Abstract
It is difficult to identify lymph vessels in tissue sections by histochemical staining, and thus a specific marker for lymphatic endothelial cells would be more practical in histopathological diagnostics. Here we have applied a specific antigenic marker for lymphatic endothelial cells in the human skin, the vascular endothelial growth factor receptor-3 (VEGFR-3), and show that it identifies a distinct vessel population both in fetal and adult skin, which has properties of lymphatic vessels. The expression of VEGFR-3 was studied in normal human skin by in situ hybridization, iodinated ligand binding, and immunohistochemistry. A subset of developing vessels expressed the VEGFR-3 mRNA in fetal skin as shown by in situ hybridization and radioiodinated vascular endothelial growth factor (VEGF)-C bound selectively to a subset of vessels in adult skin that had morphological characteristics of lymphatic vessels. Monoclonal antibodies against the extracellular domain of VEGFR-3 stained specifically endothelial cells of dermal lymph vessels, in contrast to PAL-E antibodies, which stained only blood vessel endothelia. In addition, staining for VEGFR-3 was strongly positive in the endothelium of cutaneous lymphangiomatosis, but staining of endothelial cells in cutaneous hemangiomas was weaker. These results establish the utility of anti-VEGFR-3 antibodies in the identification of lymphovascular channels in the skin and in the differential diagnosis of skin lesions involving lymphatic or blood vascular endothelium.
Angiogenesis, the formation of new blood vessels from vascular endothelium, is a key event in several biological processes, including wound healing and tumor development. 1 The regulation of angiogenesis depends on a balance between stimulatory and inhibitory factors affecting the proliferation and differentiation of endothelial cells. 2 Vascular endothelial growth factor (VEGF), which belongs to the platelet-derived growth factor family, is currently known as the major inducer of angiogenesis and vessel permeability. 3 Other members of the family, closely related to VEGF, include PlGF, VEGF-B, VEGF-C, and VEGF-D. 4-9
The biological activities of VEGF and VEGF-C are exerted via binding to tyrosine kinase receptors. Selective binding of these factors occurs to VEGF receptor (VEGFR)-1 (Flt-1) and VEGFR-3 (Flt4), respectively, and both of the factors also bind to VEGFR-2 (Flk-1/KDR). 5,6,10-15 The recently identified specific receptor for VEGF-B is VEGFR-1 (B. Olofsson et al, manuscript in preparation), whereas VEGF-D binds both VEGFR-2 and VEGFR-3. 7
In the skin of transgenic mice, overexpression of the VEGF-C cDNA has been shown to selectively induce lymphatic endothelial cell proliferation and hyperplasia of the lymphatic vasculature. 16 Furthermore, in differentiated chick chorioallantoic membrane, purified mature VEGF-C also induced growth of lymphatic vessels, having very little effect on blood capillaries. 17 In the present work, we have analyzed the binding of VEGF-C and expression of VEGFR-3 in adult human skin, in cutaneous lymphangiomatosis and hemangioma samples using iodinated ligand binding, in situ hybridization, and immunohistochemistry for the identification of the specific receptors.
Materials and Methods
In Situ Hybridization
Skin from 17- and 18-week-old human fetuses was obtained from legal abortions induced with prostaglandins. The gestational age was confirmed from the foot length. 18 The study was approved by the Ethical Committee of the Helsinki University Hospital. The skin samples were fixed in 4% paraformaldehyde for about 20 h before dehydration and paraffin embedding. The human antisense and sense VEGFR-3 RNA probes were generated from linearized pBluescriptIISK+ plasmid (Stratagene, La Jolla, CA), containing an EcoRI-SphI fragment corresponding to nucleotides 1 through 595 of the human VEGFR-3 (Flt4) cDNA. 12 Radiolabeled RNA was synthesized using T3 and T7 polymerases and 35S-labeled UTP (Amersham Corp., Arlington Heights, IL). VEGFR-2 RNA probe was generated from linearized pBluescriptIISK+ plasmid containing an EcoRI-HindIII fragment covering bp 6 through 715. 15 In situ hybridization of the paraffin sections was performed as described previously. 19 Alkaline hydrolysis was omitted for the VEGFR-3 probe. The high-stringency wash was for 90 minutes at 65°C in 1× standard saline citrate containing 30 mmol/L dithiothreitol. The slides were exposed for 4 weeks, developed, and stained with hematoxylin. Control hybridizations with sense strand did not give a specific signal above background.
Iodinated Growth Factor Binding
Recombinant human (rh) VEGF165 or the 21-kd mature form of VEGF-C was labeled with 125I using the Iodo-Gen reagent (Pierce, Rockford, IL) and purified by gel filtration on PD-10 columns (Pharmacia, Uppsala, Sweden). The specific activities were 2.2 × 105 cpm/ng and 1.0 × 105 cpm/ng for rh-VEGF and rh-VEGF-C, respectively. The iodinated growth factors were tested for specific binding using PAE-VEGFR-1 and PAE-VEGFR-3 cells 20 and soluble receptor-immunoglobulin proteins. 7
The skin samples obtained were frozen immediately and kept at −70°C. Frozen sections were cut at 7 μm and then mounted onto silane-coated slides and stored in airtight boxes at −70°C. After thawing, the sections were incubated for 30 minutes at room temperature in the blocking solution, (minimum essential medium (Life Technologies, Inc., Grand Island, NY), 0.5 mg/ml bovine serum albumin, 20 mmol/L HEPES pH 7.4, 1 mmol/L phenylmethylsulfonyl fluoride, and 4 μg/ml leupeptin). The blocking buffer was then removed, and the sections were covered by a droplet of the same buffer containing 10 pmol/L 125I-labeled rh-VEGF or 125I-labeled rh-VEGF-C. Adjacent sections were incubated in the same concentration of iodinated growth factor in the presence of 1 nmol/L of the corresponding nonradioactive growth factor, to define nonspecific binding. Cross-competition of binding was assessed in the presence of 1 nmol/L rh-VEGF-C for 125I-labeled VEGF or 1 nmol/L rh-VEGF for 125I-labeled rh-VEGF-C binding.
After a 90-minute incubation in a humidified chamber at room temperature, the sections were rinsed five times (3 minutes each time) on ice, once with binding buffer and four times with phosphate-buffered saline. Sections were then fixed for 10 minutes in 2% paraformaldehyde, 2% glutaraldehyde in 0.1 mol/L phosphate buffer pH 7.4, rinsed for 2 to 5 seconds in dH20, and dried at room temperature for approximately 2 hours. The dried sections were covered with NTB-2 emulsion (Eastman Kodak Co., Rochester, NY) and stored at 4°C for 2 weeks, developed, and stained.
Immunohistochemistry
Human skin from the leg, neck, and lower lip obtained after surgical removal or from buccal mucosa biopsies was frozen immediately, sectioned, stored at −70°C, and used for immunohistochemistry; one case of lymphangiomatosis and two cases of intramuscular hemangiomas were obtained in the same fashion. Paraffin-embedded biopsy specimens of six cases of lymphangiomatosis taken from the limb skin of young men were also studied. 21 The monoclonal antibodies (mAbs) used were against CD31 (platelet/endothelial cell adhesion molecule 1; DAKO Immunoglobulins, Glostrup, Denmark), an as-yet molecularly undefined endothelial antigen (PAL-E; Sanbio, Uden, The Netherlands), laminin (Sigma Chemical Co., St. Louis, MO), von Willebrand factor/factor VIII-related antigen (vWF, 6.3 μg/ml; DAKO Immunoglobulins), and mAb 9D9 developed against the extracellular domain of VEGFR-3 expressed in a baculovirus system. 22
Adjacent 5-μm cryosections were air-dried and fixed in cold acetone for 10 minutes. The sections were incubated with blocking serum (5% normal horse serum) and then with anti-VEGFR-3 at a concentration of 1.1 μg/ml, anti-CD31 (diluted 1:200), anti-vWF (diluted 1:200), PAL-E (0.15 μg/ml), or anti-laminin (diluted 1:2000) for 2 h in a humid atmosphere at room temperature. A subsequent incubation for 30 minutes in biotinylated anti-mouse serum was followed by a 30-minute incubation using reagents of the Vectastain Elite avidin-biotin complex (ABC)/HPR kit (Vector Laboratories, Burlingame, CA). A 60-minute incubation with ABC was found to be optimal. Peroxidase activity was developed with 3-amino-9-ethyl carbazole (Sigma Chemical Co.) for 10 minutes. Finally, the sections were stained with hematoxylin. Negative controls were done by omitting the primary antibody, by using irrelevant primary antibody of the same isotype, or by blocking the anti-VEGFR-3 by overnight incubation with a 10-fold molar excess of the immunogen. Five-μm-thick sections of paraffin-embedded tissue from cutaneous lymphangiomatosis were deparaffinized and heated in a microwave oven in 10 mmol/L citrate buffer, pH 6.0, at 780 W for 5 minutes, followed by 450 W for 10 minutes. The sections were then incubated in methanol containing 30% H2O2 for 30 minutes and processed as the cryosections.
For differential staining of lymph and blood vessels, the following double-staining protocol was used. Frozen 4-μm sections were fixed in acetone; incubated with undiluted PAL-E mAb supernatant for 1 hour, biotinylated horse anti-mouse antibody (Vectastain, dilution 1:200) for 30 minutes, and ABC-peroxidase (Vectastain, 1:100) for 45 minutes; and developed with 3-amino-9-ethyl carbazole for 10 minutes. For the second step, the sections were incubated with anti-CD31 mAb for 1 hour (1:2000), followed by incubation with rabbit anti-mouse immunoglobulin conjugated with alkaline phosphatase for 30 minutes (DAKO Immunoglobulins, 1:40), and developed with Fast Blue (Sigma Chemical Co.) for 20 minutes. All procedures were done at room temperature, and the sections were rinsed with phosphate-buffered saline between each step. In adjacent sections, VEGFR-3 expression was visualized by immunostaining with the 9D9 mAb, according to a procedure previously described for signal enhancement. 23 Briefly, acetone-fixed cryosections were incubated for 1 hour with anti-VEGFR-3 mAb 9D9, followed by biotinylated horse anti-mouse antibody for 30 minutes (1:200), ABC-peroxidase for 30 minutes (1:100), biotinylated tyramine solution (1:2000) containing 0.4 vol % of 30% H2O2 and ABC-peroxidase (1:100) for 20 minutes, and 3-amino-9-ethyl carbazole for 10 minutes, all at room temperature. Sections were counterstained with Harris’ hematoxylin. After the staining procedures, all samples were examined by a trained pathologist.
Results
Localization of VEGF Receptors in Fetal Skin by in Situ Hybridization
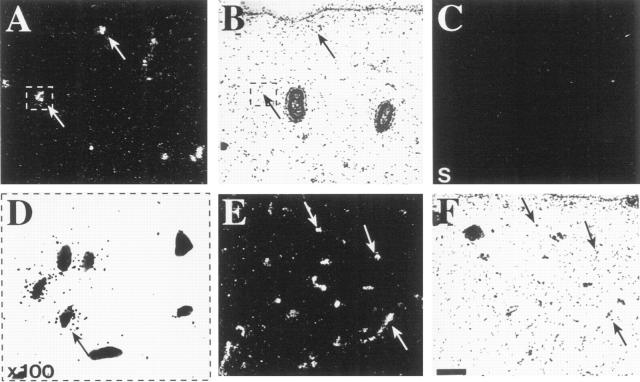
To compare the expression of the VEGF-C receptors in normal fetal skin, analysis of their mRNAs was performed by in situ hybridization of adjacent sections. VEGFR-3 was expressed in putative developing lymphatic vessels mainly in the upper part of the developing dermis (Figure 1 ▶ , A and B, arrows). Control hybridization with the probe from the VEGFR-3 sense strand did not give any specific signal above background (Figure 1C) ▶ . When viewed at higher magnification, the cells showing the VEGFR-3 signal were often devoid of surrounding vessel wall structures; eg, no basement membrane could be identified around these cells (Figure 1D) ▶ . VEGFR-2 showed a strong hybridization signal from numerous vessels located at various levels of the dermis (Figure 1 ▶ , E and F).
Figure 1.
Expression of VEGFR-3 and VEGFR-2 in fetal skin analyzed by in situ hybridization. A, B, and D: Hybridization with the VEGFR-3 antisense probe. The signal originates from a subset of vessels of the dermis (arrows). C: Control hybridization with the VEGFR-3 sense strand. D: In a higher magnification of the area marked in (A) and (B), the autoradiographic grains are shown to be localized to the endothelial cells of a developing vessel. E and F: Hybridization for VEGFR-2. The probe gives signal in developing vessel structures at several levels of the dermis (arrows). B, D, and F: Bright-field photographs of the same sections. Bar: 0.1 mm.
Distribution of VEGF-C and VEGF Binding Sites in Adult Skin
To enable identification of the specific binding sites of VEGF-C and VEGF in the skin, iodinated recombinant human growth factors were used for in vivo ligand binding and autoradiography of frozen sections. The signal from the receptor-bound radioactive VEGF-C was visualized specifically in a horizontal zone of the subpapillary lymphatic plexus, which occurs in association with the superficial venous system at the junction of papillary and reticular dermis (Figure 2 ▶ , A to C). These lymphatic vessels were seen as long extended structures, often collapsed, and had thin walls compared with the thicker walls of nearby blood vessels. Expression was also detected in the lymphatic vessels of the deep dermal plexus at the dermal-subcutaneous junction (data not shown). In these sections, the binding was displaced with the addition of 1 nmol/L rh-VEGF-C to the incubation medium (Figure 2 ▶ , D to F).
Figure 2.
Radioactive VEGF-C and VEGF binding in human adult skin visualized by autoradiography. A, B, and C: Binding of 125I-labeled rh-VEGF-C. The signal is localized to the thin lymphatic vessels of the subpapillary plexus (arrows). D, E, and F: Binding sites are blocked when a 100-fold excess of cold ligand is added to the incubation medium. Some unspecific binding is seen originating from keratin squames in the epidermis (D, arrowhead). G, H, and I: Specific 125I-labeled rh-VEGF binding to vascular endothelium, which extends throughout the dermis. (C and I: Higher magnifications (×100) of the marked vessel structures binding the radioactive ligands VEGF-C and VEGF, respectively. Dark-field (A, D, and G) and bright-field (B, C, E, F, H, and I) exposures are shown. Bar: 0.1 mm for A, B, and D to H.
Incubation of the skin sections with iodinated VEGF revealed binding to all discernible vessels of every type throughout the dermis (Figure 2 ▶ , G to I). In these sections, the binding was displaced from vessel endothelia with the addition of 1 nmol/L rh-VEGF. However, even in the presence of a 100-fold excess of cold VEGF, a weak signal was still detected in occasional cells that were not parts of the endothelial structures of the vessels. Unexpectedly, cross-competition of radioactive VEGF-C binding with VEGF or vice versa did not significantly change the binding patterns obtained (data not shown).
VEGFR-3 Immunohistochemistry
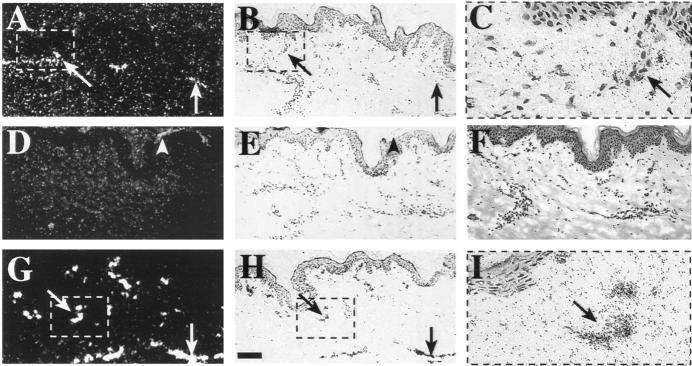
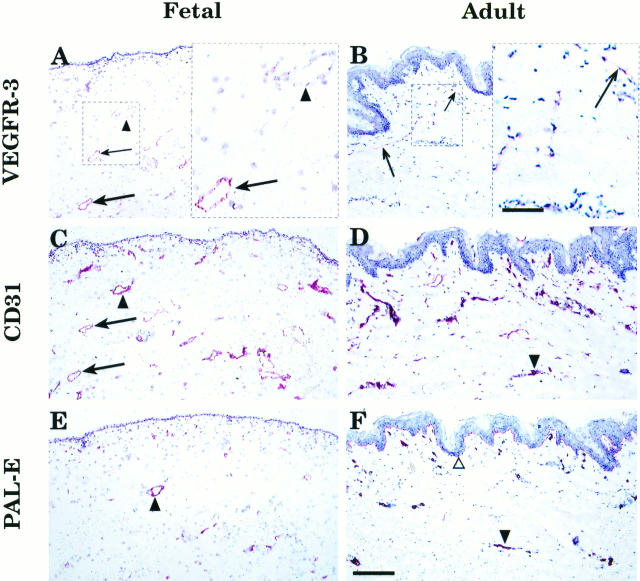
Staining of the fetal skin using the anti-VEGFR-3 mAb showed circular lymphatic structures in the corium layer of the developing dermis (Figure 3A ▶ , arrows). This pattern was similar to that seen in the in situ hybridization (compare with Figure 1 ▶ , A and B). Adjacent skin sections were stained with anti-CD31 (Figure 3C) ▶ , which decorated all endothelial cells, and PAL-E (Figure 3E) ▶ , which detects endothelial cells in blood vessels. In adult skin, a few horizontally organized lymphatic vessels were stained by the anti-VEGFR-3 (Figure 3B ▶ , arrows). These vessels were difficult to trace in the adjacent sections stained by anti-CD31 (Figure 3D) ▶ and PAL-E (Figure 3F) ▶ . According to previously published data, the PAL-E−/CD31+ vascular structures were defined as lymphatic vessels. 24 Indeed, comparison of the stainings confirmed that different subpopulations of the CD31-positive vessels were highlighted by anti-VEGFR-3 and PAL-E mAbs. To further confirm the identity of the lymphatic vessels, anti-VEGFR-3 staining of adult buccal mucosa was combined with double staining for CD31 and PAL-E. 24 This analysis directly confirmed that practically all vessels positive for VEGFR-3 were of the PAL-E−/CD31+ type (Figure 4 ▶ , A and B). In addition to the blood vessel endothelium, PAL-E antibody also stained the basal lamina of the epidermis and buccal mucosa (Figures 3F and 4B ▶ ▶ , open triangles). In contrast to the blood vessels, the VEGFR-3-positive vessels had only very weak or no staining for laminin, which was used as a basal lamina marker (Figure 4D) ▶ . 25
Figure 3.
Comparison of VEGFR-3 expression with two vascular endothelial markers in fetal and adult skin. Arrows indicate vessels in adjacent sections of fetal and adult skin, respectively, which stain for VEGFR-3 (A and B) and CD31 (C and D), but not for PAL-E (E and F), and are therefore presumably lymphatic. Arrowheads identify vessels, which only react with antibodies against the two blood vascular endothelial antigens. In adult skin, the thin VEGFR-3-positive lymphatic vessels could be identified by morphology and lack of a basal lamina (B), but the same vessel could not be traced in the adjacent sections stained for CD31 (D) or PAL-E (F). Bar: 0.17 mm. Insets (A and B, right) illustrate higher magnifications of the lymphatic vessels (arrows) in marked areas of the fetal and adult skin sections, respectively. Bar: 57 μm.
Figure 4.
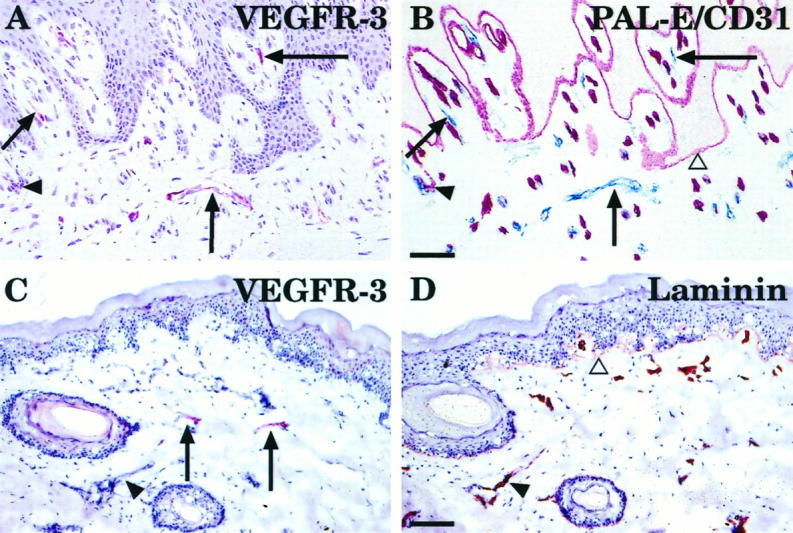
Comparison of VEGFR-3 expression with double staining for two vascular endothelial markers and with anti-laminin staining. A sample of adult buccal mucosa was stained with the anti-VEGFR-3 mAb (A) and an adjacent section, first with PAL-E (B, red-colored endothelia) and then with anti-CD31 (B, blue-colored endothelia), to distinguish lymphatic structures from blood vessels. PAL-E also decorates the epidermal basal lamina (B, open triangle). Comparison of VEGFR-3 and laminin expression in adult skin is shown in (C) and (D). Arrows indicate lymphatic vessels of adult skin, which stain for VEGFR-3 (C). Arrowheads show blood vessels with basement membranes, which stain for laminin (D), but only very weakly for VEGFR-3. Bars: 35 μm (A and B) and 0.11 mm (C and D).
VEGFR-3-Positive Vessels in Lymphangiomatosis and in Hemangioma
Lymphatic endothelium was also analyzed in six cases of the rare condition of lymphangiomatosis of the skin. Staining for VEGFR-3 was detected in thin endothelium-lined anastomosing channels dissecting through dermal connective tissue (Figure 5A) ▶ . A negative control staining was done by blocking anti-VEGFR-3 with the immunogen (Figure 5B) ▶ . The same structures in the adjacent section were vWF negative (Figure 5D ▶ , arrows), although blood vessels in the same section were vWF positive (Figure 5D ▶ , arrowheads). Both blood vessels (Figure 5C ▶ , arrowheads) and lymphatic vessels (Figure 5C ▶ , arrows) were stained for CD31. In three fixed, paraffin-embedded cutaneous capillary hemangiomas, endothelial cells lining the blood capillaries containing red cells and also endothelial cells apparently not associated with capillaries had very little or no VEGFR-3 signal, but were clearly positive for CD31 (Figure 5 ▶ , E and F, arrowheads). Only some vessels, which were devoid of red cells and thus presumably lymphatic, expressed VEGFR-3 in the tissue surrounding the capillary hemangiomas (Figure 5E ▶ , arrows). However, when adjacent cryosections of two intramuscular hemangiomas were stained for VEGFR-3 (Figure 5G) ▶ and for the endothelial marker CD31 (Figure 5H) ▶ , the majority of the CD31-positive vessels with lumens were found to express at least some VEGFR-3-positive staining (Figure 5H ▶ , arrows). The identity of such vessels is unknown at present.
Figure 5.
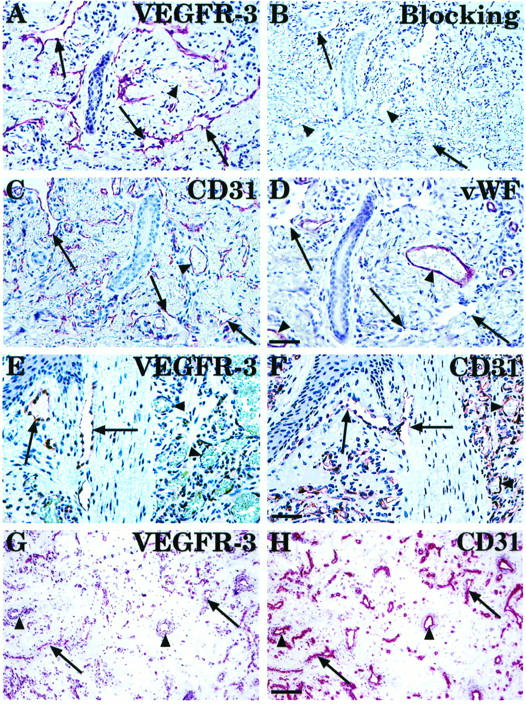
Comparison of VEGFR-3 expression with vascular endothelial markers in lymphangiomatosis (A to D) and in capillary cutaneous (E and F) and intramuscular (G and H) hemangiomas. Arrows indicate collapsed, VEGFR-3-positive thin endothelium-lined anastomosing channels dissecting through dermal connective tissue in a lymphangiomatosis sample (A). Negative control was done by blocking anti-VEGFR-3 with the immunogen (B). Adjacent sections were also stained for CD31 (C) and vWF (D). Note that in contrast to the buccal mucosa sample (Figure 4) ▶ , CD31 identifies the endothelial cells lining lymphatic spaces in lymphangiomatosis, whereas the vWF staining is weak or absent. In cutaneous hemangioma, the well-formed capillaries with prominent endothelial cells show very little or no expression of VEGFR-3 (E), but are strongly CD31 positive (F). In a frozen section of intramuscular hemangioma, weak VEGFR-3 staining is detected (G, arrows) in part of the CD31-positive vessels (H). Bars: 65 μm (A to D, 48 μm (E and F), and 140 μm (G and H).
Discussion
A uniformly structured, dense subpapillary, and wide-meshed deep dermal lymphatic network is a common feature of all skin regions. Although a reliable detection technique has been developed to demonstrate dermal lymph vessel networks in skin samples, 26 a specific marker for lymphatic endothelial cells would be useful, eg, in the diagnosis of lymphangiomas. Here we present such a marker, the VEGFR-3, using mAbs and show that it identifies the VEGF-C growth factor receptor and a distinct vessel population both in fetal and adult skin, which has properties of lymphatic vessels.
The mRNA expression patterns of the VEGFRs were distinct but partially overlapping in the fetal skin analyzed by in situ hybridization. Expression of VEGFR-3 appeared to be restricted to a subpopulation of vessels in the upper dermis, which may correspond to the developing superficial lymphatic plexus of the fetal skin. The mRNA signal for the major mitogenic VEGF receptor, VEGFR-2, was observed in all vessels throughout the developing skin. This finding is in agreement with a previous report that the distribution of these receptors in different fetal organs partially overlaps, yet certain endothelia lack one or two of the three receptors. 19 These results are also consistent with the suggested specific roles of each of these receptors in the vascular system of the skin.
The results of iodinated ligand binding experiments are consistent with the results obtained by in situ hybridization. The iodinated VEGF-C was shown to bind preferentially to the lymphatic vessels in which VEGFR-3 was shown to be expressed by in situ hybridization. These results are in agreement with earlier findings that VEGFR-3 expression was shown to become restricted to the lymphatic vessels in adult mouse tissues and some high endothelial venules in adult human tissues. 27 However, skin was not examined in the previous study. Somewhat surprisingly, although VEGF-C and VEGF compete for VEGFR-2 binding, 125I-labeled rh-VEGF-C binding to the skin was not detectably decreased by the addition of cold VEGF (unpublished data), suggesting that in this tissue sample, VEGF-C bound mostly to VEGFR-3. This cannot be fully explained with the threefold-greater affinity of VEGF-C toward VEGFR-3 when compared with binding to VEGFR-2. 20 However, a 100-fold excess of VEGF-C abolished completely VEGF-C-specific binding to its receptor(s).
In the case of 125I-labeled rh-VEGF, binding was detected in most of the vessel structures, including those that bound radioactive VEGF-C. VEGF has been reported to have about a 5- to 10-fold greater affinity for VEGFR-1 than to VEGFR-2, so that the observed signal in this experiment could originate mostly from binding to VEGFR-1. 13,28-30 Thus, if VEGFR-1 were expressed in the same endothelia, blocking of VEGFR-2 sites with VEGF-C would not necessarily change the binding patterns observed. On the other hand, as VEGF is shown to compete more efficiently for VEGFR-2 binding than VEGF-C, added VEGF-C might not be able to compete for all receptor sites, and this would leave some signal unquenched. 20
Immunohistochemical staining of fetal and adult skin samples showed that anti-VEGFR-3 mAbs identify a subpopulation of vessels positive for the pan-endothelial marker CD31, but negative for PAL-E, which has been previously suggested to define lymphatic vessels. 24 The lack of staining for the basal lamina antigen laminin was consistent with such a conclusion. Cutaneous lymphangiomatosis was chosen for immunohistochemistry, because the disorder is characterized by proliferation of presumed lymphatic endothelium. 21 Besides the skin, lymphangiomatosis often involves bone, soft tissue, or viscera during the first 20 years of life. 31 Whereas the dermal lymph vessels were relatively weakly stained in healthy adult skin using the anti-VEGFR-3, the endothelia of lymphangiomatosis lesions were strongly stained. This is consistent with earlier results showing enhanced VEGFR-3 mRNA expression in lymphangiomas. 27 Furthermore, these results support the theory that the lymphangiomatosis lesions indeed develop from aberrant rests of lymphatic tissue with obliteration of draining lymphatics. 32
Capillary hemangioma is a benign acquired vascular tumor, consisting of multiple thick- and thin-walled vascular structures. 33 It occurs frequently in the skin and sometimes in the internal organs. Compared with normal capillaries, the vessels in capillary hemangiomas have a more prominent endothelial lining. The antibody against VEGFR-3 gave little or no staining of CD31-positive endothelial cells decorating blood vessels in fixed, paraffin-embedded samples of cutaneous capillary hemangiomas. Also, the scattered endothelial cells or clusters of them in well-formed collagen stroma contained very little or no VEGFR-3. However, weak staining of small vessels was obtained in two frozen sections of intramuscular hemangioma lesions, suggesting that these lesions contain endothelial cells in vessels that share phenotypic properties with the lymphatic vessels. One possibility is that such vessels are less differentiated than mature blood vessels, thus resembling early embryonic vessels, which express all three VEGFRs. 27-30
In summary, it may be concluded on the basis of specific radioactive ligand binding, receptor in situ hybridization, and immunohistochemistry that VEGFR-3 is distributed in a manner consistent with the known lymphatic vascular pattern in human skin and that the anti-VEGFR-3 mAbs should prove useful in studies of skin diseases affecting the lymphatic or blood vascular system.
Acknowledgments
We thank Drs. Marja-Terttu Matikainen and Päivi Heikkilä for help with the antibodies and histopathology; Dr. Arja-Leena Kariniemi for critical comments on the manuscript; and Eija Koivunen, Pipsa Ylikantola, and Lia Schalkwijk for cutting the skin sections.
Footnotes
Address reprint requests to Dr. Kari Alitalo, Molecular/Cancer Biology Laboratory, Haartman Institute, University of Helsinki (Haartmaninkatu 3), SF-00014 Helsinki, Finland. E-mail: kari.alitalo@helsinki.fi.
Supported by the Finnish Cancer Organizations, the Finnish Academy, the Sigrid Juselius Foundation, the State Technology Development Center and the European Union (Biomedicine grant PL 963380). AL was supported by a grant from the Paulo Foundation, and TAP was supported by the Helsinki University Central Hospital Research Fund.
Athina Lymboussaki and Taina A. Partanen contributed equally to this work.
References
- 1.Folkman J, Shing Y: Angiogenesis. J Biol Chem 1992, 267:10931-10934 [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997, 18:4-25 [DOI] [PubMed] [Google Scholar]
- 4.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG: Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA 1991, 88:9267-9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Gray A, Yuan J, Luoh S-M, Avraham H, Wood W: Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci USA 1996, 93:1988-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA: Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998, 95:548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson RF, Alitalo K, Eriksson U: Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA 1996, 93:2576-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada Y, Nezu J, Shimane M, Hirata Y: Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics 1997, 42:483-488 [DOI] [PubMed] [Google Scholar]
- 10.de Vries C, Escobedo J, Ueno H, Houck H, Ferrara N, Williams L: The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255:989-991 [DOI] [PubMed] [Google Scholar]
- 11.Matthews W, Jordan C, Gavin M, Jenkins N, Copeland N, Lemischka I: A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci USA 1991, 88:9026-9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, Alitalo R, Alitalo K: FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res 1993, 52:5738-5743 [PubMed] [Google Scholar]
- 13.Quinn T, Peters K, de Vries C, Ferrara N, Williams L: Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA 1993, 90:7533-7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibuya M: Role of VEGF-flt receptor system in normal and tumor angiogenesis. Adv Cancer Res 1995, 67:281-316 [DOI] [PubMed] [Google Scholar]
- 15.Terman B, Dougher-Vermazen M, Carrion M, Dimitrov D, Armellino D, Gospodarowich D, Böhlen P: Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 1992, 187:1579-1586 [DOI] [PubMed] [Google Scholar]
- 16.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain R, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 17.Oh S, Jeltsch M, Birkenhäger R, McCarthy J, Weich H, Christ B, Alitalo K, Wilting J: VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol 1997, 188:96-109 [DOI] [PubMed] [Google Scholar]
- 18.Munsick R: Human fetal extremity lengths in the interval from 9 to 21 menstrual weeks of pregnancy. Am J Obstet Gynecol 1984, 149:883-887 [DOI] [PubMed] [Google Scholar]
- 19.Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico M, Terman B, Alitalo K: The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med 1993, 178:2077-2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K: Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 1997, 13:3898-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez CS, Calonje E, Ferrar D, Browse N, Fletcher CDM: Lymphangiomatosis of the limbs. Am J Surg Pathol 1995, 2:125-133 [DOI] [PubMed] [Google Scholar]
- 22.Jussila L, Valtola R, Partanen TA, Salvén P, Heikkilä P, Matikainen M-T, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K: Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor 3. Cancer Res 1998, 58:1599-1604 [PubMed] [Google Scholar]
- 23.Kerstens H, Poddighe P, Hanselaar A: A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem 1995, 43:347-352 [DOI] [PubMed] [Google Scholar]
- 24.de Waal R, van Altena M, Erhard H, Weidle U, Nooijen P, Ruiter D: Lack of lymphangiogenesis in human primary cutaneous melanoma. Am J Pathol 1997, 150:1951-1957 [PMC free article] [PubMed] [Google Scholar]
- 25.Autio-Harmainen H, Karttunen T, Apaja-Sarkkinen M, Dammert K, Risteli L: Laminin and type IV collagen in different histological stages of Kaposi’s sarcoma and other vascular lesions of blood vessel origin. Am J Surg Pathol 1988, 12:469-476 [DOI] [PubMed] [Google Scholar]
- 26.Lubach D, Wawrzyniak-Schulz A, Neukam D, Nissen S: The extension technique: a new method of demonstrating initial lymph vessels in excised human skin. Br J Dermatol 1990, 123:179-185 [DOI] [PubMed] [Google Scholar]
- 27.Kaipainen A, Korhonen J, Mustonen T, Van Hinsberg V, Fang G, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller N, Werner R, Ullrich A: High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72:835-846 [DOI] [PubMed] [Google Scholar]
- 29.Breier G, Clauss M, Risau W: Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn 1995, 204:228-239 [DOI] [PubMed] [Google Scholar]
- 30.Peters K, De Vries C, Williams L: Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci USA 1993, 90:8915-8919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peh W, Ngan H: Lymphography: still useful in the diagnosis of lymphangiomatosis. Br J Radiol 1993, 66:28-31 [DOI] [PubMed] [Google Scholar]
- 32.Ramani P, Shah A: Lymphangiomatosis. Am J Surg Pathol 1993, 17:329-335 [PubMed] [Google Scholar]
- 33.Wade T, Kamino H, Ackerman A: A histologic atlas of vascular lesions. J Dermatol Surg Oncol 1978, 4:845-850 [DOI] [PubMed] [Google Scholar]