Abstract
Activated protein C (APC) acts as an anticoagulant by inhibiting coagulation factors Va and VIIIa. Although the liver appears to be the primary site of protein C (PC) synthesis, the demonstration that other components of this system are produced extrahepatically raises the possibility that PC itself is synthesized in other tissues. We therefore used quantitative reverse transcription-polymerase chain reaction, in situ hybridization, and immunohistochemistry to screen various murine tissues for PC expression. Relatively high levels of PC mRNA were detected in the kidney (35% of liver) and testis (22% of liver). PC mRNA and antigen were demonstrated in tubular epithelial cells in the renal cortex, in spermatogenic cells in the testis, and in epithelial cells in the epididymis. Low but significant levels of PC mRNA were detected in the epididymis (1.7% of the level in liver), brain (1.1% of liver), and lung (0.8% of liver). PC antigen was demonstrated in bronchial epithelial cells in the lung, in pyramidal neurons in the cerebrum, and in Purkinje cells in the cerebellum. The extrahepatic expression of PC mRNA (ie, in the kidney) was significantly decreased in mice with renal disease (eg, in MRL lpr/lpr mice with autoimmune lupus nephritis, in db/db mice with diabetic nephropathy, and in endotoxin-treated mice with acute renal injury). The decreased renal expression of PC may contribute to the increased procoagulant potential of the kidney during septic and inflammatory processes and to the progression of kidney disease associated with these conditions.
Protein C (PC) is a vitamin K-dependent plasma glycoprotein precursor of the serine protease, activated protein C (APC). 1 It is activated by thrombin bound to thrombomodulin, an endothelial cell surface protein. 2 APC inhibits coagulation by inactivating coagulation factors Va and VIIIa 3 and may stimulate fibrinolysis by reducing thrombin generation, which leads to decreased generation of TAFI (thrombin activatable fibrinolysis inhibitor). 4 It may also stimulate fibrinolysis 4 by reducing the activity of plasminogen activator inhibitor-1. 5,6 The physiological importance of PC is demonstrated by the observations that life-threatening thrombotic complications occur in infants with homozygous PC deficiency, 7 and that patients with heterozygous PC deficiency have a high incidence of venous thrombosis. 8 Moreover, administration of neutralizing monoclonal antibodies to PC induces thrombosis in mice, 9 and infusion of APC prevents the lethal effects of Escherichia coli in baboon models of Gram-negative sepsis 10 and is an effective treatment for certain forms of human septic shock. 11 These results imply that the PC pathway may influence both coagulation and inflammation.
Although the liver appears to be the principal site of PC synthesis, 12 a few recent studies suggest that it also may be produced extrahepatically. 13,14 For example, several components of the PC anticoagulant system have been shown to be synthesized in the male reproductive tissues. In this regard, PC and protein S (a co-factor of APC 15 ) have been detected in Leydig cells of the human testis, 16,17 whereas PC inhibitor was detected throughout the male reproductive system 18 and in tubular cells of the human kidney. 19 These observations thus raise the possibility that the PC pathway may contribute to the maintenance of tubular fluidity in the kidney and that it may be involved in various reproductive processes.
In this report, quantitative reverse transcription-polymerase chain reaction (RT-PCR) 20,21 is used to more completely identify extrahepatic sites of PC synthesis in the mouse and to determine whether expression of this potent anticoagulant molecule is altered in murine models of thrombotic disease. Although PC gene expression was the highest in the liver, high concentrations also were apparent in the kidneys and testes, and low but significant levels were demonstrated in the epididymis, brain, and lung. The kidneys of mice with autoimmune lupus nephritis (MRL lpr/lpr), with diabetic nephropathy (db/db), and with sepsis after endotoxin (lipopolysaccharide (LPS)) treatment expressed considerably lower amounts of PC mRNA than the normal controls. The decreased renal expression of PC may increase the local procoagulant potential of the kidney and thus contribute to the fibrin-mediated progression of renal damage associated with chronic kidney diseases and sepsis.
Materials and Methods
Animals and Tissue Preparation
Female MRL lpr/lpr mice 22 and their normal counterparts (MRL +/+) were obtained from Scripps Clinic Rodent Breeding Colony (La Jolla, CA), whereas male obese/diabetic mice (C57BL/KsJ db/db) 23,24 and their lean counterparts (C57BL/KsJ +/?) were obtained from The Jackson Laboratories (Bar Harbor, ME). Adult mice (2 to 6 months old) were sacrificed, and various tissues were surgically removed and immersed in chilled 4% paraformaldehyde. The tissues were fixed in 4% paraformaldehyde by incubation at 4°C overnight, embedded in paraffin blocks, and sectioned at a thickness of 2 to 5 μm using a microtome. The sections were then mounted onto polylysine slides and stored at room temperature pending analysis. Portions of the freshly removed tissues also were minced and then immediately frozen in liquid nitrogen for preparation of total RNA. Total RNA was prepared by the acid guanidinium thiocyanate-phenol-chloroform method 25 and then quantitated by measuring absorption at 260 nm. The integrity of the 18S and 28S ribosomal RNA was monitored by inspection under UV light after electrophoresing 10 μg of total RNA through a 1.2% agarose/formaldehyde gel.
In separate experiments, LPS (50 μg/mouse; E. coli serotype O111:B4; Sigma Chemical Co., St. Louis, MO) was diluted in 200 μl of saline (Baxter, Deerfield, IL) and injected intraperitoneally into adult male CB6 mice (BALB/c/ByJ × C57BL6/J; Scripps Clinic Rodent Breeding Colony). Control mice were injected with an equivalent volume of saline alone. At 2, 4, 8, and 24 hours after LPS, the mice were sacrificed, and kidney and liver tissues were removed as described above.
Quantitative RT-PCR
We have developed quantitative RT-PCR assays to determine the concentration of specific mRNAs in murine tissues, 21,26 and these procedures were adapted to the analysis of PC mRNA. Briefly, a synthetic DNA template to be used as a standard for the quantitation of PC mRNA was first constructed. This template consisted of the sequence of an upstream primer for mouse PC (5′- GGCAGACGACCACATGCGCTGCAAGTCCAC-3′) followed by the sequence of a downstream primer on the complementary strand (5′-CCAGGAAGTGTGGATGAGCACCCCTCCGCA-3′). 27 The complementary RNA (cRNA) standard was then in vitro transcribed from this template using the Riboprobe Gemini II (Promega, Madison, WI), and a fixed amount was mixed with 1 μg of total tissue RNA and then reverse transcribed using a GeneAmp RNA PCR kit (Perkin-Elmer/Cetus, Norwalk, CT). Serial twofold dilutions of the RT mixture were amplified using PC (described above) or β-actin-specific primers (sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; antisense, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′) 28 in the presence of 32P-end-labeled sense primer (5 × 105 cpm). After PCR amplification for 28 cycles (ie, denaturation at 95°C for 1 minute, primer annealing at 60°C for 1 minute, and extension at 72°C for 1 minute), 20-μl aliquots of the PCR products were electrophoresed on a 2.5% agarose gel. The appropriate bands corresponding to the standard cRNA product (189 bp for PC; 293 bp for β-actin) and the target mRNA product (241 bp for PC; 349 bp for β-actin) were excised from the gel, and the incorporated radioactivity was determined using a scintillation counter. The number of molecules of PC mRNA was then determined by extrapolation using the cRNA standard curve as previously described. 20 Variations in sample loading were assessed by measuring β-actin mRNA.
Riboprobe Preparation
A BamHI/KpnI fragment of the mouse PC cDNA was obtained by RT-PCR from 1 μg of total mouse liver RNA using the following specific primers: 5′-GGATCCAAGAGATGCGGCCAGGCAGC and 3′-GGTACCCCGAAAGAAGACCACC. The resulting fragment containing nucleotides 150 to 1249 of mouse PC cDNA 27 was subcloned into the vector pGEM-3Z (Promega). This vector was linearized and used as a template for in vitro transcription of radiolabeled antisense or sense riboprobes using SP6 or T7 RNA polymerase (Promega), respectively, in the presence of 35S-labeled UTP (>1200 Ci/mmol; Amersham Corp., Arlington Heights, IL). Templates were removed by digestion with RQ1 DNase (Promega) for 15 minutes at 37°C, and the riboprobes were purified by phenol extraction and ethanol precipitation. Mouse urokinase-type plasminogen activator (u-PA) mRNA was used as a control in some experiments. The riboprobes for mouse u-PA were prepared as described previously. 21
In Situ Hybridization
In situ hybridizations were performed as described previously. 21,29 After hybridization, the slides were dehydrated by immersion in a graded alcohol series containing 0.3 mol/L NH4Ac and then dried and either placed directly on XAR-5 film (Eastman Kodak, Rochester, NY) for regional in situ autoradiography or coated with emulsion for high-resolution analysis. For regional autoradiography, the films were developed and photographed after incubation in the dark at room temperature for 2 weeks. For high-resolution analysis, the slides were coated with NTB2 emulsion (Kodak; 1:2 in water) and exposed in the dark at 4°C for 4 to 12 weeks. The slides were developed for 2 minutes in D19 developer (Kodak), fixed, washed in water, and counterstained with hematoxylin and eosin. No specific hybridization signal could be detected in parallel sections using 35S-labeled sense probes for nonspecific hybridization in each experiment (data not shown).
Immunohistochemistry
Immunohistochemical staining was performed using the HISTOSTAIN-SP Kit (Zymed Laboratories, South San Francisco, CA) as described previously. 21 Briefly, the paraffin-embedded tissues were deparaffinized, treated with 2% hydrogen peroxide to quench endogenous peroxidase activity, and dehydrated. The sections were then permeabilized by sequential treatment with 0.2% and 0.5% Triton X-100 in Tris-buffered saline. To unmask tissue antigens, sections were incubated at 37°C with prewarmed 0.23% (w/v) pepsin (2830 U/mg; Worthington Biochemical Corp., Freehold, NJ) in 0.01 N HCl for 8 minutes. After incubation with 10% normal goat serum for 30 minutes, the slides were incubated with primary rabbit antibodies (25 μg/ml of anti-human PC immunoglobulin G; Sigma Chemical Co.) containing 0.1% bovine serum albumin for 16 to 18 hours at 4°C, followed by incubation for 1 hour at 25°C. According to the manufacturer, this polyclonal antibody shows no reaction with PC-depleted human plasma using immunoblotting methods. In control experiments, tissues were incubated with preimmune (normal) rabbit immunoglobulin G instead of primary antibody. The slides were then washed and treated sequentially with biotinylated goat anti-rabbit immunoglobulin G (Zymed Laboratories), streptavidin-peroxidase conjugate (Zymed Laboratories) and aminoethylcarbazole chromagen containing 0.03% hydrogen peroxide (Zymed Laboratories). After rinsing in distilled water for 3 minutes, the slides were counterstained with Gill modified hematoxylin for 20 seconds, rinsed well with tap water, and mounted in GVA mounting solution (Zymed Laboratories).
Results
Tissue Distribution of PC mRNA in Murine Tissues
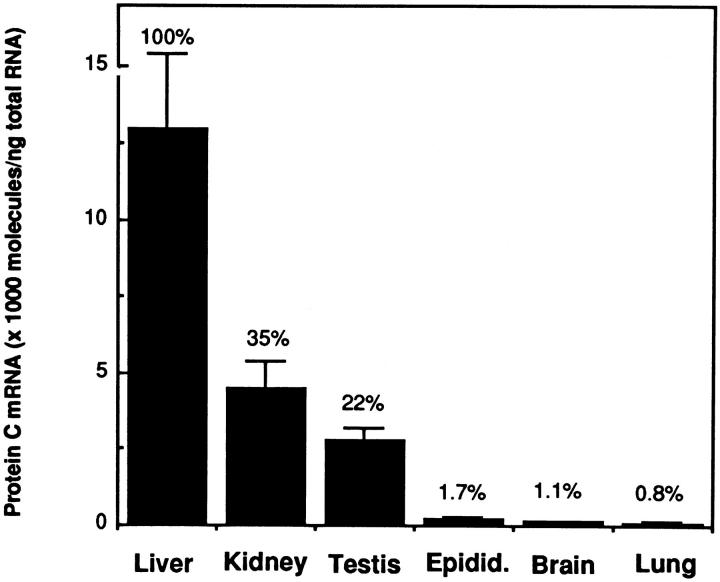
Experiments were performed to determine the concentration of PC mRNA in various mouse tissues. Total RNA was extracted from murine tissues, and the concentration of PC mRNA was determined by quantitative RT-PCR (see Materials and Methods). In these experiments, 1 μg of total tissue RNA and a fixed amount of the cRNA standard (ie, 1 × 107 molecules for the liver; 1 × 106 molecules for the kidney and testis; and 1 × 105 molecules for the epididymis, brain, and lung) were combined and then quantitated by RT-PCR. The results are shown in Figure 1 ▶ . As expected, the liver contained the highest concentration of PC mRNA (1.3 ± 0.24 × 107 molecules of PC mRNA/μg total tissue RNA). However, the kidney and the testis also contained relatively high amounts, representing 35% and 22%, respectively, of the amount detected in the liver. Low but significant levels of PC mRNA also were detected in the epididymis (1.7% of the liver), brain (1.1% of the liver), and lung (0.8% of the liver). PC mRNA was not detected in the heart, thymus, spleen, gut, pancreas, adrenal, skeletal muscle, adipose tissue, uterus, and aorta (data not shown).
Figure 1.
Distribution of PC mRNA in tissues of the normal mouse. The concentration of PC mRNA in various tissues from adult CB6 mice was determined by quantitative RT-PCR as described in Materials and Methods. Data are means (bars, SD) of three different experiments. The numbers above each bar represent the relative percentage of PC mRNA in that tissue compared with the level in the liver. Epidid.: Epididymis.
Localization of PC mRNA and Antigen in Murine Tissues
To identify the cellular sites of PC synthesis in the above tissues, tissue sections were hybridized to 35S-labeled probes and analyzed by regional in situ autoradiography and by high-resolution in situ hybridization (Figure 2) ▶ . The tissue sections also were stained immunohistochemically using rabbit anti-human PC antibody as described in Materials and Methods (Figures 2 and 3) ▶ ▶ . As expected, hepatocytes in the liver expressed abundant PC mRNA and antigen (data not shown). In the kidney, PC mRNA was expressed exclusively in the cortex (Figure 2A) ▶ , in contrast to the expression of u-PA mRNA, which was localized primarily to the medulla (Figure 2B ▶ 21). High-resolution in situ hybridization analysis revealed that the PC mRNA in the renal cortex was localized specifically to epithelial cells of the proximal and distal convoluted tubules (Figure 2 ▶ , C and D). Immunohistochemical staining demonstrated similar localization of PC antigen (red stain) in the kidney (Figure 2 ▶ , E and F). No expression of PC mRNA or antigen was observed in glomerular endothelial cells or mesangial cells or in epithelial cells of the straight portions of tubules in the renal medulla. In the testis, PC mRNA was detected in spermatogenic cells present in both seminiferous tubules (Figure 2G) ▶ and in efferent ducts (data not shown). Epithelial cells in the epididymis also expressed abundant PC mRNA (Figure 2H) ▶ and antigen (not shown). However, no signal was detected in interstitial cells or in Leydig cells in the testis (not shown).
Figure 2.
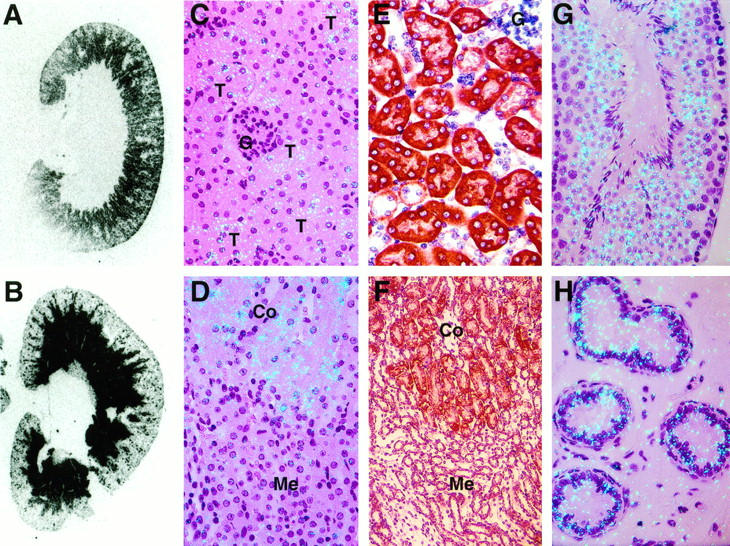
Histological analysis of PC mRNA and antigen in the kidney and male reproductive organs of the normal mouse. Tissue sections from adult CB6 mice were analyzed by in situ hybridization for PC or u-PA mRNA and by immunohistochemical staining for PC antigen. A and B: Analysis of kidney sections by regional in situ autoradiography for PC (A) or u-PA (B) mRNAs. The slides were incubated on X-ray film in the dark at room temperature and developed after 2 weeks of exposure. C and D: High-resolution in situ hybridization analysis of kidney sections for PC mRNA (magnification, ×400). E and F: Immunohistochemical staining for PC antigen in kidney sections (magnification: E, ×400; F, ×200). In F, PC antigen is indicated by the red-brown stain. G and H: In situ hybridization for PC mRNA in male reproductive organs. G, testis (magnification, ×400); H, epididymis (magnification, ×400). Slides for C and D were exposed for 8 weeks at 4°C, and those for E and F were exposed for 12 weeks at 4°C. The slides were then stained with hematoxylin and eosin. C to H: G, glomeruli; T, tubules; Co, cortex; Me, medulla.
Figure 3.
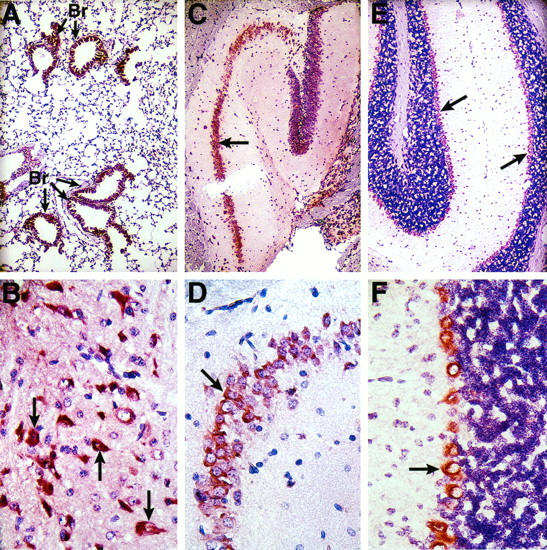
Histological analysis of PC antigen in the lung and brain of the normal mouse. Tissue sections from adult CB6 mice were analyzed by immunohistochemistry for PC antigen (red-brown stain) as described in Materials and Methods. A: Lung (magnification, ×100). Br denotes bronchioles. B: Cerebral cortex (magnification, ×400). Arrows indicate typical pyramidal neurons. C and D: Hippocampal area in the cerebrum. Arrows indicate positive staining in neurons in the pyramidal layer of the hippocampal cortex. Magnification: C, ×100; D, ×400. E and F: Cerebellar cortex. Arrows indicate positive staining in Purkinje cells lining the outside granular layer in the cerebellar cortex. In control experiments, parallel sections were analyzed using a preimmune (normal) rabbit immunoglobulin G instead of anti-PC antibody. No staining was observed in those sections (data not shown). Magnification: E, ×100; F, ×400.
We also failed to detect a specific signal for PC mRNA in the brain and lung by in situ hybridization (data not shown), even though PC mRNA was detected within these tissues by quantitative RT-PCR. However, PC antigen was detected in the bronchial epithelial cells of the lung (Figure 3A ▶ , red-brown stain), in pyramidal neurons in the cerebral cortex (Figure 3B) ▶ , in neurons in the pyramidal layer of the hippocampal cortex (Figure 3 ▶ , C and D), and in Purkinje cells lining the outer surface of the granular layer in the cerebellar cortex (Figure 3 ▶ , E and F). No specific staining for PC antigen was observed in tissues that did not contain detectable levels of PC mRNA when analyzed by RT-PCR (ie, in the heart, thymus, spleen, adrenal gland, and aorta; data not shown). Although PC synthesis was previously demonstrated in human umbilical vein endothelial cells, 30 no PC mRNA or antigen was observed in endothelial cells in any of the tissues examined (data not shown).
Decreased Renal Expression of PC mRNA in Mice with Kidney Disease
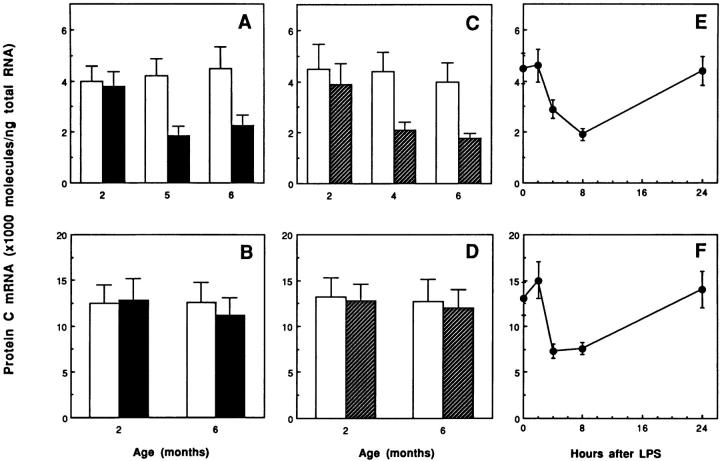
Figure 1 ▶ demonstrates that the kidney is a major site of PC synthesis. The kidney is also one of the primary sites of thrombosis in a variety of disorders. We therefore used quantitative RT-PCR (Figure 4) ▶ and in situ hybridization (Figure 5) ▶ to determine whether PC gene expression is altered in the kidneys of mice with renal disease (ie, female MRL lpr/lpr mice, a model of lupus nephritis; db/db mice, a model of type II diabetes; and LPS-treated mice, a model of sepsis). No significant differences in PC mRNA levels were observed in kidneys from lupus-prone or diabetes-prone mice 2 months of age (Figure 4 ▶ , A and C). These observations are consistent with the fact that histopathological changes in the kidneys of female MRL lpr/lpr mice and db/db mice usually do not become apparent until 4 to 6 months of age. 22,31-33 However, marked decreases in PC mRNA (50 to 60% reduction) were observed in kidneys of these mice at 5 to 6 months of age (Figure 4 ▶ , A and C). These age-related decreases in PC expression seem to be specific for the diseased kidneys, given that no changes in PC mRNA levels were observed in similarly aged control kidneys or in livers from the control versus the disease-prone animals (Figure 4 ▶ , B and D). In situ hybridization analysis revealed that the reduction of PC mRNA in the lupus-prone mice occurred specifically in the tubular epithelial cells of kidneys having the characteristic histopathological changes associated with lupus nephritis (compare Figure 5 ▶ , A and B). Similarly, the kidneys of 6-month-old db/db mice (ie, with pathological changes characteristic of diabetic nephropathy including mesangial matrix accumulation in glomeruli and hyaline material in the interstitium; Figure 5D ▶ ) also showed decreased expression of PC mRNA in tubular cells (Figure 5D) ▶ compared with their normal counterparts (Figure 5C) ▶ .
Figure 4.
Changes in PC mRNA levels in the kidneys and livers of mice with renal disease. Total kidney and liver RNAs were extracted from female MRL lpr/lpr mice and their controls (MRL +/+) at 2, 5, and 6 months of age; from male db/db mice and their normal counterparts (C57BL/KsJ +/?) at 2, 4, and 6 months of age; and from control (0 time point) and LPS (50 μg)-treated normal CB6 mice at 2, 4, 8, and 24 hours after injection. PC mRNA was determined using quantitative RT-PCR analysis as described in Materials and Methods. A and B: Kidneys (A) and livers (B) from control (open bar) and female MRL lpr/lpr (closed bar) mice. C and D: Kidneys (C) and livers (D) from control (open bar) and db/db (hatched bar) mice. E and F: Kidneys (E) and livers (F) from LPS-treated mice. Data are means (bars, SD) of three different experiments.
Figure 5.
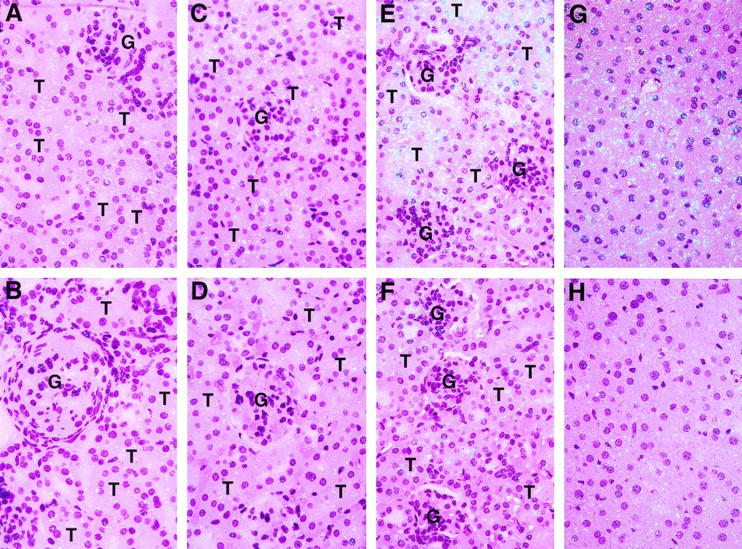
High-resolution in situ hybridization analysis of PC mRNA in kidneys and livers of mice with renal disease. Kidney sections from control (MRL +/+; A) and lupus-prone (female, MRL lpr/lpr; B) mice of 5 months of age, from control (C57BL/KsJ +/?; C) and db/db (D) mice 6 months of age, and from control (saline-treated; E) and LPS-treated (8 hours; F) mice were analyzed by high-resolution in situ hybridization as described in Materials and Methods. Liver sections (G and H) from control (G) and LPS-treated (8 hours; H) mice were also examined by high-resolution in situ hybridization analysis. G, glomeruli; T, tubules. Slides for A to F were exposed for 8 weeks at 4°C, whereas those for (G) and (H) were exposed for 4 weeks at 4°C. The slides were then stained with hematoxylin and eosin; magnification, ×400.
Finally, the effect of LPS on PC mRNA expression in the kidney and liver was examined. In this model, PC mRNA levels were significantly decreased (50 to 60% reduction) in both the kidney and liver at 4 to 8 hours after LPS injection (Figure 4 ▶ , E and F). This was a transient effect, with PC mRNA levels returning to control levels by 24 hours (Figure 4 ▶ , E and F). High-resolution in situ hybridization demonstrated that LPS caused a marked and specific decrease in PC mRNA in tubular epithelial cells of the kidney (compare Figure 5 ▶ , E and F) and in hepatocytes of the liver (compare Figure 5 ▶ , G and H).
Discussion
Although the liver is regarded as the major site of PC synthesis, 12,13,27,34 a few recent reports suggest that PC also may be synthesized extrahepatically. 13,14 For example, Northern blotting revealed PC mRNA in several rat tissues although at much lower levels than the liver, 14 and PC antigen was detected in human male reproductive tissues (eg, testis, epididymis, and prostate). 16 In this regard, human seminal plasma contains PC antigen at concentrations that are approximately 5% of those in plasma. 16 In this report, we not only extend these studies to the mouse, but we also use more sensitive PCR approaches to demonstrate PC gene expression in other tissues, and in situ hybridization approaches to localize it to specific cells. Our observations also support the conclusion that PC is not a liver-specific protein.
The level of extrahepatic PC mRNA expression was the highest in the kidney and testis, representing 35% and 22%, respectively, of that in the liver (Figure 1) ▶ . Histological approaches revealed abundant amounts of PC mRNA and antigen in the epithelial cells of the convoluted tubules in the renal cortex (Figure 2) ▶ . Interestingly, in the human kidney, renal tubular cells also appear to synthesize PC inhibitor, 19 and they are the major source of u-PA, another target of the PC inhibitor. 35 However, the cells responsible for the synthesis of u-PA are located in the straight portion of tubules in the renal medulla 36 (Figure 2) ▶ . The different patterns of expression of PC and u-PA in the kidney (Figure 2) ▶ imply that different portions of the renal tubules may synthesize different anticoagulant and/or fibrinolytic proteins. It should be noted that protein S is also synthesized in the kidney, at least in the rat. 14,37,38 The relatively high concentration of PC in the kidney and the demonstration that the kidney also produces the PC inhibitor, u-PA and protein S, suggest that the PC pathway may function as a local anticoagulant in the renal circulation.
In the testis, PC mRNA and antigen were detected in spermatogenic cells and in epithelial cells of the epididymis in the mouse (Figure 2) ▶ in agreement with the human studies. However, we did not detect it in Leydig cells in the testis, as observed in the human case. 16 The different PC mRNA expression pattern in human and murine testis may reflect species variations. In any case, these results raise the possibility that the PC present in reproductive tissues may contribute to the fluidity of the seminal plasma.
Low concentrations of PC mRNA also were demonstrated in the lung (Figure 1) ▶ , and PC antigen was specifically detected in bronchial epithelial cells (Figure 3) ▶ . However, no specific signal for PC mRNA was detected in these cells. The function of PC in the lung remains to be determined. Low levels of PC mRNA also were detected in the murine brain (Figure 1) ▶ . Immunohistochemical staining analysis revealed that PC antigen was expressed specifically in pyramidal neurons in the cerebral cortex and in the hippocampal area and in Purkinje cells in the cerebellum (Figure 3) ▶ . Again, we were unable to demonstrate a specific signal for PC mRNA in these cells. The failure to demonstrate PC mRNA in specific cells of the lung and brain suggests that its concentration in these cells is below the detection level of in situ hybridization. Although the function of PC in the brain is unknown, a number of other PC pathway genes appear to be expressed in the central nervous system in vivo. These include prothrombin, 39 protein S, 37 and thrombomodulin, an important co-factor for the activation of PC. 40 Interestingly, the distribution of PC antigen in the murine cerebrum is very similar to that observed for protein S in rabbits. 37 These observations suggest that the PC pathway may act as a local anticoagulant in the cerebral and cerebellar circulation. It should be noted that tissue plasminogen activator is also synthesized by a variety of neurons in the brain 41 and appears to be induced in Purkinje neurons after cerebellar motor learning. 42 Thus, the plasminogen activator-plasmin system may also contribute to neuronal plasticity. In this context, it has been reported that APC inactivates plasminogen activator inhibitor-1, 5,6 the primary inhibitor of plasminogen activators. Taken together, these observations raise the possibility that the PC system contributes to the physiology of the central nervous system.
Experiments were performed in an attempt to relate changes in the extrahepatic expression of PC to tissue function and disease. The kidney was selected for these studies not only because of its relatively high level of expression of PC, but also because of the availability of numerous murine models of renal disease. Thus, we investigated the expression of PC in three different murine models of renal disease (ie, in autoimmune lupus nephritis, in diabetic nephropathy, and in acute renal injury in sepsis). Significant decreases in PC mRNA levels were observed in kidneys from lupus-prone (MRL lpr/lpr) and diabetes-prone (db/db) mice compared with control kidneys (Figures 4 and 5) ▶ ▶ . Again, these decreases in PC expression seemed to be specific for the diseased kidneys, because no differences in PC mRNA levels were observed in similarly aged control kidneys or in livers of control, lupus-prone, or diabetes-prone mice (Figure 4) ▶ .
Fibrin deposits are often detected in the renal microvasculature of lupus nephritic lesions, 43 and a fibrin cap and occluded capillaries are often formed in the glomeruli of diabetic nephropathy. These alterations may lead to tubular dysfunction in these renal pathologies. 44 Fibrin itself may promote the progression of renal damage by occluding microvessels, by inducing glomerular cell proliferation, and by a direct cytotoxic effect on mesangial cells. 45 In this regard, the observed reduction in renal PC expression may result in an increase in the local procoagulant potential of the kidney, leading to a predisposition to renal microthrombosis and in the development of renal injuries under these pathological conditions. Interestingly, PC activity has been reported to decrease in patients with chronic renal insufficiency 46 and uremia, 47 possibly because of the presence of an inhibitor. Although these observations are consistent with the possibility that the observed reduction in PC expression could contribute to the development of several types of human renal disease, other reports show increased PC antigen in nephrotic syndrome. 48,49 Thus, elucidation of the exact role of the PC system in human renal disease will require detailed analyses of PC activity, antigen, and mRNA.
Marked decreases in PC mRNA expression also were demonstrated in kidneys from endotoxin-treated mice (Figures 4 and 5) ▶ ▶ . However, in this instance, the decrease was not restricted to the kidneys, because PC gene expression in the liver was reduced to a similar extent. Interestingly, plasma PC levels were observed to be consistently decreased in patients with sepsis. 50,51 Although it was suggested that these decreases resulted from increased PC consumption, our observations support the alternative hypothesis that endotoxin may act by decreasing the rate of PC synthesis in the liver and kidney, the two organs that contribute most to the composition of the plasma. Endotoxin has been reported to alter the expression of a variety of hemostatic genes. For example, endotoxin administration frequently results in an increase in tissue factor procoagulant activity and in antifibrinolytic potential (ie, plasminogen activator inhibitor-1 activity) in vitro 52,53 and in vivo. 54,55 Moreover, PC activation and/or thrombomodulin expression decreases when endothelial cells in culture are exposed to endotoxin 52 or tumor necrosis factor-α. 56,57 We previously showed that microvascular fibrin deposition was selectively induced in the kidney by endotoxin injection into normal mice, primarily because of the dramatic induction of plasminogen activator inhibitor-1 and tissue factor and the reduction of u-PA in the kidney. 21 The decrease in renal PC expression after endotoxin injection may also contribute to the increase in local procoagulant potential, eventually leading to fibrin deposition in the renal microvasculature. The molecular mechanism by which PC expression is decreased in these renal pathologies is unknown. However, in preliminary studies, we observed that injection of tumor necrosis factor-α into normal mice also decreased PC mRNA levels in the liver and kidney (data not shown). Tumor necrosis factor-α is known to be one of the primary inflammatory mediators in sepsis, and its expression appears to be elevated in lupus nephritis 58,59 and in obesity/non-insulin-dependent diabetes mellitus. 60 Taken together, these results suggest that tumor necrosis factor-α may be one of the cytokines involved in the decreased expression of PC under these pathological conditions.
In summary, our data not only demonstrate PC gene expression in a variety of murine tissues, but also show that the expression of this potent anticoagulant molecule is altered in thrombotic diseases of the kidney. Our results thus suggest that PC may contribute to the evolution of these pathologies. The nature of factors that control the expression and activity of PC in the kidney and other tissues, and the exact function of the PC pathway in physiological and pathological processes of the kidney, testis, epididymis, brain, and lung, remain to be determined.
Acknowledgments
The authors thank T. Thinnes for expert technical assistance and M. McRae for excellent secretarial assistance.
Footnotes
Address reprint requests to Dr. David J. Loskutoff, Department of Vascular Biology, VB-3, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037. E-mail:loskutof@scripps.edu.
Supported by National Institutes of Health Grant HL-47819 (to DJL).
References
- 1.Kisiel W: Human plasma protein C. Isolation, characterization and mechanism of activation by α-thrombin. J Clin Invest 1979, 64:761-769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esmon CT: The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem 1989, 264:4743-4746 [PubMed] [Google Scholar]
- 3.Marlar RA, Kleiss AJ, Griffin JH: Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood 1982, 59:1067-1072 [PubMed] [Google Scholar]
- 4.Bajzar L, Manuel R, Nesheim ME: Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem 1995, 270:14477-14484 [DOI] [PubMed] [Google Scholar]
- 5.van Hinsbergh VWM, Bertina RM, van Wijngaarden A, van Tilburg NH, Emeis JJ, Haverkate F: Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood 1985, 65:444-451 [PubMed] [Google Scholar]
- 6.Sakata Y, Loskutoff DJ, Gladson CL, Hekman CM, Griffin JH: Mechanism of protein C-dependent clot lysis: role of plasminogen activator inhibitor. Blood 1986, 68:1218-1223 [PubMed] [Google Scholar]
- 7.Seligsohn U, Berger A, Abend M, Rubin L, Attias D, Zivelin A, Rapaport SI: Homozygous protein C deficiency manifested by massive venous thrombosis in the newborn. N Engl J Med 1984, 310:559-562 [DOI] [PubMed] [Google Scholar]
- 8.Bovill EG, Bauer KA, Dickerman JD, Callas P, West B: The clinical spectrum of heterozygous protein C deficiency in a large New England kindred. Blood 1989, 73:712-717 [PubMed] [Google Scholar]
- 9.Kurosawa-Ohsawa K, Kimura M, Kume-Iwaki A, Tanaka T, Tanaka S: Anti-protein C monoclonal antibody induces thrombus in mice. Blood 1990, 75:2156-2163 [PubMed] [Google Scholar]
- 10.Taylor FB, Chang A, Jr, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE: Protein C prevents the coagulopathic, and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest 1987, 79:918-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerson WT, Dickerman JD, Bovill EG, Golden E: Severe acquired protein C deficiency in purpura fulminans associated with disseminated intravascular coagulation: treatment with protein C concentrate. Pediatrics 1993, 91:418-422 [PubMed] [Google Scholar]
- 12.Beckmann RJ, Schmidt RJ, Santerre RF, Plutzky J, Crabtree GR, Long GL: The structure and evolution of a 461 amino acid human protein C precursor and its messenger RNA, based upon the DNA sequence of cloned human liver cDNAs. Nucleic Acids Res 1985, 13:5233-5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okafuji T, Maekawa K, Nawa K, Marumoto Y: The cDNA cloning and mRNA expression of rat protein C. Biochim Biophys Acta 1992, 1131:329-332 [DOI] [PubMed] [Google Scholar]
- 14.Jamison CS, McDowell SA, Marlar RA, Friezner Degen SJ: Developmental expression of protein C and protein S in the rat. Thromb Res 1995, 78:407-419 [DOI] [PubMed] [Google Scholar]
- 15.Esmon CT: The regulation of natural anticoagulant pathways. Science 1987, 235:1348-1352 [DOI] [PubMed] [Google Scholar]
- 16.He X, Shen L, Bjartell A, Malm J, Lilja H, Dahlback B: The gene encoding vitamin K-dependent anticoagulant protein C is expressed in human male reproductive tissues. J Histochem Cytochem 1987, 235:1348-1352 [DOI] [PubMed] [Google Scholar]
- 17.Malm J, He X, Bjartell A, Shen L, Dahlback B: Synthesis of vitamin K-dependent protein S in the Leidig cells of human testis. Biochem J 1994, 302:845-850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurell M, Christensson A, Abrahamsson P, Stenflo J, Lilja H: Protein C inhibitor in human body fluids: seminal plasma is rich in inhibitor antigen deriving from cells throughout the male reproductive system. J Clin Invest 1992, 89:1094-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radtke K-P, Fernández JA, Greengard JS, Tang WW, Wilson CB, Loskutoff DJ, Scharrer I, Griffin JH: Protein C inhibitor is expressed in tubular cells of human kidney. J Clin Invest 1994, 94:2117-2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang AM, Doyle MV, Mark DF: Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci USA 1989, 86:9717-9721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto K, Loskutoff DJ: Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest 1996, 97:2440-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theofilopoulos AN, Dixon FJ: Murine models of systemic lupus erythematosus. Adv Immunol 1985, 37:269-330 [DOI] [PubMed] [Google Scholar]
- 23.Hummel KP, Dickie MM, Coleman DL: Diabetes, a new mutation in the mouse. Science 1966, 153:1127-1128 [DOI] [PubMed] [Google Scholar]
- 24.Coleman DL, Hummel KP: Studies with the mutation, diabetes, in the mouse. Diabetologia 1967, 3:238-248 [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 26.Samad F, Yamamoto K, Loskutoff DJ: Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo: induction by tumor necrosis factor-α and lipopolysaccharide. J Clin Invest 1996, 97:37-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tada N, Sato M, Tsujimura A, Iwase R, Hashimoto-Gotoh T: Isolation and characterization of a mouse protein C cDNA. J Biochem 1992, 111:491-495 [DOI] [PubMed] [Google Scholar]
- 28.Tokunaga K, Taniguchi H, Yoda K, Shimizu M, Sakiyama S: Nucleotide sequence of a full-length cDNA for mouse cytoskeletal β-actin mRNA. Nucleic Acids Res 1986, 14:2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keeton M, Eguchi Y, Sawdey M, Ahn C, Loskutoff DJ: Cellular localization of type 1 plasminogen activator inhibitor messenger RNA and protein in murine renal tissue. Am J Pathol 1993, 142:59-70 [PMC free article] [PubMed] [Google Scholar]
- 30.Tanabe S, Sugo T, Matsuda M: Synthesis of protein C in human umbilical vein endothelial cells. J Biochem 1991, 109:924-928 [DOI] [PubMed] [Google Scholar]
- 31.Datta SK: Murine lupus. Methods Enzymol 1988, 162:385-412 [DOI] [PubMed] [Google Scholar]
- 32.Like AA, Lavine RL, Poffenbarger PL, Chick WL: Studies in the diabetic mutant mouse. VI. Evolution of glomerular lesions and associated proteinuria. Am J Pathol 1972, 66:193-204 [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MP, Clements RS, Hud E, Cohen JA, Ziyadeh FN: Evolution of renal function abnormalities in the db/db mouse that parallels the development of human diabetic nephropathy. Exp Nephrol 1996, 4:166-171 [PubMed] [Google Scholar]
- 34.Long GL, Belagaje RM, MacGillivray RTA: Cloning and sequencing of liver cDNA coding for bovine protein C. Proc Natl Acad Sci USA 1984, 81:5653-5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stief TW, Radtke K, Heimburger N: Inhibition of urokinase by protein C-inhibitor (PCI): evidence for identity of PCI and plasminogen activator inhibitor 3 (PAI 3). Biol Chem Hoppe-Seyler 1987, 368:1427-1433 [DOI] [PubMed] [Google Scholar]
- 36.Sappino A-P, Huarte J, Vassalli J-D, Belin D: Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J Clin Invest 1991, 87:962-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X, Shen L, Bjartell A, Dahlback B: The gene encoding vitamin K-dependent anticoagulant protein S is expressed in multiple rabbit tissues as demonstrated by Northern blotting, immunocytochemistry and in situ hybridization. J Histochem Cytochem 1995, 43:85-96 [DOI] [PubMed] [Google Scholar]
- 38.Yasuda F, Hayashi T, Tanitame K, Nishioka J, Suzuki K: Molecular cloning and functional characterization of rat plasma protein S. J Biochem 1995, 117:374-383 [DOI] [PubMed] [Google Scholar]
- 39.Dihanich M, Kasser M, Reinhard E, Cunningham D, Monard D: Prothrombin mRNA is expressed by cells of the nervous system. Neuron 1991, 6:575-581 [DOI] [PubMed] [Google Scholar]
- 40.Wong VLY, Hofman FM, Ishii H, Fisher M: Regional distribution of thrombomodulin in human brain. Brain Res 1991, 556:1-5 [DOI] [PubMed] [Google Scholar]
- 41.Sappino A-P, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli J-D: Extracellular proteolysis in the adult murine brain. J Clin Invest 1993, 92:679-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeds NW, Williams BL, Bickford PC: Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science 1995, 270:1992-1994 [DOI] [PubMed] [Google Scholar]
- 43.Kant KS, Pollak VE, Weiss MA, Glueck HI, Miller MA, Hess EV: Glomerular thrombosis in systemic lupus erythematosus: prevalence and significance. Medicine 1981, 60:71-86 [DOI] [PubMed] [Google Scholar]
- 44.Viberti G, Wiseman MJ, Pinto JR, Messent J: Diabetic nephropathy. Kahn CR Weir GC eds. Joslin’s Diabetes Mellitus. 1994, :pp 691-737 Lea & Febiger, Philadelphia [Google Scholar]
- 45.Tsumagari T, Tanaka K: Effects of fibrinogen degradation products on glomerular mesangial cells in culture. Kidney Int 1984, 26:712-718 [DOI] [PubMed] [Google Scholar]
- 46.Faioni EM, Franchi F, Krachmalnicoff A, Valsecchi C, Vigano G, Remuzzi G, Mannucci PM: Low levels of the anticoagulant activity of protein C in patients with chronic renal insufficiency: an inhibitor of protein C is present in uremic plasma. Thromb Haemostasis 1991, 66:420-425 [PubMed] [Google Scholar]
- 47.Sorensen PJ, Knudsen F, Nielsen AH, Dyerberg J: Protein C assays in uremia. Thromb Res 1989, 54:301-310 [DOI] [PubMed] [Google Scholar]
- 48.Cosio FG, Harker C, Batard MA, Brandt JT, Griffin JH: Plasma concentrations of the natural anticoagulants protein C and protein S in patients with proteinuria. J Lab Clin Med 1985, 106:218-222 [PubMed] [Google Scholar]
- 49.Mannucci PM, Valsecchi C, Bottasso B, D’Angelo A, Casati S, Ponticelli C: High plasma levels of protein C activity and antigen in the nephrotic syndrome. Thromb Haemostasis 1986, 55:31-33 [PubMed] [Google Scholar]
- 50.Hesselvik JF, Malm J, Dahlback B, Blomback M: Protein C, protein S, and C4b-binding protein in severe infection, and septic shock. Thromb Haemostasis 1991, 65:126-129 [PubMed] [Google Scholar]
- 51.Fijnvandraat K, Derkx B, Peters M, Bijlmer R, Sturk A, Prins MH, van Deventer SJH, ten Cate JW: Coagulation activation and tissue necrosis in meningococcal septic shock: severely reduced protein C levels predict a high mortality. Thromb Haemostasis 1995, 73:15-20 [PubMed] [Google Scholar]
- 52.Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU: Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest 1987, 79:124-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawdey M, Podor TJ, Loskutoff DJ: Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells: induction by transforming growth factor-β, lipopolysaccharide, and tumor necrosis factor-α. J Biol Chem 1989, 264:10396-10401 [PubMed] [Google Scholar]
- 54.Mackman N, Sawdey MS, Keeton MR, Loskutoff DJ: Murine tissue factor gene expression in vivo: tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol 1993, 143:76-84 [PMC free article] [PubMed] [Google Scholar]
- 55.Collen D, Rijken DC, Van Damme J, Billiau A: Purification of human tissue-type plasminogen activator in centigram quantities from human melanoma cell culture fluid and its conditioning for use in vivo. Thromb Haemostasis 1982, 48:294-296 [PubMed] [Google Scholar]
- 56.Conway EM, Rosenberg RD: Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol 1988, 8:5588-5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lentz SR, Tsiang M, Sadler JE: Regulation of thrombomodulin by tumor necrosis factor-α: comparison of transcriptional and posttranscriptional mechanisms. Blood 1991, 77:542-550 [PubMed] [Google Scholar]
- 58.Boswell JM, Yui MA, Burt DW, Kelley VE: Increased tumor necrosis factor and IL-1β gene expression in the kidneys of mice with lupus nephritis. J Immunol 1988, 141:3050-3054 [PubMed] [Google Scholar]
- 59.Brennan DC, Yui MA, Wuthrich RP, Kelley VE: Tumor necrosis factor and IL-1 in New Zealand Black/White mice: enhanced gene expression and acceleration of renal injury. J Immunol 1989, 143:3470-3475 [PubMed] [Google Scholar]
- 60.Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 1993, 259:87-91 [DOI] [PubMed] [Google Scholar]