cells by treatment with PMA. A: DD of mRNAs from indicated times of PMA treatment. Arrow: Position of the stc sequence. B: Top: Northern blot analysis with human stc cDNA of stc mRNA expression during PMA-induced neural differentiation. Bottom: Hybridization with a human glyceraldehyde-3-phosphate dehydrogenase probe to demonstrate equal loading. The same filter was stripped and reprobed.
Northern blot analysis using the full-length human stc cDNA as a probe revealed a rapid upregulation of stc expression in Paju cells treated with PMA. Only a very weak signal was seen in untreated Paju cells, whereas after only 3 hours of PMA induction an increased level of stc mRNA was recorded. After 48 hours of PMA treatment, the expression of stc declined but remained above that seen in untreated cells (Figure 1B) ▶ .
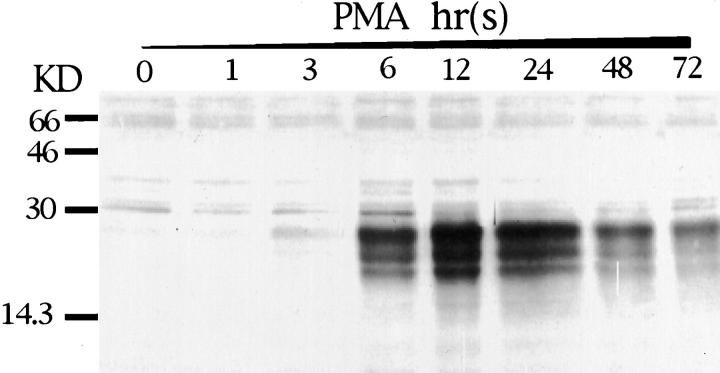
Western blot analysis with antibodies to STC of lysates from PMA-treated Paju cells revealed several strongly reactive bands of 20 to 30 kd after 6 hrs of PMA induction. The intensity of the bands of lower molecular weight gradually declined, and after 72 hours of PMA treatment, a major band of apparent molecular weight of 29 kd remained (Figure 2) ▶ . The initial variation in apparent molecular weight of the bands seen by Western blotting during early PMA treatment may be attributed to an initially immature glycosylation. Treatment of the cell lysates with neuraminidase before sodium dodecyl sulfate-polyacrylamide gel electrophoresis partially removed the lower molecular weight bands in the Western blot, also indicating the glycosylation of STC (data not shown).
Figure 2.
Western blot analysis with rabbit antibodies to human STC of lysates of Paju cells treated with PMA for indicated times.
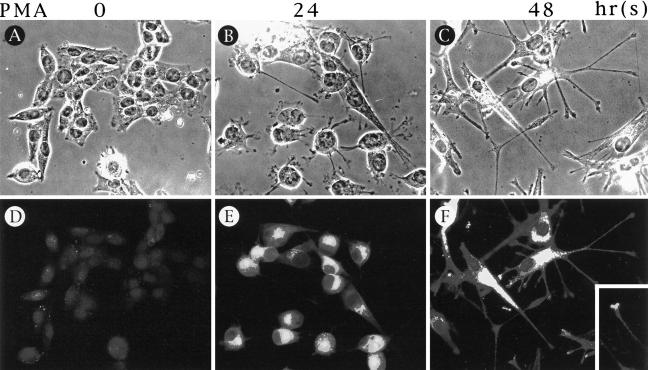
When fixed and permeabilized Paju cells were stained by indirect immunofluorescence with rabbit antibodies to human STC, a strong paranuclear reactivity with a Golgi-like distribution was seen after 24 hours of PMA treatment (Figure 3) ▶ . In fully differentiated Paju cells treated for 48 hours with PMA, the granular staining of STC was also seen in the neuronal extensions (Figure 3F) ▶ and even in the growth cone-like structures (Figure 3 ▶ , inset). No appreciable staining was seen in uninduced Paju cells (Figure 3D) ▶ . Control staining with preabsorbed antibody or normal rabbit serum did not show any activity (data not shown).
Figure 3.
Immunofluorescencent staining of Paju cells after 0, 24, and 48 hours of PMA treatment with STC antibodies (D to F); A to C are the corresponding phase-contrast pictures.
Expression of STC in Brain Neurons
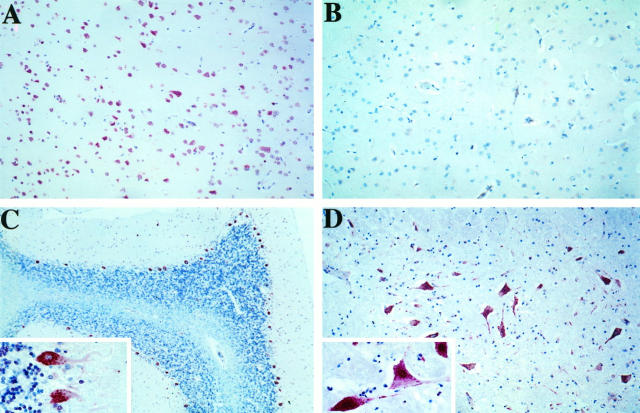
Based on the strong expression of STC in differentiated Paju cells, we performed immunohistochemical staining with anti-STC antibodies on sections from different parts of normal human brain (Figure 4) ▶ . A strong reactivity was apparent in cortical neurons (Figure 4A) ▶ , in Purkinje cells of the cerebellum (Figure 4C) ▶ , and in large neurons of the dentate nucleus (Figure 4D) ▶ . The strongest reactivity was seen in the perinuclear cytoplasm. Also the nuclei, but not in the nucleoli, and the neuronal processes frequently displayed positive staining. The STC reactivity appeared frequently in a granular distribution like that seen in differentiated Paju cells. In addition to the large neurons of the dentate nucleus, weak nuclear staining was also seen in the small neurons of the granular layer of the cerebellar cortex. No staining was observed in the glial cells, and only a weak reactivity was seen in the vascular endothelium. Preabsorption of the rabbit antibodies to STC with recombinant protein completely removed the reactivity against neurons (Figure 4B) ▶ but not to the endothelium, suggesting a nonspecific nature in the endothelial staining of this antibody.
Figure 4.
Immunohistochemical stainings of different parts of normal human brain. A: Parietal cortex. B: Parietal cortex stained with STC antibodies preabsorbed with recombinant STC protein (control). C: Cerebellum. C, inset: Larger magnification of Purkinje cells. D: Nucleus dentatus. D, inset: Larger magnification of the neuronal staining.
Given that expression of STC was confined to differentiating Paju cells but was not seen in proliferating cells, we asked whether the expression of STC in brain neurons also correlated to their terminal differentiation. Because the heteroantiserum against human STC also reacts with the highly homologous mouse STC, we investigated by immunohistochemistry sections from brains of embryonic (15-day embryos), newborn, and adult mice. No specific staining was seen in the embryonic brain (Figure 5A) ▶ . Only a very weak reactivity was appreciated in some brain neurons of the newborn mouse (Figure 5B) ▶ . The terminally differentiated large neurons of adult mouse brain displayed an immunoreactivity for STC, which corresponds with that seen in human brain (Figure 5C) ▶ . Preabsorbed antibody used as control staining did not give any activity (Figure 5 ▶ , D and E).
Figure 5.
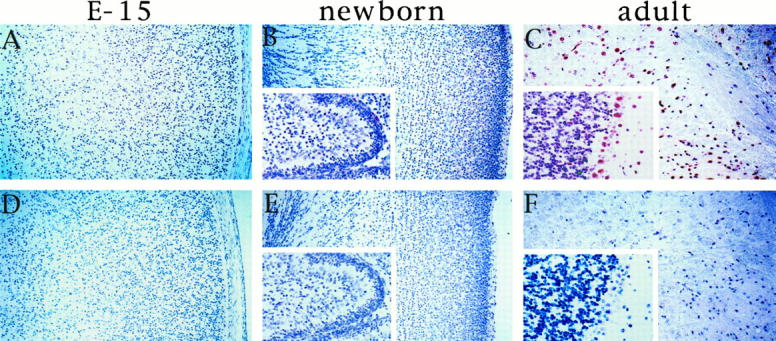
Immunohistochemical stainings of mouse brain with antibodies to STC (A to C) and with preabsorbed antibodies (D to F). A and D: Brain tissue from 15-day-old embryo. B and E: Cortex from a newborn mouse, inset: Cerebellum. C and F: Adult mouse parietal cortex, inset: Cerebellum.
Discussion
In this study, we have shown that terminally differentiated neurons in mouse and human brain express high levels of STC. STC is a 29-kd glycoprotein that until recently was considered an exclusive hormone for the regulation of calcium homeostasis in bony fish. The recently cloned cDNAs for the human and mouse stc have disclosed the expression of STC in mammals. 8,9 A high degree of sequence homology between fish and mammalian stc also indicates an evolutionary conservation of the stc gene. Although the fish STC is produced by a specialized organ, the glands of Stannius, the major sources of STC in higher vertebrates are still to be defined.
We used DD-RT-PCR to screen for gene expression in relation to neuronal differentiation in the human neural crest-derived cell line Paju and found a dramatically upregulated expression of stc mRNA coupled to neural sprouting by treatment with PMA. Immunostaining by indirect immunofluorescence with antibodies to STC did not show appreciable amounts of STC in untreated cultures of Paju cells, whereas, after 6 hours in the presence of PMA, a paranuclear accumulation of STC reactivity corresponding to the Golgi area was seen. A granular distribution of STC throughout the cytoplasm and also in the dendritic processes including the growth cones was seen in terminally differentiated cells. STC also gradually accumulated in the culture medium of PMA-treated Paju cells, but whether this results from active secretion or passive release is under investigation (Zhang et al, manuscript in preparation). Hypercalcemia triggers production of STC in fish. 2,3 Cultivation of Paju cells in the presence of high Ca2+ concentrations or calcium ionophore A23187 did not, however, induce synthesis of detectable amounts of STC (data not shown), indicating that the expression of the stc gene in the Paju cell line is coupled to the genetic program of neural differentiation.
Immunostaining of sections from different parts of normal human brain revealed a strong expression of STC only in the neurons, whereas the glial cells did not contain detectable STC. The large neurons, including the Purkinje cells of the cerebellum, displayed a cytoplasmic granular reactivity extending to the axons with a similar pattern seen in differentiated Paju cells. Nuclear staining was particularly evident in the small neurons in the granular layer of the cerebellar cortex, but also some large neurons in the basal ganglia showed both cytoplasmic and nuclear staining.
The immunostaining of adult mouse brain gave a pattern similar to that seen in humans, with strong reactivity in the fully differentiated large neurons. Staining brain sections from fetal and newborn mice in which the neurons are still proliferating gave a very weak or absent reactivity. As in the Paju model, the expression of STC in brain neurons appears to be linked to their terminal maturation.
The only known function of STC is its regulatory influence on Ca2+ homeostasis. In fish, STC protects from dangerous hypercalcemia. 20 Studies on STC in mammals so far have shown that addition of STC reduces the Ca2+ uptake and increases phosphate absorption in pig duodenal and in rat intestinal epithelium. 12 Infusion of recombinant human STC in rats inhibits the renal phosphate excretion and reduces calcium reabsorption. 11 Given this, it appears plausible that the high level of STC found in terminally differentiated brain neurons is associated with neuronal calcium homeostasis. It should, however, be emphasized that STC mRNA has also been found in ovary, prostate, and thyroid, 8 which indicates that STC may have other endocrine functions unrelated to calcium homeostasis.
Calcium has a well established pleiotropic effect on the nervous system. In addition to the regulatory influence on ion fluxes in neural signal transmission, 21-23 changes in intracellular calcium can modify events such as release of neurotransmitters, 24 axon sprouting, 25 and neuronal survival. 26 Increased intracellular level of free Ca2+ is considered a major pathogenic mechanism in ischemic brain damage, 15,27 and excessive Ca2+ influx is suggested to be important in excitotoxic neuronal cell death. 28
It is conceivable that, as in intestinal and kidney tubular epithelia, STC may also regulate transmembrane Ca2+ fluxes in neurons and may contribute to the protection against hypercalcemia in terminally differentiated neurons. Our very recent observations lend some support to this notion. Immunohistochemical staining of human brain sections from areas of early (4-hour) ischemia revealed redistribution of STC in cortical neurones from a mainly perinuclear reactivity to membranes and axons (Lindsberg et al., manuscript in preparation). Moreover, Fierro and Llano 29 recently reported a steep increase in the ability to buffer depolarization-evoked intracellular calcium changes in Purkinje cells from 15-day-old rats as compared with cells from 6-day-old animals. Given the increased expression of STC in relation to Purkinje cell maturation reported here, it is tempting to speculate that STC may contribute to the rising calcium buffering capacity observed in rat Purkinje cells during the neonatal period.
Together with our previous findings, 13,30 these observations suggest that the generation of terminally maturated neuronal cells with limited proliferative potential may involve expression of genes conferring increased resistance to internal and external cell damage to ensure extended cell survival.
Acknowledgments
We thank Hilkka Toivonen, Anneli Asikainen, and Hannele Laaksonen for technical assistance; Dr. James Chambers for sequencing the DD-RT-PCR clones; and Dr. Jackson S. Wan, for bioinformation support.
Footnotes
Address reprint requests to Dr. Leif C. Andersson, Department of Pathology, Haartman Institute, University of Helsinki, P.O. Box 21, 00014 Helsinki, Finland. E-mail: Leif.Andersson@helsinki.fi.
Supported by The Sigrid Jusélius Foundation, The Finnish Academy of Science, University of Helsinki, The Center for International Mobility Organisation, The Finnish Cancer Society, and The Cancer Society in Stockholm.
References
- 1.Stannius H: Uber nebenniere bei knochenfischen. Arch Anat Physiol 1839, 6:97-101 [Google Scholar]
- 2.Wagner GF, Gellersen B, Friesen HG: Primary culture of teleocalcin cells from rainbow trout corpuscles of Stannius: regulation of teleocalcin secretion by calcium. Mol Cell Endocrinol 1989, 62:31-39 [DOI] [PubMed] [Google Scholar]
- 3.Wagner GF, Milliken C, Friesen HG, Copp DH: Studies on the regulation and characterization of plasma stanniocalcin in rainbow trout. Mol Cell Endocrinol 1991, 79:129-138 [DOI] [PubMed] [Google Scholar]
- 4.Fenwick JC, So YP: A perfusion study of the effect of stanniectomy on the net influx of calcium 45 across an isolated eel gill (1). J Exp Zool 1974, 188:125-131 [DOI] [PubMed] [Google Scholar]
- 5.Lafeber FP, Flik G, Wendelaar Bonga SE, Perry SF: Hypocalcin from Stannius corpuscles inhibits gill calcium uptake in trout. Am J Physiol 1988, 254:891-896 [DOI] [PubMed] [Google Scholar]
- 6.Lu M, Wagner GF, Renfro JL: Stanniocalcin stimulates phosphate reabsorption by flounder renal proximal tubule in primary culture. Am J Physiol 1994, 267:1356-1362 [DOI] [PubMed] [Google Scholar]
- 7.Sundell K, Bjornsson BT, Itoh H, Kawauchi H: Chum salmon (Oncorhynchus keta) stanniocalcin inhibits in vitro intestinal calcium uptake in Atlantic cod (Gadus morhua). J Comp Physiol B 1992, 162:489-495 [DOI] [PubMed] [Google Scholar]
- 8.Chang AC, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR: A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol 1995, 112:241-247 [DOI] [PubMed] [Google Scholar]
- 9.Chang AC, Dunham MA, Jeffrey KJ, Reddel RR: Molecular cloning and characterization of mouse stanniocalcin cDNA. Mol Cell Endocrinol 1996, 124:185-187 [DOI] [PubMed] [Google Scholar]
- 10.Haddad M, Roder S, Olsen HS, Wagner GF: Immunocytochemical localization of stanniocalcin cells in the rat kidney. Endocrinology 1996, 137:2113-2117 [DOI] [PubMed] [Google Scholar]
- 11.Wagner GF, Vozzolo BL, Jaworski E, Haddad M, Kline RL, Olsen HS, Rosen CA, Davidson MB, Renfro JL: Human stanniocalcin inhibits renal phosphate excretion in the rat. J Bone Miner Res 1997, 12:165-171 [DOI] [PubMed] [Google Scholar]
- 12.Madsen KL, Tavernini MM, Yachimec C, Mendrick DL, Alfonso PJ, Buergin A, Olsen HS, Antonaccio MJ, Thomson A, Fedorak RN: Stanniocalcin: a novel protein regulating calcium and phosphate transport across mammalian intestine. Am J Physiol 1998, 37:96-102 [DOI] [PubMed] [Google Scholar]
- 13.Zhang KZ, Westberg JA, Holtta E, Andersson LC: BCL2 regulates neural differentiation. Proc Natl Acad Sci USA 1996, 93:4504-4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biessels G, Gispen WH: The calcium hypothesis of brain aging and neurodegenerative disorders: significance in diabetic neuropathy. Life Sci 1996, 59:379-387 [DOI] [PubMed] [Google Scholar]
- 15.Lazarewicz JW: Calcium transients in brain ischemia: role in neuronal injury. Acta Neurobiol Exp (Warsz) 1996, 56:299-311 [DOI] [PubMed] [Google Scholar]
- 16.Liang P, Pardee AB: Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 1992, 257:967-971 [DOI] [PubMed] [Google Scholar]
- 17.Wan J, Sharp S, Poirier G, Wagaman P, Chambers J, Pyati J, Hom YL, Galindo J, Huvar A, Peterson P, Jackson M, Erlander M: Cloning differentially expressed mRNAs. Nat Biotechnol 1996, 14:1685-1691 [DOI] [PubMed] [Google Scholar]
- 18.Olsen HS, Cepeda MA, Zhang QQ, Rosen CA, Vozzolo BL: Human stanniocalcin: a possible hormonal regulator of mineral metabolism. Proc Natl Acad Sci USA 1996, 93:1792-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Boguslawsky K: Immunohistochemical detection of progesterone receptors in paraffin sections: a novel method using microwave oven pretreatment. APMIS 1994, 102:9:641–646 [DOI] [PubMed]
- 20.Wagner GF, Hampong M, Park CM, Copp DH: Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol 1986, 63:481-491 [DOI] [PubMed] [Google Scholar]
- 21.Racay P, Lehotsk J: Intracellular and molecular aspects of Ca(2+)-mediated signal transduction in neuronal cells. Gen Physiol Biophys 1996, 15:273-289 [PubMed] [Google Scholar]
- 22.Davenport RW, Dou P, Mills LR, Kater SB: Distinct calcium signaling within neuronal growth cones and filopodia. J Neurobiol 1996, 31:1-15 [DOI] [PubMed] [Google Scholar]
- 23.Rehder V, Williams CV, Kater SB: Functional compartmentalization of the neuronal growth cone: determining calcium’s place in signaling cascades. Perspect Dev Neurobiol 1996, 4:215-226 [PubMed] [Google Scholar]
- 24.Bliss TV, Collingridge GL: A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993, 361:31-39 [DOI] [PubMed] [Google Scholar]
- 25.Collins F, Schmidt MF, Guthrie PB, Kater SB: Sustained increase inintracellular calcium promotes neuronal survival. J Neurosci 1991, 11:2582-2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi DW: Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1:623-634 [DOI] [PubMed] [Google Scholar]
- 27.Kristian T, Siesj BK: Calcium-related damage in ischemia. Life Sci 1996, 59:357-367 [DOI] [PubMed] [Google Scholar]
- 28.Choi DW: Excitotoxic cell death. J Neurobiol 1992, 23:1261-1276 [DOI] [PubMed] [Google Scholar]
- 29.Fierro L, Llano I: High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J Physiol (Lond) 1996, 496:617-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang KZ, Junnikkala S, Erlander MG, Guo HQ, Westberg JA, Meri S, Andersson LC: Up-regulated expression of decay-accelerating factor (CD55) confers increased complement resistance to sprouting neural cells. Eur J Immunol 1998, 28:1189-1196 [DOI] [PubMed] [Google Scholar]